Abstract
Chronic pain in adults has been associated with early-life stress. To examine the pronociceptive effect of early-life stress, we evaluated cutaneous and muscle nociception and activity in muscle nociceptors in an animal model of neonatal stress, limited bedding, in the rat. In this model, litters are exposed to limited bedding between postnatal days 2 – 9 (neonatal limited bedding, NLB) and controls to standard bedding. In adult NLB-treated rats, mechanical nociceptive threshold in skeletal muscle was ficantly lower (~22%) than in controls. Furthermore, administration of prostaglandin E2 (PGE2) in skin as well as produced markedly prolonged hyperalgesia, an effect prevented by spinal intrathecal injection of oligodeoxynucleotide antisense to protein kinase CΣ (PKCε), a second messenger in nociceptors that has been implicated in the induction and maintenance of chronic pain. In electrophysiological studies, mechanical threshold of muscle nociceptors was reduced by ~31% and conduction velocity significantly increased (~28%). These findings indicate that neonatal stress induces apersistent hyperalgesia and nociceptor sensitization manifest in the adult and that the second messenger PKCε may be a target against which therapies might be directed to treat a chronic pain syndrome that is associated with early-life traumatic stress.
Keywords: Stress, Skeletal muscle, Hyperalgesia, Neonatal stress, Limited bedding, Nociceptors, Conduction velocity, PKCε
INTRODUCTION
Adults exposed to emotional stress as young children (e.g. neglect, abandonment or emotional deprivation) may exhibit a state of enhanced pain sensitivity [16,24,57,59,62]. In particular, there is an increased severity of chronic widespread pain syndromes (e.g. irritable bowel syndrome, temporomandibular disorder and fibromyalgia syndrome) in response to stressful life events [9,29,42,43,45,49,54,69]). For example, in adults who, as children, were raised in institutional care facilities or who experienced maternal death, independent of adult psychological distress or social class [37].
Animal studies have established that maternal interactions with their offspring, early in life, also affect stress responses in the adult [41,55,56]. For example, neonatal limited bedding (NLB) produces fragmented and aberrant maternal nurturing behavior [36] that increases basal plasma corticosterone in 9-day old pups [6]. And rats exposed to prolonged maternal separation (3–12 h) or maternal deprivation (separation ≥24 h) during the neonatal period, also disrupt mother-pup interactions [35], exhibit exaggerated stress responses and increased anxiety [47]. While maternal-pup separation stress has been reported to produce increased thermal threshold in the skin in adult females [66], or no change in thermal or mechanical nociceptive thresholds in adult male and female rats [38], it produces a greater response in the second (but not first) phase of the formalin test [64], and visceral hyperalgesia in the colorectal distension model of visceral nociception [13]. In an effort to further our understanding of the mechanisms underlying altered nociception produced by early life stressors we employed a well-established model of early-life stress, NLB, which produces a life-long enhanced neuroendocrine stress response [28].
METHODS
Animals
Primiparous timed-pregnant Sprague Dawley female rats were obtained from Charles River (Hollister, CA). After delivery, dams were housed with their litter in standard cages on postnatal days 0 – 1. On postnatal day 2, litters were assigned to limited bedding (NLB) or standard care conditions. Behavioral and electrophysiological experiments were performed on 200 – 350 g (age: 50 – 75 d) male rats from these litters. Behavioral data collection was not performed “blind” to treatment condition.
The animals used in these experiments were housed in the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12 h light/dark cycle (lights on 7 am – 7 pm) and environmentally controlled conditions; ambient room temperature (21° – 23°C), with food and water available ad libitum. Their care and use in experiments conformed to National Institutes of Health guidelines and measures were taken to minimize pain and discomfort. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Neonatal limited bedding (NLB) stress
Using a well-establish limited bedding model [28], beginning on postnatal day 2, mother rats and their pups were placed in cages fitted with a stainless steel mesh bottom (Techniplast S.p.A, Philadelphia, PA), raised ~2.5 cm from the floor of the home cage, to allow collection of urine and feces [28]. The nesting/bedding material provided consisted of one sheet of paper towel (~112 × 22 cm). Litters were left undisturbed during post-natal days 2 – 9. From postnatal day 10 – 20 mother and pups were housed in cages with standard bedding material (Paperchip® animal bedding, Shepherd Specialty Papers, Watertown, TN); on postnatal day 21 pups were weaned and females culled.
Mechanical nociceptive threshold in muscle
Mechanical nociceptive threshold was quantified using a Chatillon digital force transducer (model DFI2, Amtek Inc., Largo, FL) [20]. Rats were lightly restrained in a cylindrical acrylic holder that allows for easy access to the hind limb, and a 6 mm diameter probe attached to the force transducer applied to the gastrocnemius muscle to deliver an increasing compression force. The nociceptive threshold was defined as the force, in Newtons, at which the rat withdrew its hind leg. Baseline withdrawal threshold was defined as the mean of 2readings taken at 5-min intervals, and nociceptive thresholds were tested after injection of prostaglandin E2 (PGE2; 1 μg in 20 ∝l 0.9% saline) into the belly of the gastrocnemius muscle. Each hind limb is treated as an independent measure and each experiment performed on a separate group of rats. All behavioral testing was done between 10 am and 4 pm.
Mechanical nociceptive threshold in skin
Mechanical nociceptive threshold in the skin was measured using an Ugo Basile Analgesymeter® (Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its paw. Rats were lightly restrained in vented, cylindrical acrylic restrainers that have openings to allow extension of the hind legs for testing nociceptive threshold. Rats were acclimatized to the testing procedure to reduce variability in the paw-withdrawal threshold, which was determined before (baseline) and after administration of PGE2 (100 ng in 2.5 ∝l, 0.9% saline). Each paw was treated as an independent measure. All behavioral testing was done between 10 am and 4 pm. While this method is generally accepted as an assay of cutaneous nociceptive threshold, we have not excluded the possibility that afferents innervating other structures (e.g. ligaments, tendons, periosteum) contribute to the nociceptive response.
