Abstract
Background and Objectives
In the past four decades numerous studies have reported the efficacy of low level light (laser) therapy (LLLT) as a treatment for diverse diseases and injuries. Recent studies have shown that LLLT can biomodulate processes in the central nervous system and has been extensively studied as a stroke treatment. However there is still a lack of knowledge on the effects of LLLT at the cellular level in neurons. The present study aimed to study the effect of 810 nm laser on several cellular processes in primary cortical neurons cultured from embryonic mouse brains.
Study Design/Materials and Methods
Neurons were irradiated with fluences of 0.03, 0.3, 3, 10, or 30 J/cm2 of 810-nm laser delivered over varying times at 25 mW/cm2 and intracellular levels of reactive oxygen species (ROS), nitric oxide and calcium were measured using fluorescent probes within 5 minutes of the end of irradiation. The changes in mitochondrial function in response to light were studied in terms of adenosine triphosphate (ATP) and mitochondrial membrane potential (MMP).
Results
Light induced a significant increase in calcium, ATP and MMP at lower fluences and a decrease at higher fluences. ROS was significantly induced at low fluences, followed by a decrease and a second larger increase at 30 J/cm2. Nitric oxide levels showed a similar pattern of a double peak but values were less significant compared to ROS.
Conclusions
The results suggest that LLLT at lower fluences is capable of inducing mediators of cell signaling processes which in turn may be responsible for the beneficial stimulatory effects of the low level laser. At higher fluences beneficial mediators are reduced and high levels of Janus-type mediators such as ROS and NO (beneficial at low concentrations and harmful at high concentrations) may be responsible for the damaging effects of high-fluence light and the overall biphasic dose response.
Keywords: low level laser therapy, LLLT, photobiomodulation, cultured cortical neurons, near-infra red laser, reactive oxygen species, nitric oxide, mitochondrial membrane potential, intracellular calcium, ATP, biphasic dose response
INTRODUCTION
Low-level light (laser) therapy (LLLT) has gained considerable interest in recent years as a therapy for the treatment of a variety of diseases and injuries by eliciting significant biological effects at a cellular and tissular level. This therapeutic modality is supported by studies of various animal models and a number of clinical trials many of which are randomized controlled trials. Different conditions for which this therapy has shown positive outcome are pain relief [1–3], inflammation [4], and wound healing [5–7]. In the last few years this therapy has been extended for the improvement of more severe conditions such as stroke, myocardial infarctions, neurodegenerative disorders and traumatic brain injury [8]. There is a growing number of reports demonstrating the promising outcome for LLLT in diseases related to the nervous system. Some of the positive effects shown in the neurons are: the promotion of axonal growth and nerve regeneration in both rat spinal cord [9,10] and peripheral nerve injuries [11] after LLLT. The efficacy of LLLT in the nervous system has been further established in animal studies showing improved neurological and functional outcomes post-stroke [12–14], post-traumatic brain injury [15], and in a mouse model of Alzheimer’s disease [16]. LLLT also improves emotional response and memory function of middle aged CD-1 mice [17]. The results of human clinical studies in patients with long term peripheral nerve injury [6] and ischemic stroke [18,19] have also been promising. Recently a study was performed on two human chronic TBI patients which showed that transcranial LED may improve cognition [20].
Although the data for therapeutic usefulness of LLLT for neurological disorders is promising, the medical and research community at large is still skeptical about this therapy. The reason for these reservations is the lack of fundamental understanding into the basic mechanistic effects of LLLT and how the cellular effects in the neurons are translated into improved brain functions. The proposed mechanism of photobiomodulation is largely assumed to involve stimulation of the cellular energy metabolism and energy production mediated by mitochondria acting as the primary cellular photoreceptor or target for absorbing the photons. Within mitochondria, cytochrome c oxidase (complex 4 of the respiratory chain) is considered to be the principal chromophore absorbing the incident light although other cytochromes, porphyrins, and heme proteins could be involved. Light absorption further leads to increased activity of cytochrome c oxidase [21], release of nitric oxide (NO) [22], and an increase in ATP levels [23,24]. Changes in intracellular signaling molecules such as calcium ions, reactive oxygen species (ROS) and redox sensitive transcription factors like NF-kB are also thought to mediate the effects of light. Previous studies from our laboratory in mouse embryonic fibroblast cells [25] have shown that 810 nm laser induced ROS-mediated NF-kB activation. However the effects of 810-nm laser on neurons have not yet been studied.
LLLT studies have demonstrated that light follows a biphasic response [26], along the lines of the frequently cited “Arndt-Schulz Law.” [27–31] The Arndt-Schulz Law, formulated in 1888, stated that various poisons, if given in very low doses, had a stimulatory effect; small dosages were beneficial, and large doses harmful. Stated in a generic manner, the law states that small doses stimulate, moderate doses inhibit, and large doses kill. Such an axiom fits well for LLLT: a biphasic dose response exists. An insufficient dose of energy would result in no response (or an insignificant one); if the dose of light is increased past the necessary threshold, then biostimulation takes place. However, if too much energy is applied, then not only does biostimulation decrease, but bioinhibition may take over. The biochemical mechanisms responsible for the biphasic dose response at the cellular level remain unknown although reactive oxygen species have been implicated.
The present study was designed to elucidate the basic cellular responses of mouse primary cortical neurons to LLLT (810 nm laser). The changes in cell signaling molecules are proposed to mediate the biomodulatory effects of LLLT on the brain. A wide range of fluences spanning three orders of magnitude was employed to increase the chances of detecting the biphasic dose response.
MATERIALS AND METHODS
Primary Mouse Cortical Neuron Isolation From Mice
All animal procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital and met the guidelines of the National Institutes of Health. Pregnant female C57BL/6 mice (aged 8–10 weeks) were sacrificed at 16 days post-conception. The cortical lobes of 6–10 embryonic brains were separated from subcortical structures in calcium-magnesium-free, Hank’s balanced salt solution containing 10 mM HEPES buffer (HBSS/HEPES) (Invitrogen–Gibco, Carlsbad, CA). After removal of the meninges, brain tissue was placed into HBSS/HEPES, triturated briefly with pipetting, and incubated at 37°C for 10 minutes with 2.5% trypsin (Invitrogen) and deoxyribonuclease I (Type IV). Cells were then further dissociated mechanically and centrifuged at 200g, at 4°C for 5 minutes. The pellet was resuspended in Neurobasal Media (NBM) (Invitrogen–Gibco) supplemented with glutamine (Sigma, St. Louis, MO), antibiotic-antimycotic solution (Sigma), and B27 supplement (Invitrogen–Gibco). Cells were plated at a density of approximately 30,000 cells/well on poly-d-lysine coated cell culture plates (Sigma) or sterile glass coverslips. The plating and maintenance media consisted of Neurobasal plus B27 supplement (NB27) This media formulation inhibits the outgrowth of glia resulting in a neuronal population which is >95% pure [32]. Experiments were performed on days 8–9 after plating the cells.