Single fiber electrophysiology
The in vivo single fiber electrophysiology technique for studying muscle afferents has been described in detail previously [10]. In brief, rats were anesthetized with sodium pentobarbital (initially 50 mg/kg, i.p., with additional doses given throughout the experiment to maintain areflexia), their trachea cannulated, and heart rate monitored. Anesthetized animals were positioned on their right side and an incision made on the dorsal skin of the left leg, between the mid-thigh and calf. Then the biceps femoris muscle was partially removed to expose the sciatic nerve and gastrocnemius muscle. The edges of the incised skin were fixed to a metal loop to provide a pool that was filled with warm mineral oil that bathed the sciatic nerve and gastrocnemius muscle.
The sciatic nerve was cut proximally to prevent flexor reflexes during electrical stimulation of sensory neurons. Fine fascicles of axons were then dissected from the distal stump, and placed on a recording electrode. Single units were first detected by mechanical stimulation of the gastrocnemius muscle with a small blunt-tipped glass bar. Bipolar stimulating electrodes were then placed and held on the center of the receptive field of the muscle afferent, by a micromanipulator (Narishige model MM-3, Tokyo, Japan). Conduction velocity of each fiber was calculated by dividing the distance between the stimulating and recording electrodes by the latency of the electrically evoked action potential. All recorded muscle afferents had conduction velocities in the range of type III (conduction velocity 2.5 – 30 m/s: 12%) or type IV (conduction velocity <2.5; 88%) fibers [18]. Mechanical threshold, determined with calibrated von Frey hairs (VFH Ainsworth, London, UK), was defined as the lowest force that elicited at least 2 spikes within 1 s, in at least 50% of trials. Sustained (60 s) suprathreshold (10 g) mechanical stimulation was accomplished by use of a mechanical stimulator that consisted of a force-measuring transducer (Entran, Fairfield, NJ, USA) with a blunt plastic tip that was applied by a micromanipulator (BC-3 and BE-8, Narishige) on the center of the afferent’s receptive field, for 60 s. Neural activity and timing of stimulus onset and termination were monitored and stored on a computer with a Micro 1401 interface (CED, Cambridge, UK) and analyzed off-line with Spike2 software (CED).
Interspike interval (ISI) analysis
ISI analysis, used to evaluate the temporal characteristics of the response of C-fiber nociceptors to sustained (60 s) suprathreshold (10 g) mechanical stimulation, was adopted from our previous study of nociceptor activity in rat models of neuropathic pain syndromes [12,61]. The ISIs for the responses of C-fibers were grouped into 100 ms bins between 0 and 499 ms; the few ISIs greater than or equal to 500 ms were not further analyzed [61]. The number of interspike intervals occurring in each bin was expressed as the percentage of the total number of ISIs in that 60 s stimulus trial. This normalization procedure allowed the distribution of ISIs from multiple fibers to be averaged together.
Coefficient of variation analysis
Since ISIs do not give an accurate estimate of the variability of neuronal firing if the mean firing rate changes over time, a common occurrence, we also calculated the coefficient of variation (CV2) which compares the relative difference between adjacent ISIs during the 60 s suprathreshold stimulus [34]. CV2is defined as the square root of 2 multiplied by the S.D. of two ISIs divided by their mean [34]:
, where ti is the latency for the ith action potential.
Thus, CV2 is a dimensionless number that is independent of absolute firing rate.
That differences in CV2 reflect physiologically meaningful differences between functionally important classes of neurons were recently demonstrated in a study that used CV2 analysis to distinguish slowly adapting type I from type II afferents [68].
Design and administration of antisense oligodeoxynucleotide to PKCε mRNA
To attenuate the expression of PKCε in nociceptors we used a 20-mer antisense oligodeoxynucleotide (ODN) sequence, 5′-GCC AGC TCG ATC TTG CGC CC-3′, directed against a unique sequence of rat PKCε mRNA. The corresponding GenBank accession number and ODN position within the cDNA sequence are XM345631 and 226–245, respectively. The mismatch ODN sequence, 5′-GCC AGC GCG ATC TTT CGC CC-3′, corresponds to the PKCε subunit antisense sequence with 2 bases mismatched (indicated in bold typeface). We have previously shown that (at a dose of 40 μg) antisense ODN with this sequence decreases PKCε protein in dorsal root ganglia [19,19,23,52,53,60]. A search of EMBL and NCBI GenBank Rattus norvegicus databases to PKCε identified no homologous sequences. Before ODN injections, rats were briefly anesthetized with 3% isoflurane, 97% oxygen and a 30-gauge hypodermic needle inserted into the subarachnoid space, at the midline, between the L4 and L5 vertebrae. ODN (40 μg/10 μl) was slowly injected over ~15 s. This procedure was repeated daily so that ODN was administered on 3 consecutive days. Control animals received injections of mismatch ODN.
Statistical analyses
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made by Student’s t-test (for one or two independent populations) or by one-way ANOVA for comparing multiple treatments, using StatView statistical software. To take uneven variances into account, for comparisons between groups of unequal numbers, Welch’s correction for the Student’s t-test was used. To compare change from baseline, one-way repeated-measures ANOVAs with a Greenhouse-Geisser adjusted P-value was used(SPSS statistical software). P < 0.05 was considered statistically significant.
RESULTS
Neonatal limited bedding stress
a) Muscle nociception
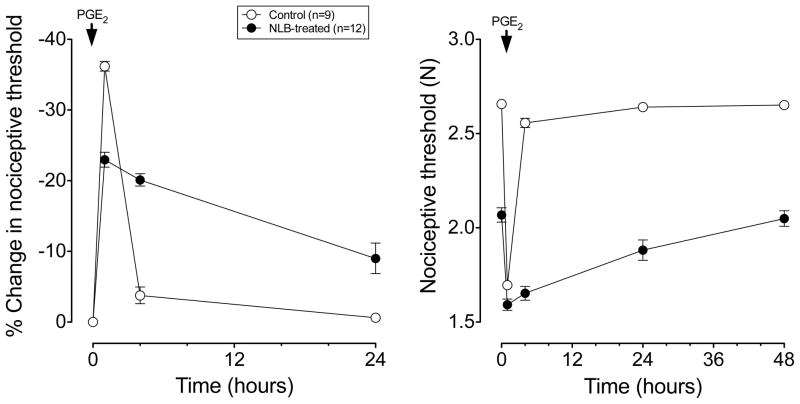
In adult rats that had been exposed neonatally to the limited bedding protocol (i.e. NLB), mechanical nociceptive threshold in skeletal muscle was lower (~22%) compared to control rats, which had standard bedding during the same neonatal period (2068±37 mN (n=12) vs. 2657±13 mN (n=10), P<0.05, Student’s t-test, Fig. 1). Next, to determine if NLB induces plasticity in nociceptor function we evaluated the hyperalgesia induced by a well-studied inflammatory mediator, PGE2, which produces a markedly prolonged hyperalgesia after adult exposure to stress [30], hyperalgesia that is mediated by a second messenger (PKCε) dependent signaling pathway [17,20,21,44]. In both naive control and NLB rats, PGE2 (1 μg, into the gastrocnemius muscle)induced mechanical hyperalgesia with a rapid onset. While in control rats nociceptive threshold returned to baseline by 4 h after intramuscular injection of PGE2 (100 ng), in adult NLB stressed rats, hyperalgesia was still present 48 h after administration of PGE2 (Fig. 1, one-way ANOVA; P < 0.05).