Laser Irradiation
The experiments were conducted with a diode laser (Photothera, Inc., Carlsbad, CA), which emits 810-nm near infrared radiation. The cells were irradiated in continuous wave mode with a power density of 25 mW/cm2 for different time periods to achieve energy densities of 0.03, 0.3, 3, 10, and 30 J/cm2.
ROS Production
The ROS was measured using MitoSox Red, a fluorescent indicator of mitochondrial superoxide. The cells were irradiated with the 810 nm laser after 10 minutes of incubation with the MitoSox Red (Invitrogen) at a final concentration of 5 µM in dark at 37°C. Five micrograms per milliliters of nuclear stain, Hoechst-33342 (Sigma) was also added. At the end of irradiation, the cells were washed twice with PBS (Sigma) and fluorescence was either measured by plate reader (Molecular Devices, Sunnyvale, CA) with excitation 510-nm and emission 580-nm or imaged with a confocal microscope (Olympus America, Inc., Center Valley, PA).
Intracellular NO Production
DAF-FM is essentially non-fluorescent until it is nitrosylated by products of oxidation of NO, resulting in DAF-FM triazole which exhibits about a 160-fold greater fluorescence quantum yield. DAF-FM diacetate (Invitrogen) and Hoechst-33342 (Sigma) were added to the cells to a final concentration of 5 µM and 5 µg/ml respectively and allowed to incubate for 30 minutes in dark at 37°C. The cells were then illuminated for different time periods with 810 laser. At the end of irradiation, the cells were washed twice with PBS (Sigma) and the fluorescence of activated DAF-FM was visualized with FITC-filter in a confocal microscope or measured in a fluorescence plate reader (excitation 495-nm and emission 520-nm).
Intracellular Calcium Assay
Fluo-4AM, which exhibits a large fluorescence intensity increase on binding to Ca2+, was used to measure the intracellular calcium levels. Cells were incubated with 5 µM of the indicator dye, fluo-4 Am (Invitrogen) and 5 µg/ml of Hoechst-33342 for 20 minutes at 37°C in calcium free Hanks buffer (Gibco). At the end of incubation, the cells were irradiated. At the end of irradiation, the cells were washed twice with PBS (Sigma) and the fluorescence was measured in a fluorescence plate reader (excitation 495-nm and emission 520-nm) or imaged in a confocal microscope.
Mitochondrial Membrane Potential (MMP) Measurement
JC-1 is a cationic dye that accumulates in mitochondria. This accumulation is dependent on the membrane potential and indicated by a fluorescence emission shift from green (~525 nm) to red (~590 nm). Consequently, mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio. After 20 minutes of incubation with JC-1 (Invitrogen) and Hoechst-33342 (final concentrations, 5 µg/ml) at 37°C, the cells were irradiated with the 810-nm laser. The cells were washed twice with the PBS (Sigma) and fluorescence was either measured by fluorescence plate reader (green fluorescence, excitation 485-nm and emission 535-nm; red fluorescence, excitation 590-nm and emission 610-nm) and imaged with a confocal microscope.
ATP Assay