Figure 1. NLB stress produces a decrease mechanical nociceptive threshold and prolongation of PGE2 hyperalgesia in skeletal muscle.
PGE2 (1 μg in 20 μl intramuscular) produced a marked decrease in mechanical nociceptive threshold in the gastrocnemius muscle 1 h post-injection that returns to near baseline in control (non-stressed) rats (open circles, n=9), when tested 4 h post-injection. In adult rats that were exposed to limited bedding on postnatal days 2 – 9 (NLB rats, see Methods, filled circles, n=12), PGE2 hyperalgesia was present 1 h post-injection, near maximal 4 h post-injection and still present 24 h post-injection. In adult NLB rats, mechanical nociceptive threshold of the gastrocnemius muscle was significantly lower (2.07±0.04 N) than the threshold in control rats (2.66±0.01 N).
b) Cutaneous nociception
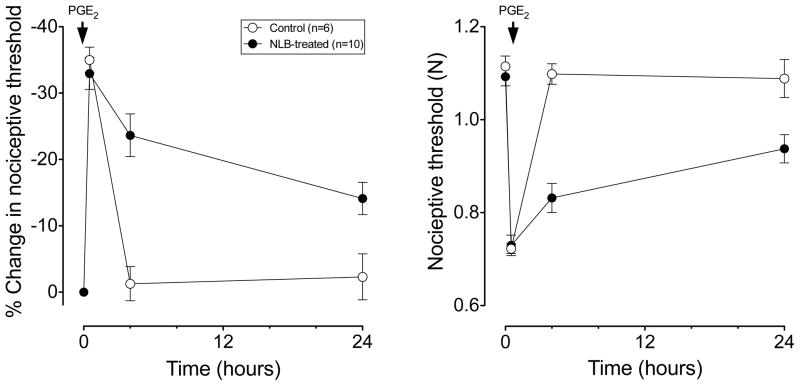
In contrast to the effect of NLB on nociceptive threshold in muscle, in adult NLB-treated rats, cutaneous nociceptive threshold was not different from that in control rats (1.09±0.02 N, n=10 vs. 1.11±0.02, n=6, respectively P=NS, Fig. 2). However, while in control rats nociceptive threshold returned to baseline by the 4th hour after PGE2 (100 ng, intradermal) administration; in NLB rats, cutaneous mechanical hyperalgesia was still present 24 h after PGE2 administration (Fig. 2, one-way ANOVA; P < 0.05). Thus, while NLB produced chronic mechanical hyperalgesia in skeletal muscle it did not affect mechanical nociceptive threshold in the skin. However, similar to muscle, NLB produced a marked prolongation of PGE2-induced cutaneous mechanical hyperalgesia.
Figure 2. NLB stress produces a prolongation in PGE2 hyperalgesia in skin.
PGE2 (1 μg in 2.5 μl, intradermally) produced a marked decrease in mechanical nociceptive threshold in the hind paw measured 1 h post-injection that returns to near baseline in control (non-stressed) rats (open circles, n=6), when tested 4 h post-injection. In adult NLB rats (filled circles, n=10), PGE2 hyperalgesia was present 1 h post-injection and was still near maximal 4 h post-injection, and still present 24 h post injection. In adult NLB rats, mechanical nociceptive threshold of the skin on the dorsum of the hind paw was not significantly different from the threshold in control rats.
Role of PKCε in NLB stress-induced hyperalgesia and priming
a) Muscle nociception
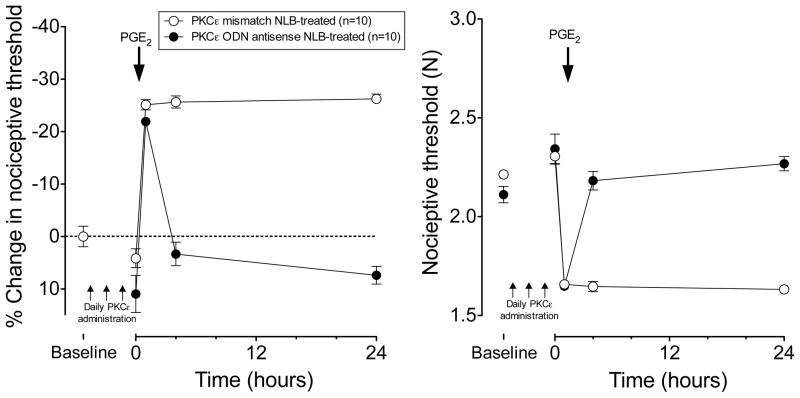
ODN antisense or mismatch to PKCε mRNA (40 μg, i.t.) was administered once daily for 3 days to adult rats that had been treated with NLB. Compared to mismatch ODN, ODN antisense to PKCε produced a small, albeit, significant increase in the mechanical nociceptive threshold in the gastrocnemius muscle of adult NLB-treated rats (Antisense treatment 2110±40 to 2340±80 mN; mismatch treatment 2210±20 to 2310±40 mN, two-way repeated measures ANOVA interaction P<0.05, followed by one-way repeated measures antisense P<0.005, mismatch P=N.S). In addition to partially reversing NLB-induced mechanical hyperalgesia, treatment with PKCε antisense completely reversed the prolongation of PGE2 hyperalgesia in the gastrocnemius muscle of NLB-treated adult rats; i.e., PGE2-induced mechanical hyperalgesia that was present 4 and 24 h after injection of PGE2 (1 μg i.m.) in mismatch ODN-treatment in NLB rats, was absent at these time points in adult NLB rats pretreated with ODN antisense against PKCε mRNA (P<0.0001 at both 4 h and 24 h time point, repeated measures ANOVA with Bonferroni post hoc test, Fig. 3). Of note, hyperalgesia in the mismatch group remains undiminished (at ~25% lower threshold) at 24 h post-PGE2 (Fig. 3) which differs from untreated NLB that shows ~10% hyperalgesia at 24h (Fig. 1).