After 5 minutes of irradiation, the cells in each well were lysed with 50 µl cell lysis buffer and the plate was placed on shaker for 2 minutes to ensure completely release of ATP. Five microliters lysate was then transferred to 96-well plates for BCA assay for protein concentration measurement, while the rest of the cell lysates were transferred to 96-well plates with white walls for ATP measurement. One hundred microliters of Cell-Titer Glo Assay (Promega, Madison, WI) was added into each sample and after about 5 minutes, the luminescence signal was measure by a luminescence plate reader (Wallac Tri-Lux beta, PerkinElmer Life and Analytical Sciences, Waltham, MA).
Statistical Analysis
All fluorescence and chemiluminescence readings were normalized to total protein (measured by BCA assay, Pierce Biotechnology, Inc., Rockford, IL). All assays were performed in triplicate with n = 9 for each sample. Excel software was used to perform Single-Factor ANOVA to evaluate the statistical significance of experimental results (P < 0.05).
RESULTS
Laser Stimulated ROS Production
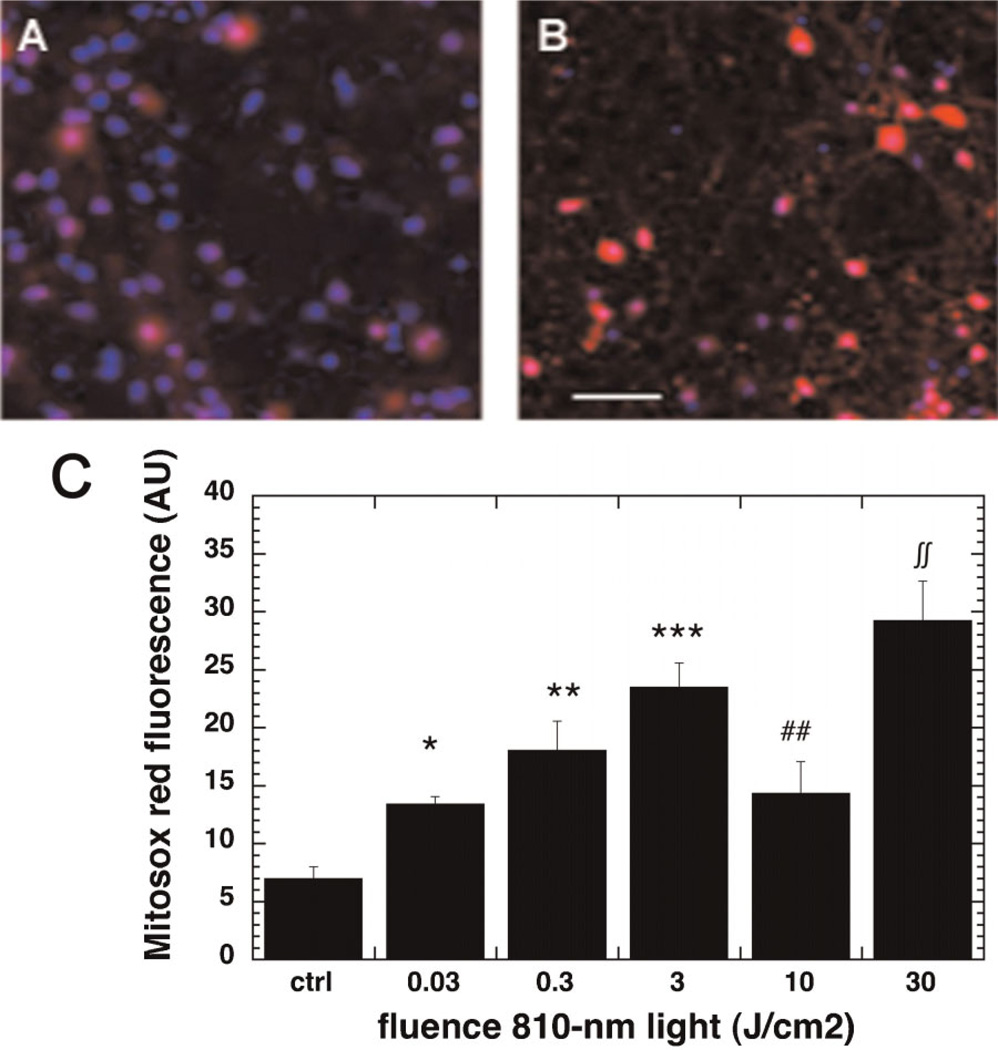
The 810-nm light induced ROS production in the mouse primary cortical neurons as shown by the micrographs in Figure 1A,B captured after 3 J/cm2. The ROS levels showed an interesting triphasic pattern depicted in Figure 1C. There was a significant initial increase with a fluence as low as 0.03 J/cm2, a further increase at 0.3 J/cm2 and a peak corresponding to a threefold increase over baseline ROS was seen at 3 J/cm2. When the fluence was further increased there was a significant decrease (compared to 3 J/cm2) in ROS observed at 10 J/cm2. When the fluence was further increased to 30 J/cm2 there was a second significant increase (compared to 10 J/cm2) in ROS to a level higher (but not significantly) than that observed at 3 J/cm2.
Fig. 1.
Effect of 810-nm laser on production of mitochondrial ROS in the cultured cortical neurons. A: Mitosox (red) and nuclear Hoechst (blue) fluorescence in control neurons. B: Mitosox and nuclear Hoechst fluorescence in neurons treated with 3 J/cm2 810-nm laser. Scale bar is 50 µm. C: Quantification by fluorescence plate reader of mean Mitosox fluorescence values from nine wells. Error bars are SD. *P < 0.05; **P < 0.01; ***P < 0.01 versus control. ##P < 0.01 versus 3 J/cm2. ∫∫P < 0.01 versus 10 J/cm2.
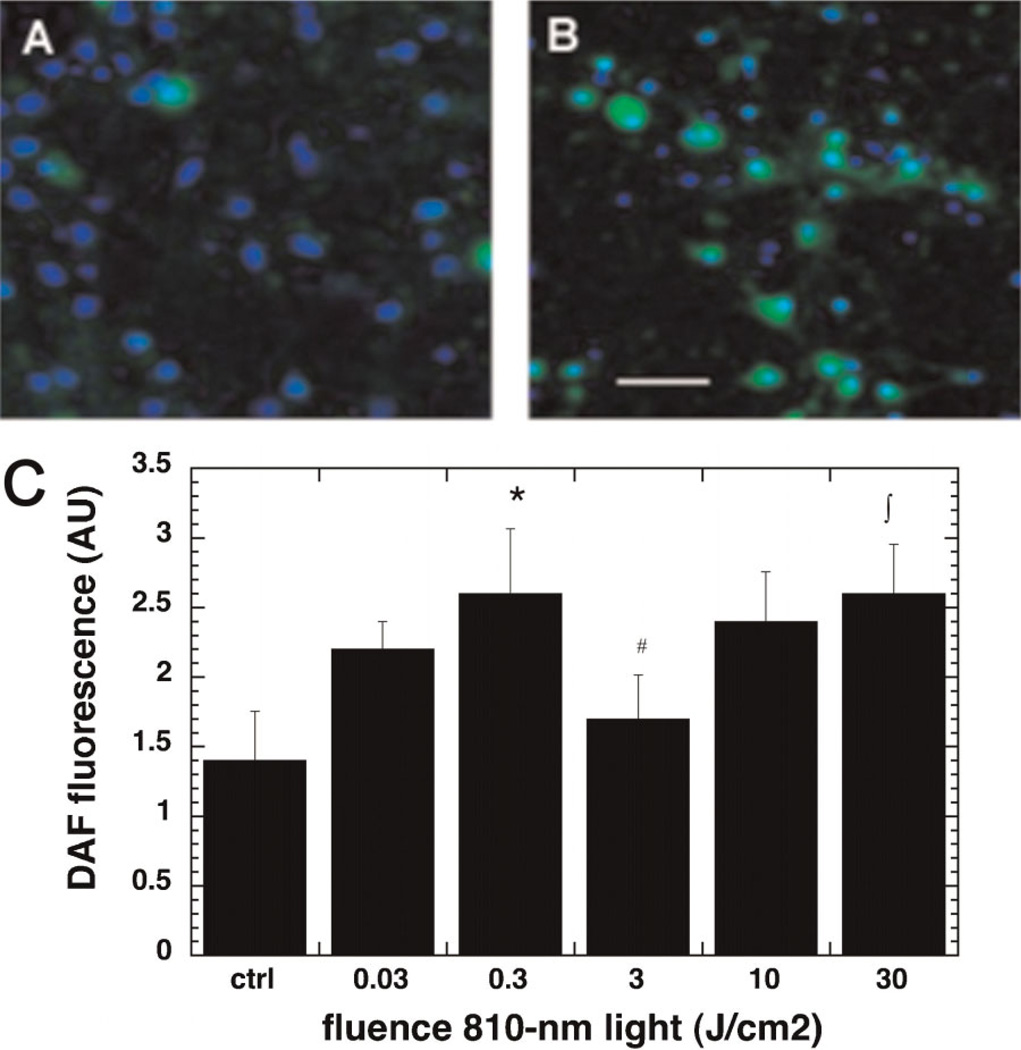
Effect of Laser on NO Release
The 810-nm light gave an increase in intracellular DAF-FM fluorescence in the cortical neurons on irradiating with light as shown in the micrographs in Figure 2A,B after a fluence of 0.3 J/cm2, indicating an increase in nitric oxide levels post irradiation. The NO levels followed the same general triphasic pattern as the ROS levels but the increase was less prominent as compared to the ROS (Fig. 2C). There was a significant increase at 0.3 J/cm2 compared to control, a significant decrease at 3 J/cm2 compared to 0.3 J/cm2, and a further increase at 30 J/cm2 compared to 0.3 J/cm2. The whole triphasic pattern appeared to be shifted towards lower fluences (the first peak was at 0.3 J/cm2 instead of 3 J/cm2 and the following trough at 3 J/cm2 instead of 10 J/cm2) compared to the pattern observed with ROS (compare Fig. 1C).