Figure 3. PKCε antisense inhibits NLB-induced hyperalgesic priming in muscle.
In adult NLB rats that had received ODN antisense against PKCε for 3 days (filled circles, n=10), PGE2–induced hyperalgesia in the gastrocnemius muscle returned to baseline by 4 h post PGE2, while in mismatch-treated rats (open circles, n=10), PGE2 hyperalgesia remained elevated 24 h after PGE2.
b) Cutaneous nociception
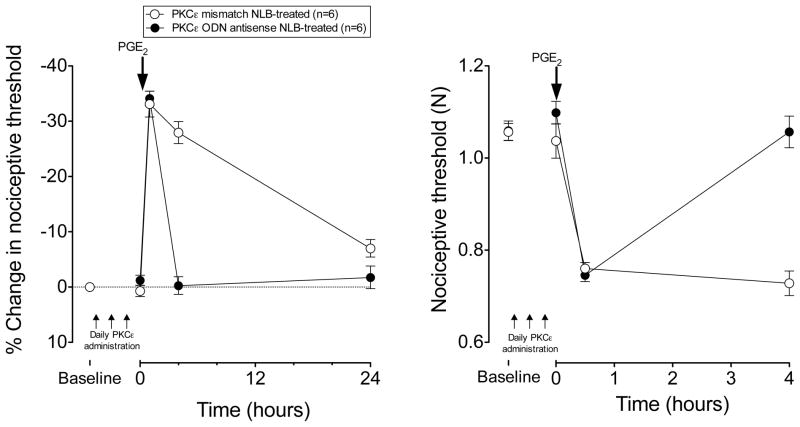
In contrast to hyperalgesia in muscle, administration of ODN antisense against PKCε or mismatch (both 40 μg, i.t.), daily for three days had no significant effect on cutaneous nociceptive threshold in adult NLB rats (antisense: 1.06±0.02 vs. 1.10±0.02 N, P=N.S.; mismatch: 1.06±0.02 vs. 1.04± 0.04 N, P=N.S., Fig. 4). However, similar to muscle hyperalgesia, administration of ODN antisense against PKCε mRNA daily for three days prior to PGE2 in adult NLB rats, prevented the prolonged PGE2-induced cutaneous mechanical hyperalgesia present in adult NLB-treated rats administered mismatch ODN (P<0.0001 at the 4 h time point, repeated measures ANOVA with Bonferroni post hoc test, Fig. 4). In contrast to muscle hyperalgesia, no significant difference between groups was observed at the 24 h post-PGE2 time point.
Figure 4. PKCε antisense inhibits NLB-induced hyperalgesic priming in skin.
In adult NLB rats that had received ODN antisense against PKCε for 3 days (filled circles, n=10), PGE2–induced hyperalgesia in the skin on the dorsum of the hind paw returned to baseline by 4 h post PGE2, while in mismatch-treated rats (open circles, n=10), PGE2 hyperalgesia remained elevated 4 h after PGE2.
Muscle nociceptors
Single-unit muscle afferent recordings of action potentials evoked by a 10-g stimulus in naive controls is shown in Fig. 5.
Figure 5. Afferent fiber response to mechanical stimulation.
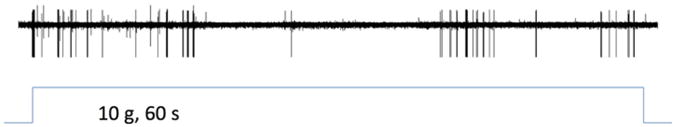
Single-unit recording of muscle afferent fiber action potentials evoked by a sustained (60 s) suprathreshold (10 g) von Frey hair mechanical stimulation.
a) Mechanical threshold
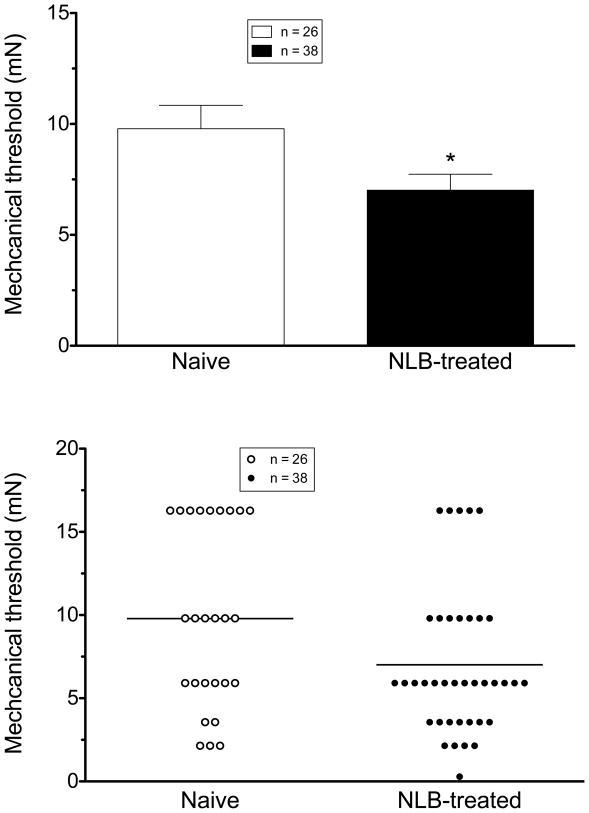
When tested by application of von Frey hairs to the peripheral receptive field in the gastrocnemius muscle, the mechanical threshold of muscle afferents in adult NLB-treated rats (7.02±0.72 mN, n=38) was significantly lower than of muscle afferents in control animals (9.78±1.05 mN, n= 26, P=0.0176, one-tailed Student’s t-test with Welch’s correction; Fig. 6); NLB stress decreases mechanical threshold ~31% for activation in skeletal muscle nociceptors.