Fig. 2.
Effect of 810-nm laser on production of intracellular NO in the cultured cortical neurons. A: DAF-DM (green) and nuclear Hoechst (blue) fluorescence in control neurons. B: DAF-DM and nuclear Hoechst in neurons treated with 0.3 J/cm2 810-nm laser. Scale bar is 50 µm. C: Quantification by fluorescence plate reader of mean DAF-FM fluorescence values from nine wells. Error bars are SD. *P < 0.05 versus control; #P < 0.05 versus 0.3 J/cm2; ∫P < 0.05 versus 3 J/cm2.
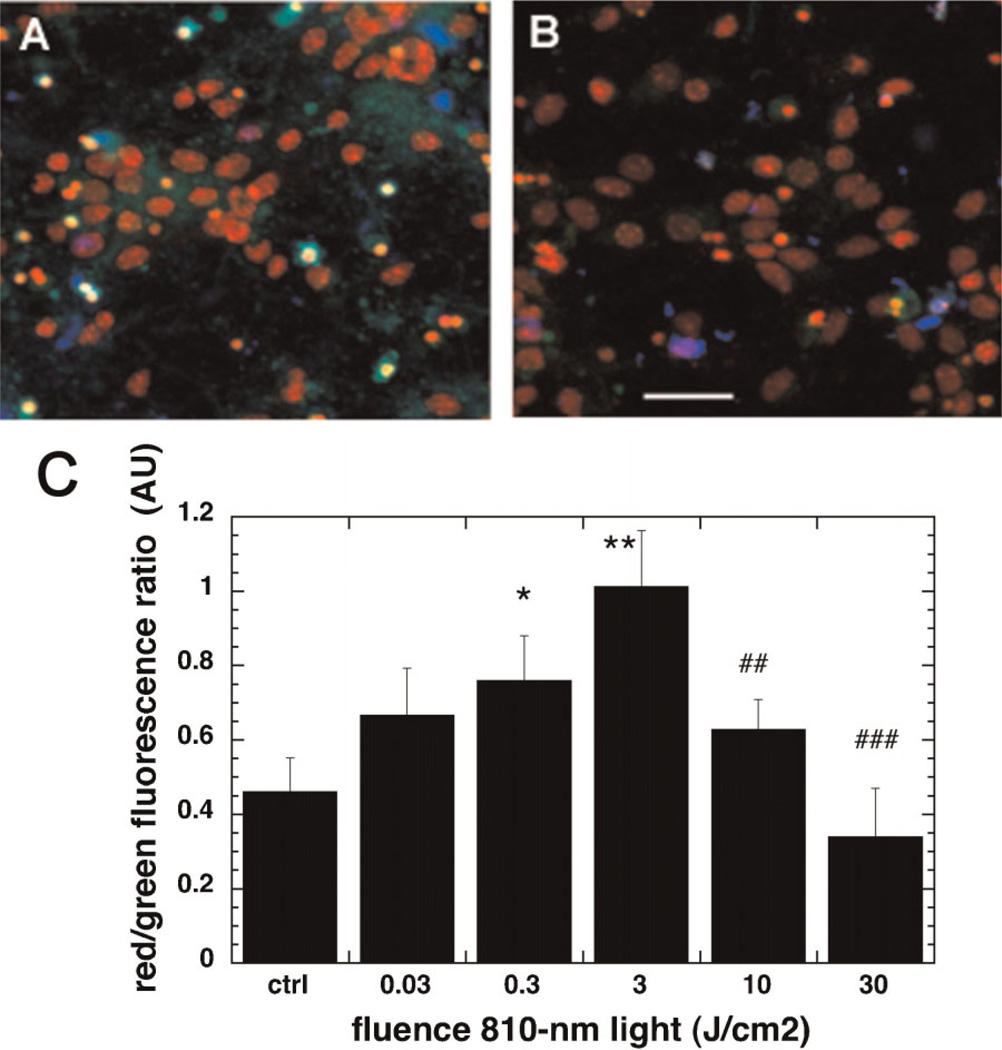
Light Induced Changes in the Mitochondrial Membrane Potential
The changes in MMP were determined by measuring the red to green ratio of the fluorescence of JC-1 dye. When the MMP is high more dye accumulates in the mitochondrial membrane which leads to dye aggregation (J-aggregates) and a red shift in the fluorescence. By contrast when MMP is low the dye disaggregates and the fluorescence is green shifted [33]. The MMP in neurons was significantly increased after 3 J/cm2 of 810-nm light as seen in the micrographs in Figure 3A,B. The dose response was biphasic in nature (Fig. 3C). There was a significant increase at 0.3 J/cm2 reaching a peak of twice basal levels at 3 J/cm2. Then there was a decrease at 10 J/cm2 and outright depolarization (significantly lower than control) of MMP was observed at 30 J/cm2.
Fig. 3.
Effect of 810-nm laser on mitochondrial membrane potential in the cultured cortical neurons. A: JC1 non-aggregated (green), JC1 aggregated (red) and nuclear Hoechst (blue) fluorescence in control neurons. B: JC1 non-aggregated, JC1 aggregated and nuclear Hoechst fluorescence in neurons treated with 3 J/cm2 810-nm laser. Scale bar is 50 µm. C: Quantification by fluorescence plate reader of the mean red/green fluorescence ratio values from nine wells. Error bars are SD. *P < 0.05; **P < 0.05 versus control. ##P < 0.01; ###P < 0.001 versus 3 J/cm2.
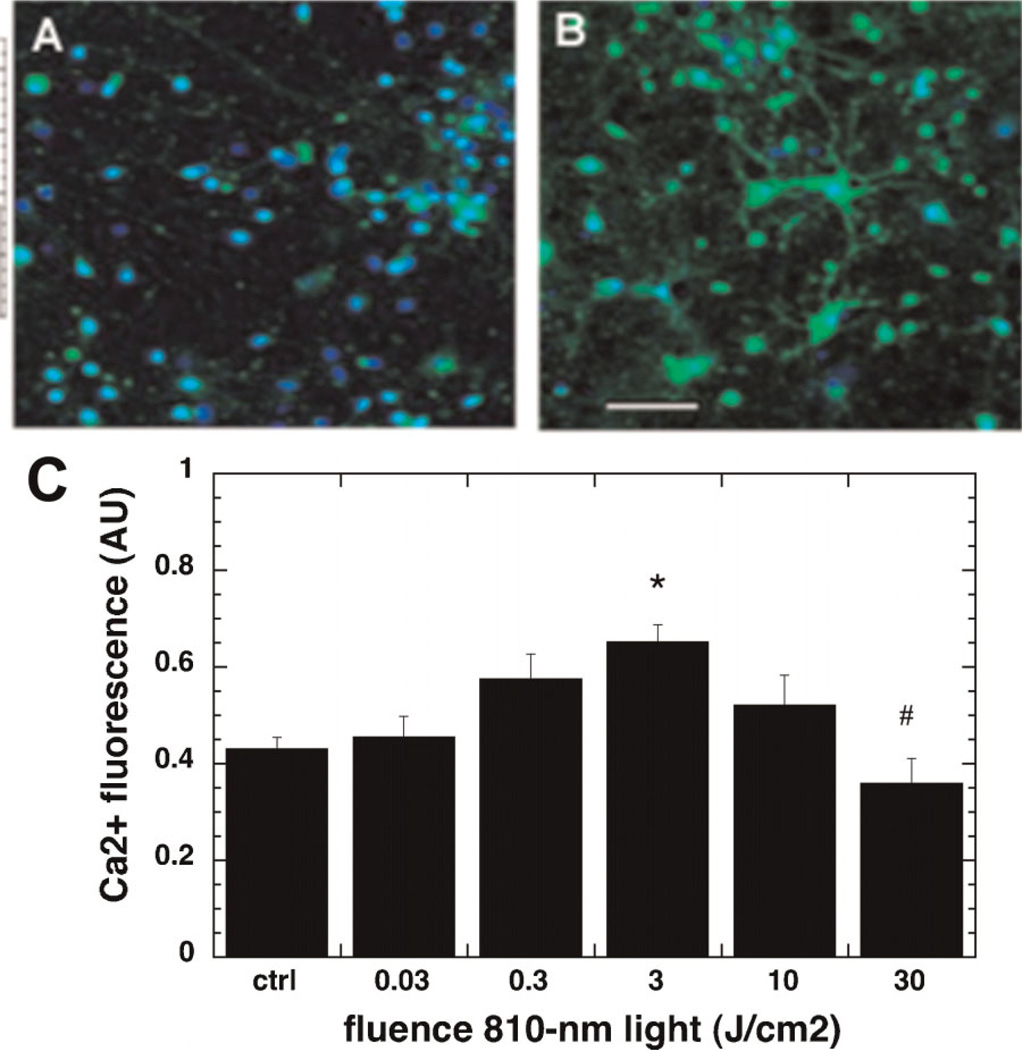
Laser Induced Increase in Intracellular Calcium Levels
Neuronal cells treated with laser showed a significant rise in the intracellular calcium levels as compared to the control as shown in the micrographs in Figure 4A,B captured after 3 J/cm2. The dose response of intracellular calcium was also biphasic as seen if Figure 4C. The increase was significant when it peaked at the fluence of 3 J/cm2. Interestingly, the levels decreased significantly in cells irradiated with 10 J/cm2 and 30 J/cm2.
Fig. 4.
Effect of 810-nm laser on intracellular calcium in the cultured cortical neurons. A: Fluo4 (green) and nuclear Hoechst (blue) fluorescence in control neurons. B: Fluo4 (green) and nuclear Hoechst (blue) fluorescence in neurons treated with 3 J/cm2 810-nm laser. Scale bar is 50 µm. C: Quantification by fluorescence plate reader of the mean red/green fluorescence ratio values from nine wells. Error bars are SD. *P < 0.05 versus control. #P < 0.05 versus 3 J/cm2.
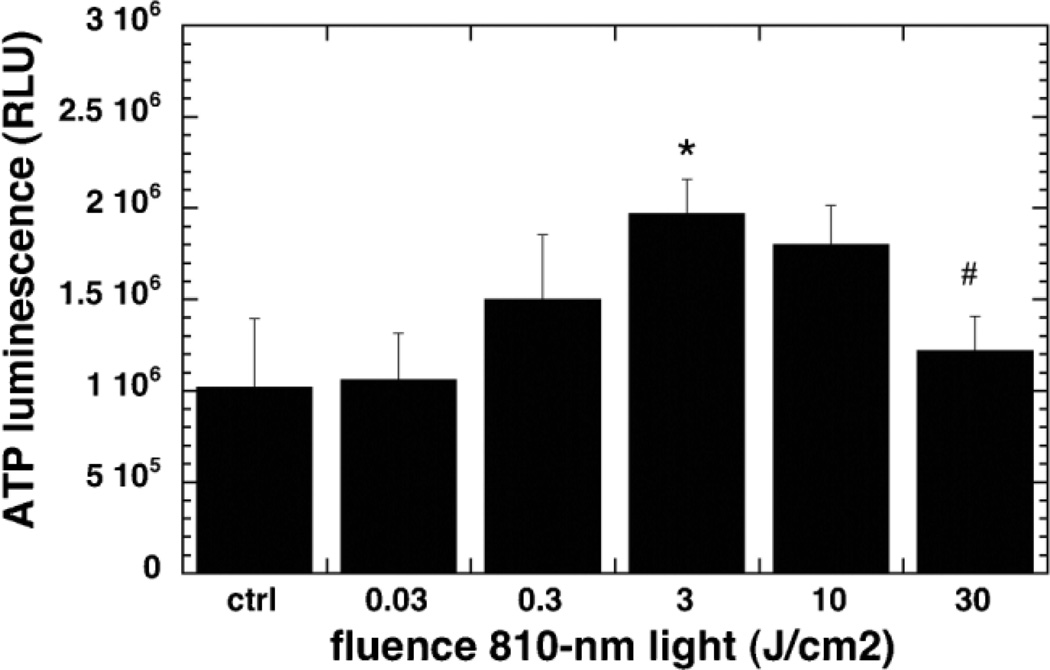
Effect of Laser Treatment on ATP Content
Figure 5 shows the ATP content per mg cell protein in the mouse cortical neurons. ATP content was found to increase on treating the neurons with 810-nm light reaching a significant peak at 3 J/cm2 as compared to the control cells. When the fluence was increased to 10 and 30 J/cm2 the levels of the ATP fell and were equal to that of the control and in the case of 30 J/cm2 significantly less than the peak at 3 J/cm2.