Figure 6. NLB stress decreases muscle nociceptor threshold.
Mechanical threshold in nociceptors innervating the gastrocnemius muscle of NLB-treated rats was significantly lower (0.71 ± 0.07 mN, n=38) than the threshold of nociceptors from naive control rats (1.0 ± 0.11 mN, n= 26, P=0.0176, one-tailed Student’s t-test with Welch’s correction; top panel). Scattergram of mechanical thresholds of individual muscle nociceptors from naive control and NLB-treated rats (bottom panel).
b) Conduction velocity
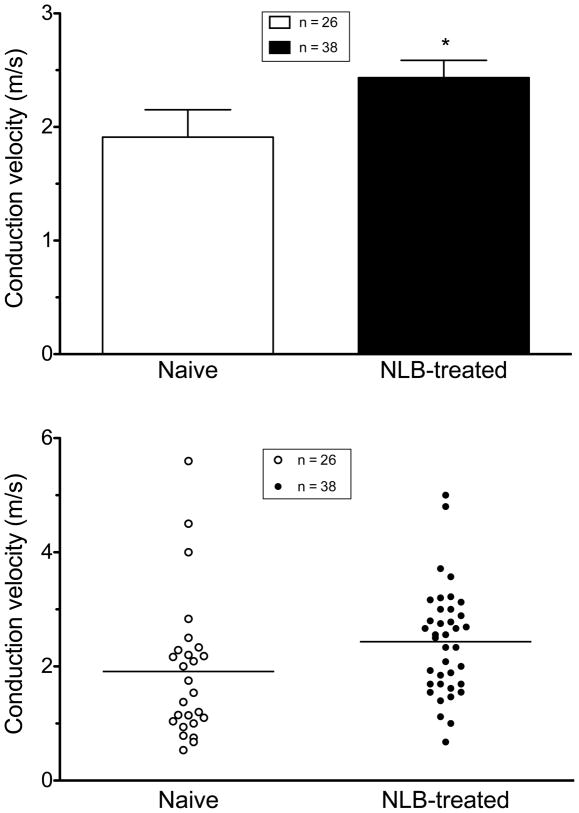
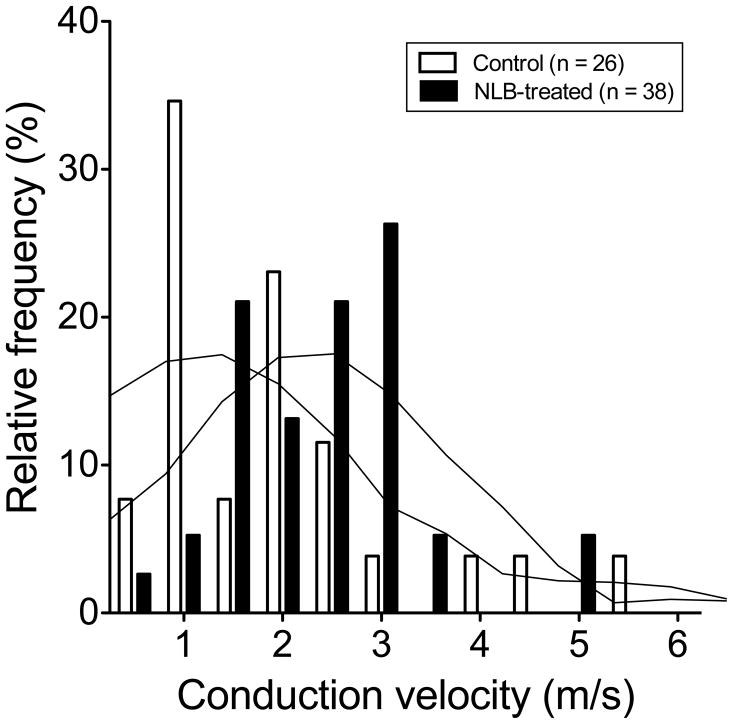
In NLB-treated rats, the conduction velocity of muscle afferents (2.44 ± 0.15 m/sec, n = 38) was significantly faster than that of afferents in control animals (1.91 ± 0.2 m/sec, n = 26, one-tailed Student’s t-test, with Welch’s correction, P=0.036, Fig. 7). The frequency distribution of conduction velocities in fibers of NLB-treated and control fibers indicates a shift in the distribution of conduction velocity, with a higher percentage of faster-conducting fibers in NLB rats (Fig. 8). Thus, while not excluding a contribution of a difference in percentage of type III and type IV fibers that were sampled in the NLB versus control groups of rats, the shift in distribution is most compatible with the suggestion that our findings are explained by an increase in conduction velocity in muscle nociceptors induced by NLB exposure. Of note, we have recently reported a similar effect on muscle nociceptor conduction velocity in a model of stress-induce hyperalgesia in the adult rat [11].
Figure 7. NLB stress increases muscle nociceptor conduction velocity.
Mean conduction velocity of muscle nociceptors in NLB-treated rats (2.44 ± 0.15 m/s, n=38) were significantly greater than from naive rats (1.91 ± 0.2 m/s, n=26, Student’s t-test with Welch’s correction, P=0.036). Scattergram of conduction velocities of muscle nociceptors in naive control and paclitaxel-treated rats (bottom panel).
Figure 8. NLB stress shifts muscle nociceptor conduction velocity frequency distribution.
A plot of frequency distribution of conduction velocity indicates that there is a shift to faster conducting fibers in NLB-treated rats. Graph overlay shows smoothing curve of data (6th order polynomial, 4 neighbor averaging) to illustrate shift in conduction velocity distribution.
c) Response to sustained stimulation
To examine excitability in muscle nociceptors from stressed rats, we evaluated their response to a sustained suprathreshold (10 g) von Frey hair stimulus. The response of muscle afferents to this sustained mechanical stimulation, in NLB-treated rats (361.1 ± 91.5 action potentials/60 sec stimulus, n= 38), although 27% greater, was not significantly different from afferents in control rats (283.3 ± 63.9 action potentials/60 sec stimulus, n= 26, respectively, P = NS, two-tailed Student’s t-test with Welch’s correction, data not shown); similarly, no significant effect of NLB was observed for the other time periods analyzed (burst: 0 – 10 s; plateau: 10 – 60 s).