Fig. 5.
Effect of 810-nm laser on intracellular ATP in the cultured cortical neurons. Quantification by luminescence plate reader of the relative light unit values per mg cell protein from Cell Titer Glo assay from nine wells. Error bars are SD. *P < 0.05 versus control. #P < 0.05 versus 3 J/cm2.
DISCUSSION
Five separate fundamental cellular responses of primary cortical neurons to the 810-nm light have been documented. The mitochondria are the putative cellular target for red and NIR photons. ROS produced in the mitochondria are involved in signaling pathways [34]. LLLT has been reported to increase ROS generation and modulate cell redox activity [35]. Recent work from our laboratory in mouse embryonic fibroblasts has shown that LLLT (810-nm laser light) activates the redox-sensitive transcription factor, NF-kB by generating ROS as signaling molecules [36]. NF-kB is known to be a transcription factor responsible for cell survival, anti-apoptosis and proliferation. However there are also reports that high fluences of light (both red and NIR) can have deleterious effects on cells by inducing apoptosis via generation of high levels of ROS [37] and via Akt/GSK3beta signaling pathway [38,39]. In a seemingly contradictory fashion LLLT delivered at lower non-inhibitory fluences has been shown to inhibit apoptosis via the same Akt/GSK3beta signaling pathway [40]. For the first time our data can go some distance towards reconciling these two apparently contradictory observations. It appears that low doses of light can activate mitochondrial respiration, possibly by dissociating NO from cytochrome c oxidase [41]. This activation of mitochondrial metabolism and respiration is manifested by increase of MMP, which is positively associated with increases in ATP. Furthermore, calcium is a physiological stimulus for ATP synthesis and has been linked with generation of ROS in mitochondria [42]. We propose that stimulatory fluences of 810-nm light release NO from mitochondria and increase ATP synthesis in concert with the production of modest levels of ROS that can activate signaling pathways but are insufficient to produce actual cellular damage and too low to initiate apoptosis. The amount of ROS generated at lower fluences (up to 3 J/cm2) was lower than that generated at higher fluences (30 J/cm2) and it appeared that there was a reduction in ROS levels at a fluence (10 J/cm2) lying between these two fluences. We propose that light-induced ROS can be produced from mitochondria both when respiration is increased (beneficial), or alternatively when it is decreased (harmful) [43]. Respiration is decreased when mitochondria are damaged by excessive ROS and in this situation mitochondrial membrane potential is also decreased as a prelude to induction of apoptosis [37]. This finding is consistent with studies in where He–Ne laser at higher fluences exceeding 60 J/cm2 were found to induce apoptosis in lung adenocarcinoma cells [44] and COS-7 cells [45]. It remains to be seen whether these two forms of ROS (the beneficial ROS observed at low fluences and the harmful ROS seen at high fluences) are qualitatively different as well as quantitatively different. For instance it may be hypothesized that the beneficial low-fluence ROS contains more superoxide, while the harmful high-fluence ROS contains more of the damaging species, hydroxyl radical or peroxynitrite.
In addition to the induction of mitochondrial ROS by light, release of NO from the mitochondria is proposed to be involved in the mechanism of action of low level laser [46]. It should be pointed out that the observed increase in NO may result from one of several pathways. First the activity of an isoform of the NO synthesis enzyme nitric oxide synthase (NOS) may be increased possibly due to an increase in intracellular calcium. Many isoforms of NOS are highly sensitive to calcium levels. Secondly the rise in NO levels could be due to release of NO by dissociation from an intracellular NO store like cytochrome c oxidase in mitochondria [41], nitrosylated heme proteins like hemoglobin or myoglobin [47,48], or from intracellular nitrosylated thiols [49]. Thirdly it has been recently demonstrated that in hypoxic conditions cytochrome c oxidase can behave as a light driven nitrite reductase producing NO from nitrite [50,51]. Further studies will be needed to tease apart the precise pathway(s) that leads to NO release after LLLT. NO is an important signaling molecule, especially in brain where it acts as a neurotransmitter [52], and has been shown to regulate expression of important neuroprotective factors like brain-derived neurotrophic factor (BDNF) [53]. NO is also reported to play an important role in adult neurogenesis [54], in the regulation of dendritic spine density [55] and morphology [56]. Thus, release of NO from neurons by LLLT could help to explain the observed neuroprotective effects of LLLT in several in vivo studies. It should be noted that several papers show that high levels of NO as for instance produced from inducible nitric oxide synthase present in inflammatory cells is thought to be harmful to neuronal survival in the brain [57]. In other words NO can act as a “Janus molecule” in the brain, beneficial at low doses and harmful at high doses [58].
Calcium ions influence nearly every aspect of cellular life and are especially important in the nervous system. Changes in intracellular calcium ion concentrations mediate signal transduction pathways that regulate neuronal functions [59]. Previous studies with RBL-2H3 mast cells [60], human fibroblast cells [15], bull sperm cells [61], and U-937 macrophages [62] have shown an increased intracellular calcium levels in response to LLLT. In the neurons, there was an increase in the intracellular calcium concentrations from 0 to 10 J/cm2 but a decline was noted when fluence was increased to 30 J/cm2. This observation is similar to the U-937 macrophage study [62] wherein an increase in calcium ion concentration was observed for lower fluence while the levels declined at higher fluence (16 J/cm2).
The mechanism of LLLT at the cellular levels is also attributed to the absorption of the light by components of respiratory chain [10]. Illumination of mitochondrial isolated from the rat liver by HeNe laser led to an increased electrochemical potential and ATP synthesis [63]. ATP content was also found to increase in human neuronal cells in culture on illumination with 808 nm laser at low fluence of 0.05 J/cm2 [64]. In agreement with these observations, we also found an increase in ATP content at low light doses, but at the higher fluence of 30 J/cm2 there was a drop in ATP content compared to the peak at 3 J/cm2. Similarly, the MMP increased at lower light doses while there was a sharp decrease at higher fluence indicating depolarization of mitochondrial membrane, which in turn, may explain the observed decrease in ATP content at higher fluences. This observation again supports the notion that lower doses of light stimulate molecules that mediate the positive signaling pathways whereas the higher doses may be toxic to the cells.
We conclude that the 810 nm light stimulates ROS production and NO release in murine cortical neurons. Low doses of laser increase ATP synthesis, mitochondrial membrane potential and intracellular calcium levels but a decline from the peak in all three is seen at high fluence (30 J/cm2). ROS, NO, calcium ions and ATP all are important mediators of cell signaling and their stimulation by low level light in vitro may explain the positive effects of light seen in vivo. The fact that higher fluences cause a bigger increase in ROS and a decrease in MMP also suggests that high doses can induce damage mitochondria and lead to toxicity. Further studies are underway to investigate the cell signaling pathways induced by laser irradiation at low fluences and the cell death mechanism at higher fluences.
ACKNOWLEDGMENTS
This work was supported by sponsored research funding from Photothera, Inc. and by NIH grant R01AI050875, Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), CDMRP Program in TBI (W81XWH-09-1-0514) and Air Force Office of Scientific Research (FA9950-04-1-0079).
Disclosure Statement: This study was partly supported by a sponsored research agreement from Photothera, Inc. Luis De Taboada and Thomas McCarthy are employees and stockholders in Photothera, Inc. Michael R Hamblin has received research support, loan of equipment and consulting fees from Photothera.
Contract grant sponsor: NIH; Contract grant number: R01AI050875; Contract grant sponsor: Center for Integration of Medicine and Innovative Technology; Contract grant number: DAMD17-02-2-0006; Contract grant sponsor: CDMRP Program in TBI; Contract grant number: W81XWH-09-1-0514; Contract grant sponsor: Air Force Office of Scientific Research; Contract grant number: FA9950-04-1-0079.
REFERENCES
- 1.Peres MF. Low-level laser therapy for neck pain. Cephalalgia. 2010;30(11):1408. doi: 10.1177/0333102410362121. [DOI] [PubMed] [Google Scholar]
- 2.Fulop AM, Dhimmer S, Deluca JR, Johanson DD, Lenz RV, Patel KB, Douris PC, Enwemeka CS. A meta-analysis of the efficacy of laser phototherapy on pain relief. Clin J Pain. 2010;26(8):729–7736. doi: 10.1097/AJP.0b013e3181f09713. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinovic LM, Cutovic MR, Milovanovic AN, Jovic SJ, Dragin AS, Letic M, Miler VM. Low-level laser therapy for acute neck pain with radiculopathy: A double-blind placebo-controlled randomized study. Pain Med. 2010;11(8):1169–1178. doi: 10.1111/j.1526-4637.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 4.Xavier M, David DR, de Souza RA, Arrieiro AN, Miranda H, Santana ET, Silva JA, Jr, Salgado MA, Aimbire F, Albertini R. Anti-inflammatory effects of low-level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med. 2010;42(6):553–558. doi: 10.1002/lsm.20896. [DOI] [PubMed] [Google Scholar]
- 5.Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of wound healing: A review of experimental studies in mouse and rat animal models. Photomed Laser Surg. 2010;28(3):291–325. doi: 10.1089/pho.2008.2446. [DOI] [PubMed] [Google Scholar]
- 6.Lucas C, Criens-Poublon LJ, Cockrell CT, de Haan RJ. Wound healing in cell studies and animal model experiments by Low Level Laser Therapy; were clinical studies justified? A systematic review. Lasers Med Sci. 2002;17(2):110–134. doi: 10.1007/s101030200018. [DOI] [PubMed] [Google Scholar]
- 7.Yasukawa A, Hrui H, Koyama Y, Nagai M, Takakuda K. The effect of low reactive-level laser therapy (LLLT) with helium-neon laser on operative wound healing in a rat model. J Vet Med Sci. 2007;69(8):799–806. doi: 10.1292/jvms.69.799. [DOI] [PubMed] [Google Scholar]
- 8.Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. Role of low-level laser therapy in neurorehabilitation. PM R. 2010;2(12) Suppl 2:S292–S305. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med. 2005;36(3):171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Dmitriev AE, Cardoso MJ, Viers-Costello AG, Borke RC, Streeter J, Anders JJ. 810 nm Wavelength light: An effective therapy for transected or contused rat spinal cord. Lasers Surg Med. 2009;41(1):36–41. doi: 10.1002/lsm.20729. [DOI] [PubMed] [Google Scholar]
- 11.Anders JJ, Geuna S, Rochkind S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol Res. 2004;26(2):233–239. doi: 10.1179/016164104225013914. [DOI] [PubMed] [Google Scholar]
- 12.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37(10):2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 13.Detaboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg Med. 2006;38(1):70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- 14.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V, Shohami E. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007;24(4):651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 16.De Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, Streeter J, Kindy MS. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J Alzheimer’s Dis. 2011;23(3):521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 17.Michalikova S, Ennaceur A, van Rensburg R, Chazot PL. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: Effects of low infrared light. Neurobiol Learn Mem. 2008;89(4):480–488. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38(6):1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 19.Lapchak PA. Taking a light approach to treating acute ischemic stroke patients: Transcranial near-infrared laser therapy translational science. Ann Med. 2010;42(8):576–586. doi: 10.3109/07853890.2010.532811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: Two case reports. Photomed Laser Surg. 2010;29:351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu WP, Wang JJ, Yu CL, Lan CC, Chen GS, Yu HS. Helium-neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J Invest Dermatol. 2007;127(8):2048–2057. doi: 10.1038/sj.jid.5700826. [DOI] [PubMed] [Google Scholar]
- 22.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36(4):307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- 23.Pastore D, Di Martino C, Bosco G, Passarella S. Stimulation of ATP synthesis via oxidative phosphorylation in wheat mitochondria irradiated with helium-neon laser. Biochem Mol Biol Int. 1996;39(1):149–157. doi: 10.1080/15216549600201151. [DOI] [PubMed] [Google Scholar]
- 24.Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175(1):95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 25.Chen AC-H, Arany PR, Huang Y-Y, Tomkinson EM, Saleem T, Yull FE, Blackwell TS, Hamblin MR. Low level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. In: Hamblin MR, Waynant RW, Anders J, editors. Mechanisms for Low-Light Therapy IV; SPIE–The International Society for Optical Engineering; San Jose, CA, USA: SPIE; 2009. pp. 0B–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubart R, Lavi R, Friedmann H, Rochkind S. Photochemistry and photobiology of light absorption by living cells. Photomed Laser Surg. 2006;24(2):179–185. doi: 10.1089/pho.2006.24.179. [DOI] [PubMed] [Google Scholar]