In NLB-treated rats, there was no change in firing frequency of muscle nociceptors in response to sustained 10 g stimulation (two-way repeated measures ANOVA, with Bonferroni post hoc test, NS, data not shown).
d) Firing pattern
Finally, to examine the effect of early-life stress on the pattern of neural activity in nociceptors, we generated inter-stimulus interval (ISI) histograms and performed coefficient of variation (CV2) analyses for muscle afferents recorded in stressed and control rats. No significant effect of NLB on ISI or CV2 distribution were observed (data not shown).
DISCUSSION
NLB-treatment produced mechanical hyperalgesia in skeletal muscle and prolongation of inflammatory mediator-induced hyperalgesia (i.e., hyperalgesic priming) [17], as well as hyperalgesic priming in the cutaneous domain, measured in adult rats. While treatment with PKCε antisense in adult NLB-treated rats only slightly reduced ongoing mechanical hyperalgesia in muscle, but not skin, it completely reversed hyperalgesic priming in both muscle and skin, implicating a role of PKCε in this effect of early-life stress. This role for PKCε in hyperalgesic priming induced by treatment with NLB is similar to that which we found in the development of cutaneous hyperalgesic priming induced by carrageenan or tumor necrosis factor 〈 [1,44,50] and muscle hyperalgesic priming induced by carrageenan, lipopolysaccharide (i.e. endotoxin) and ergonomic stimuli (i.e. eccentric exercise and occupational level exposure to vibration) [4,20–22].
The partial reversal of lowered muscle mechanical nociceptive threshold present in adult NLB-treated rats, by PKCε antisense suggests that mechanisms other than PKCε in the peripheral nociceptive muscle afferent contribute to the lowered muscle mechanical threshold in NLB-treated adult rats. However, these other mechanisms are, at present, unknown. Of note, the prolonged muscle hyperalgesia in NLB rats that received mismatch ODN (Fig. 3) compared to NLB rats (Fig. 1) may be related to the additional stress produced by the ODN injection protocol; neonatal limited bedding rats have both a greater basal corticosterone level [6] and an exaggerated response to stress [28], and since chronic stress in adult rats enhances inflammatory mediator-induced hyperalgesia [22], this may account for the prolonged hyperalgesic response in NLB rats receiving mismatch ODN compared to un-injected NLB rats.
There is considerable evidence that stressful early-life events produce long-lasting deleterious health effects in humans (e.g. increased incidence of cardiovascular disease, anxiety and depression) that persist into adulthood [26,58]. Furthermore, early-life stress is associated with development of chronic pain syndromes in adults [16,24,59,62]. While the mechanisms that contribute to these chronic pain syndromes are mostly unknown, early-life stressors, which disrupt stress axis responses, are associated with chronic pain in adults. For example, women with fibromyalgia (a syndrome characterized by chronic widespread pain) exhibited flattened diurnal cortisol with greater cortisol responses upon awakening [67] and higher diurnal cortisol levels [51], while other studies found lowered morning salivary cortisol in individuals with chronic fatigue syndrome (another chronic condition in which pain is relatively common) who had experienced childhood trauma [32]. Animal studies have provided further insight into mechanisms mediating disruption of the hypothalamic-pituitary-adrenal (HPA) axis by neonatal exposure to stress. Compared to adults, stressful stimuli only elicit a small response in the HPA axis during the early postnatal period (postnatal days 3–12) due to an active suppression by the sympathoadrenal axis [65]. For example, NLB produces an increase in basal corticosterone plasma levels, which is increased by mild environmental stimuli (e.g., handling) that have no effect on plasma corticosterone in control animals [8], and it also alters gene expression in the HPA axis, e.g. decreased glucocorticoid receptor and corticotrophic releasing hormone (CRH) mRNA expression in the hypothalamus, but increased stress-induced CRH release [6].
In addition to affecting HPA axis function, evidence from animal studies has indicated that early-life stress also affects sympathoadrenal axis function. For example, plasma epinephrine and norepinephrine levels are greater in maternally separated rats [25], and guanethidine-induced chemical sympathectomy, which destroys sympathetic innervation of the adrenal gland, attenuates the increase in corticosterone production [65]. It is well established that stress exacerbates pain in several chronic diseases (e.g. fibromyalgia syndrome [2], chronic widespread pain [3], rheumatic diseases [31] and irritable bowel syndrome [27]), and we have shown in an animal model that a long-lasting increase in activity of both the HPA and sympathoadrenal stress axes contributes to a generalized inflammatory mediator-induced hyperalgesia [30,39,40]. Furthermore, we have shown that enhanced sympathoadrenal axis activity (i.e., increased plasma epinephrine level) maintains stress-induced enhancement of mechanical hyperalgesia for at least 4 weeks [40]. In these studies, in adult rats, we did not evaluate whether this enhancement of hyperalgesia persisted for long periods of time, due to the technical limitations of assessing mechanical nociceptive threshold in very large rats. However, in the current study, we have shown that early-life stress produces an enhancement of the nociceptive response months after exposure. It is also possible that early life stress affects the phenotype of nociceptors during the critical post-natal period of development. For example, increasing nerve growth factor (NGF) during postnatal days 2–14 doubled the proportion of nociceptors that respond to noxious heat and mechanical stimuli (conversely, decreasing NGF reduced the proportion of these nociceptors by about two-thirds while increasing the proportion of low mechanical threshold neurons) [46]. Of note in this regard, neonatal maternal separation increases NGF levels in rat brain [14], as well as plasma levels in maternally-deprived rhesus macaques [15]. Furthermore, visceral hypersensitivity present in adult rats that were exposed to maternal deprivation stress is correlated with increased levels of colonic NGF [7]; this hypersensitivity is prevented by treatment with anti-NGF antibodies, while administration of NGF daily (post-natal day 2 – 14), to normally reared rats, produced visceral sensitivity in adult rats [7]. While these observations suggest that stress-induced increase in NGF levels in neonates plays a role in producing long-term phenotypic changes in the nociceptor, other mediators may be also involved. For example, maternal separation induces decreased plasma testosterone, estradiol and leptin in male rats and a long-term decrease in hippocampal glucocorticoid receptor levels [48], but a greater HPA axis response to acute stress [63].
Importantly, we observed a decrease in muscle, but not cutaneous, mechanical nociceptive threshold in adult NLB rats. This stress-induced mechanical hyperalgesia in skeletal muscle, is accompanied by changes in the electrophysiological properties of muscle nociceptors, including significantly decreased mechanical threshold (~31%) and increased conduction velocity (~27%). It is likely that these changes in skeletal muscle primary afferent nociceptor function, in particular the lowered nociceptive threshold produced by NLB, contribute to muscle hyperalgesia in the adult NLB-treated rat. Of note, these changes in the function of the nociceptor appear to be permanent, since electrophysiological studies were performed on adult rats, 7 – 9 weeks after exposure to NLB. The mechanism underlying neonatal stress-induced decreased nociceptive threshold in muscle but not in skin, is not yet known. However, NGF, which sensitizes muscle high-threshold mechanosensitive (i.e. nociceptive)group IV afferents [33], and synthesized in muscle tissue [5], if it is increased by exposure to stress in muscle (as it is in plasma [15], colon [7] and brain {[14]. However, the exact basis of the differences between muscle and cutaneous nociceptors responsible for the different effects of neonatal stress on the function of these two types of nociceptors remain to be elucidated
In summary, have shown that neonatal stress induces apersistent decrease in skeletal, but not cutaneous, mechanical nociceptive threshold and that this behavioral musculoskeletal is associated with changes in nociceptor function in muscle is associated with enhanced activity in muscle nociceptors, namely, a lowered mechanical threshold, and increased conduction velocity. Furthermore, this early-life stress produces hyperalgesic priming in skin as well as muscle. These changes provide the first demonstration of neonatal stress-induced changes in primary afferent nociceptor function, changes that could contribute to the enhanced nociception observed in this model of an adult pain syndrome induced early life stress.
Acknowledgments
We thank Dr. Robert Gear for help with CV2 calculations. This research was supported by a grant from NIAMS AR054635.
Footnotes
The authors do not have a conflict of interest.
References
- 1.Fibromyalgia problem case. Monotherapy is mostly insufficient. MMW Fortschr Med. 2002;144:52. [PubMed] [Google Scholar]
- 2.Ablin J, Neumann L, Buskila D. Pathogenesis of fibromyalgia - a review. Joint Bone Spine. 2008;75:273–279. doi: 10.1016/j.jbspin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Ablin JN, Cohen H, Clauw DJ, Shalev R, Ablin E, Neumann L, Sarzi-Puttini P, Buskila D. A tale of two cities - the effect of low intensity conflict on prevalence and characteristics of musculoskeletal pain and somatic symptoms associated with chronic stress. Clin Exp Rheumatol. 2010 [PubMed] [Google Scholar]
- 4.Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci. 2010;32:819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amano T, Yamakuni T, Okabe N, Sakimura K, Takahashi Y. Production of nerve growth factor in rat skeletal muscle. Neurosci Lett. 1991;132:5–7. doi: 10.1016/0304-3940(91)90418-s. [DOI] [PubMed] [Google Scholar]
- 6.Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain. 2010;151:460–466. doi: 10.1016/j.pain.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Green PG, Levine JD. Stress enhances muscle nociceptor activity in the rat. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.04.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Levine JD. Mechanically-evoked C-fiber activity in painful alcohol and AIDS therapy neuropathy in the rat. Mol Pain. 2007;3:Article No. 5. doi: 10.1186/1744-8069-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res. 2007;1153:68–77. doi: 10.1016/j.brainres.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Cirulli F, Alleva E, Antonelli A, Aloe L. NGF expression in the developing rat brain: effects of maternal separation. Brain Res Dev Brain Res. 2000;123:129–134. doi: 10.1016/s0006-8993(00)02844-4. [DOI] [PubMed] [Google Scholar]
- 15.Cirulli F, Francia N, Branchi I, Antonucci MT, Aloe L, Suomi SJ, Alleva E. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34:172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 17.de Coupade C, Brown AS, Dazin PF, Levine JD, Green PG. beta(2)-Adrenergic receptor-dependent sexual dimorphism for murine leukocyte migration. J Neuroimmunol. 2007;186:54–62. doi: 10.1016/j.jneuroim.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehl B, Hoheisel U, Mense S. The influence of mechanical stimuli and of acetylsalicylic acid on the discharges of slowly conducting afferent units from normal and inflamed muscle in the rat. Exp Brain Res. 1993;92:431–440. doi: 10.1007/BF00229031. [DOI] [PubMed] [Google Scholar]
- 19.Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011 doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dina OA, Messing RO, Levine JD. Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. Eur J Neurosci. 2006;24:197–204. doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- 24.Engel GL. Psychogenic pain and pain-prone patient. Am J Med. 1959;26:899–918. doi: 10.1016/0002-9343(59)90212-8. [DOI] [PubMed] [Google Scholar]
- 25.Esquivel BB, Levin G, Rivarola MA, Suarez MM. Maternal separation and lesion of adtn alters anxiety and adrenal activity in male rats. Int J Neurosci. 2009;119:1319–1336. doi: 10.1080/00207450902931847. [DOI] [PubMed] [Google Scholar]
- 26.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 27.Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8:274–281. doi: 10.2174/156652408784533760. [DOI] [PubMed] [Google Scholar]
- 28.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giske L, Bautz-Holter E, Sandvik L, Roe C. Relationship between pain and neuropathic symptoms in chronic musculoskeletal pain. Pain Med. 2009;10:910–917. doi: 10.1111/j.1526-4637.2009.00622.x. [DOI] [PubMed] [Google Scholar]
- 30.Green PG, Alvarez P, Gear RW, Mendoza D, Levine JD. Further validation of a model of fbromyalgia syndrome in the rat. J Pain. 2011 doi: 10.1016/j.jpain.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassett AL, Clauw DJ. The role of stress in rheumatic diseases. Arthritis Res Ther. 2010;12:123. doi: 10.1186/ar3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry. 2009;66:72–80. doi: 10.1001/archgenpsychiatry.2008.508. [DOI] [PubMed] [Google Scholar]