- 28.Chow R. Laser acupuncture studies should not be included in systematic reviews of phototherapy. Photomed Laser Surg. 2006;24(1):69. doi: 10.1089/pho.2006.24.69. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins D, Abrahamse H. Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg. 2006;24(6):705–714. doi: 10.1089/pho.2006.24.705. [DOI] [PubMed] [Google Scholar]
- 30.Hawkins DH, Abrahamse H. The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation. Lasers Surg Med. 2006;38(1):74–83. doi: 10.1002/lsm.20271. [DOI] [PubMed] [Google Scholar]
- 31.Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: Lasers, scanners, and NASA’s light-emitting diode array system. J Clin Laser Med Surg. 2001;19(1):29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 32.Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42(5):674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 33.Reers M, Smith TW, Chen LB. J-Aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30(18):4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 34.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 35.Tafur J, Mills PJ. Low-intensity light therapy: Exploring the role of redox mechanisms. Photomed Laser Surg. 2008;26(4):323–328. doi: 10.1089/pho.2007.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen AC-H, Huang Y-Y, Arany PR, Hamblin MR. Role of reactive oxygen species in low level light therapy. In: Hamblin MR, Waynant RW, Anders J, editors. Mechanisms for Low-Light Therapy IV; © 2009 SPIE–The International Society for Optical Engineering; San Jose, CA, USA: SPIE; 2009. pp. 716502–716511. [Google Scholar]
- 37.Wu S, Xing D, Gao X, Chen WR. High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol. 2009;218(3):603–611. doi: 10.1002/jcp.21636. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Wu S, Xing D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3beta signaling pathway. J Cell Physiol. 2010 doi: 10.1002/jcp.22367. [DOI] [PubMed] [Google Scholar]
- 39.Sun X, Wu S, Xing D. The reactive oxygen species-Src-Stat3 pathway provokes negative feedback inhibition of apoptosis induced by high-fluence low-power laser irradiation. FEBS J. 2010;277(22):4789–4802. doi: 10.1111/j.1742-4658.2010.07884.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Zhang Y, Xing D. LPLI inhibits apoptosis upstream of Bax translocation via a GSK-3beta-inactivation mechanism. J Cell Physiol. 2010;224(1):218–228. doi: 10.1002/jcp.22123. [DOI] [PubMed] [Google Scholar]
- 41.Lane N. Cell biology: Power games. Nature. 2006;443(7114):901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 42.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 43.Brookes PS. Mitochondrial H(+) leak and ROS generation: An odd couple. Free Radic Biol Med. 2005;38(1):12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Chen TS, Xing D, Wang JJ, Wu YX. Measuring dynamics of caspase-3 activity in living cells using FRET technique during apoptosis induced by high fluence low-power laser irradiation. Lasers Surg Med. 2005;36(1):2–7. doi: 10.1002/lsm.20130. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Wu S, Xing D. The reactive oxygen species-Src-Stat3 pathway provokes negative feedback inhibition of apoptosis induced by high-fluence low-power laser irradiation. FEBS J. 2010;277(22):4789–4802. doi: 10.1111/j.1742-4658.2010.07884.x. [DOI] [PubMed] [Google Scholar]
- 46.Hamblin MR. The role of nitric oxide in low level light therapy. Proc SPIE. 2008;6846 684602. [Google Scholar]
- 47.Zhang R, Mio Y, Pratt PF, Lohr N, Warltier DC, Whelan HT, Zhu D, Jacobs ER, Medhora M, Bienengraeber M. Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism. J Mol Cell Cardiol. 2009;46(1):4–14. doi: 10.1016/j.yjmcc.2008.09.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: Potential role in cardioprotection. J Mol Cell Cardiol. 2009;47:256–263. doi: 10.1016/j.yjmcc.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borutaite V, Budriunaite A, Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta. 2000;1459(2–3):405–412. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 50.Poyton RO, Ball KA. Therapeutic photobiomodulation: Nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med. 2011;11(57):154–159. [PubMed] [Google Scholar]
- 51.Ball KA, Castello PR, Poyton RO. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy. J Photochem Photobiol B. 2011;102(3):182–191. doi: 10.1016/j.jphotobiol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Vincent SR. Nitric oxide neurons and neurotransmission. Prog Neurobiol. 2010;90(2):246–255. doi: 10.1016/j.pneurobio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Patel NJ, Chen MJ, Russo-Neustadt AA. Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. Eur J Pharmacol. 2010;633(1–3):1–9. doi: 10.1016/j.ejphar.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Lu D, Mahmood A, Zhang R, Copp M. Upregulation of neuro-genesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg. 2003;99(2):351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- 55.Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 2009;19(2):146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Morales-Medina JC, Mejorada A, Romero-Curiel A, Flores G. Alterations in dendritic morphology of hippocampal neurons in adult rats after neonatal administration of N-omega-nitro-L-arginine. Synapse. 2007;61(9):785–789. doi: 10.1002/syn.20406. [DOI] [PubMed] [Google Scholar]
- 57.Gahm C, Holmin S, Wiklund PN, Brundin L, Mathiesen T. Neuroprotection by selective inhibition of inducible nitric oxide synthase after experimental brain contusion. J Neurotrauma. 2006;23(9):1343–1354. doi: 10.1089/neu.2006.23.1343. [DOI] [PubMed] [Google Scholar]
- 58.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 59.Miller RJ. Multiple calcium channels and neuronal function. Science. 1987;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- 60.Yang WZ, Chen JY, Yu JT, Zhou LW. Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem Photobiol. 2007;83(4):979–984. doi: 10.1111/j.1751-1097.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 61.Lubart R, Friedmann H, Levinshal T, Lavie R, Breitbart H. Effect of light on calcium transport in bull sperm cells. J Photochem Photobiol B. 1992;15(4):337–341. doi: 10.1016/1011-1344(92)85139-l. [DOI] [PubMed] [Google Scholar]
- 62.Young S, Dyson M, Bolton P. The effect of light on calcium uptake in macrophages. Laser Ther. 1990;2:53–57. [Google Scholar]
- 63.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 64.Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg. 2007;25(3):180–182. doi: 10.1089/pho.2007.2064. [DOI] [PubMed] [Google Scholar]