- 33.Hoheisel U, Unger T, Mense S. Sensitization of rat dorsal horn neurons by NGF-induced subthreshold potentials and low-frequency activation. A study employing intracellular recordings in vivo. Brain Res. 2007;1169:34–43. doi: 10.1016/j.brainres.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 34.Holt GR, Softky WR, Koch C, Douglas RJ. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J Neurophysiol. 1996;75:1806–1814. doi: 10.1152/jn.1996.75.5.1806. [DOI] [PubMed] [Google Scholar]
- 35.Huot RL, Brennan PA, Stowe ZN, Plotsky PM, Walker EF. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann N Y Acad Sci. 2004;1032:234–236. doi: 10.1196/annals.1314.028. [DOI] [PubMed] [Google Scholar]
- 36.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143:92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Kalinichev M, Easterling KW, Holtzman SG. Repeated neonatal maternal separation alters morphine-induced antinociception in male rats. Brain Res Bull. 2001;54:649–654. doi: 10.1016/s0361-9230(01)00485-3. [DOI] [PubMed] [Google Scholar]
- 39.Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 41.Korosi A, Baram TZ. Plasticity of the stress response early in life: Mechanisms and significance. Dev Psychobiol. 2010;52:661–670. doi: 10.1002/dev.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korszun A, Papadopoulos E, Demitrack M, Engleberg C, Crofford L. The relationship between temporomandibular disorders and stress-associated syndromes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:416–420. doi: 10.1016/s1079-2104(98)90366-3. [DOI] [PubMed] [Google Scholar]
- 43.Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, Schmulson M, Mayer EA. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–1326. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 44.Levine JD, Reichling DB. Fibromyalgia: the nerve of that disease. J Rheumatol Suppl. 2005;75:29–37. [PubMed] [Google Scholar]
- 45.Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46:697–702. doi: 10.1682/jrrd.2009.01.0006. [DOI] [PubMed] [Google Scholar]
- 46.Lewin GR, Mendell LM. Regulation of cutaneous C-fiber heat nociceptors by nerve growth factor in the developing rat. J Neurophysiol. 1994;71:941–949. doi: 10.1152/jn.1994.71.3.941. [DOI] [PubMed] [Google Scholar]
- 47.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 48.Llorente R, Miguel-Blanco C, Aisa B, Lachize S, Borcel E, Meijer OC, Ramirez MJ, De Kloet ER, Viveros MP. Long term sex-dependent psychoneuroendocrine effects of maternal deprivation and juvenile unpredictable stress in rats. J Neuroendocrinol. 2011;23:329–344. doi: 10.1111/j.1365-2826.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 49.Martin AL, Halket E, Asmundson GJ, Flora DB, Katz J. Posttraumatic stress symptoms and the diathesis-stress model of chronic pain and disability in patients undergoing major surgery. Clin J Pain. 2010;26:518–527. doi: 10.1097/AJP.0b013e3181e15b98. [DOI] [PubMed] [Google Scholar]
- 50.Miao FJ, Benowitz NL, Levine JD. Endogenous opioids suppress activation of nociceptors by sub-nanomolar nicotine. Br J Pharmacol. 2001;133:23–28. doi: 10.1038/sj.bjp.0704031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicolson NA, Davis MC, Kruszewski D, Zautra AJ. Childhood maltreatment and diurnal cortisol patterns in women with chronic pain. Psychosom Med. 2010;72:471–480. doi: 10.1097/PSY.0b013e3181d9a104. [DOI] [PubMed] [Google Scholar]
- 52.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 53.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 54.Paras ML, Murad MH, Chen LP, Goranson EN, Sattler AL, Colbenson KM, Elamin MB, Seime RJ, Prokop LJ, Zirakzadeh A. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302:550–561. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- 55.Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol. 2001;38:239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- 56.Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 57.Raphael KG, Widom CS. Post-traumatic stress disorder moderates the relation between documented childhood victimization and pain 30 years later. Pain. 2011;152:163–169. doi: 10.1016/j.pain.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 59.Sachs-Ericsson N, Kendall-Tackett K, Hernandez A. Childhood abuse, chronic pain, and depression in the National Comorbidity Survey. Child Abuse Negl. 2007;31:531–547. doi: 10.1016/j.chiabu.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Summer GJ, Puntillo KA, Miaskowski C, Dina OA, Green PG, Levine JD. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7:884–891. doi: 10.1016/j.jpain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Tanner KD, Reichling DB, Gear RW, Paul SM, Levine JD. Altered temporal pattern of evoked afferent activity in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003;118:809–817. doi: 10.1016/s0306-4522(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 62.Tietjen GE. Is there a link between abuse in childhood and pain disorders? Expert Rev Neurother. 2010;10:1625–1627. doi: 10.1586/ern.10.152. [DOI] [PubMed] [Google Scholar]
- 63.Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uhelski ML, Fuchs PN. Maternal separation stress leads to enhanced emotional responses to noxious stimuli in adult rats. Behav Brain Res. 2010;212:208–212. doi: 10.1016/j.bbr.2010.03.055. [DOI] [PubMed] [Google Scholar]
- 65.Walker CD. Chemical sympathectomy and maternal separation affect neonatal stress responses and adrenal sensitivity to ACTH. Am J Physiol. 1995;268:R1281–8. doi: 10.1152/ajpregu.1995.268.5.R1281. [DOI] [PubMed] [Google Scholar]
- 66.Weaver SA, Diorio J, Meaney MJ. Maternal separation leads to persistent reductions in pain sensitivity in female rats. J Pain. 2007;8:962–969. doi: 10.1016/j.jpain.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Weissbecker I, Floyd A, Dedert E, Salmon P, Sephton S. Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2006;31:312–324. doi: 10.1016/j.psyneuen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 68.Wellnitz SA, Lesniak DR, Gerling GJ, Lumpkin EA. The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J Neurophysiol. 2010;103:3378–3388. doi: 10.1152/jn.00810.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wood PB. Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses. 2004;62:420–424. doi: 10.1016/j.mehy.2003.10.013. [DOI] [PubMed] [Google Scholar]