Abstract
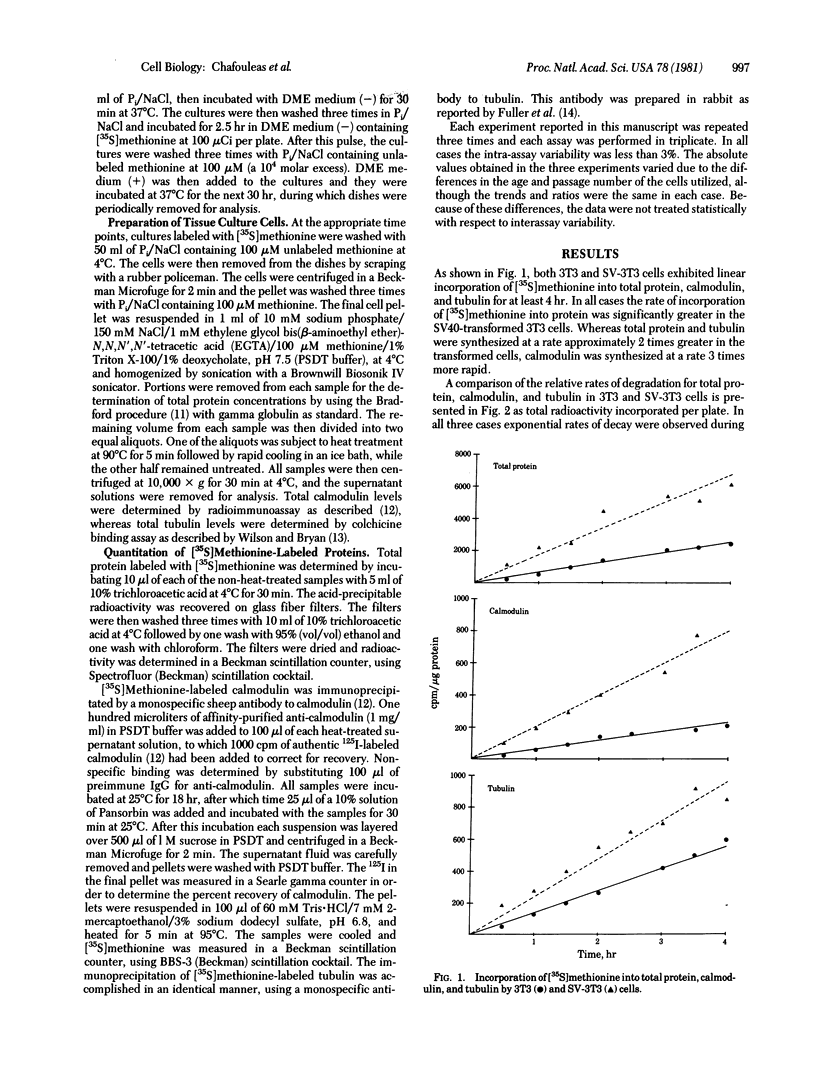
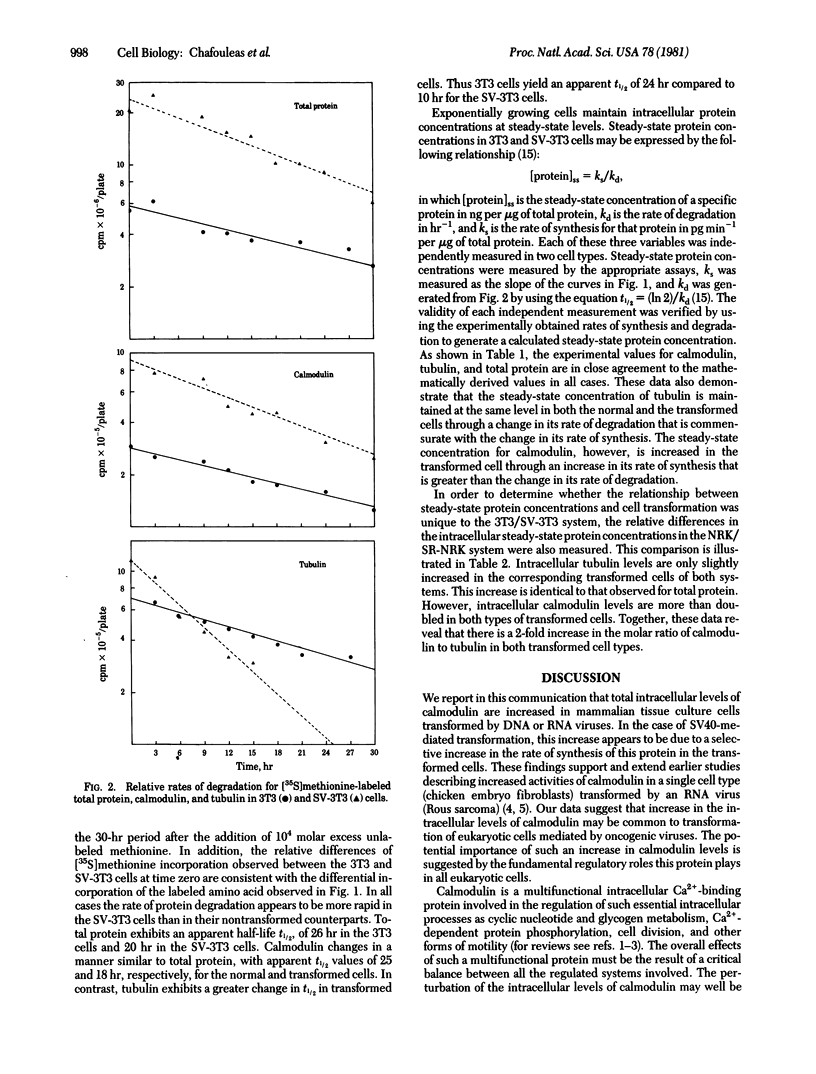
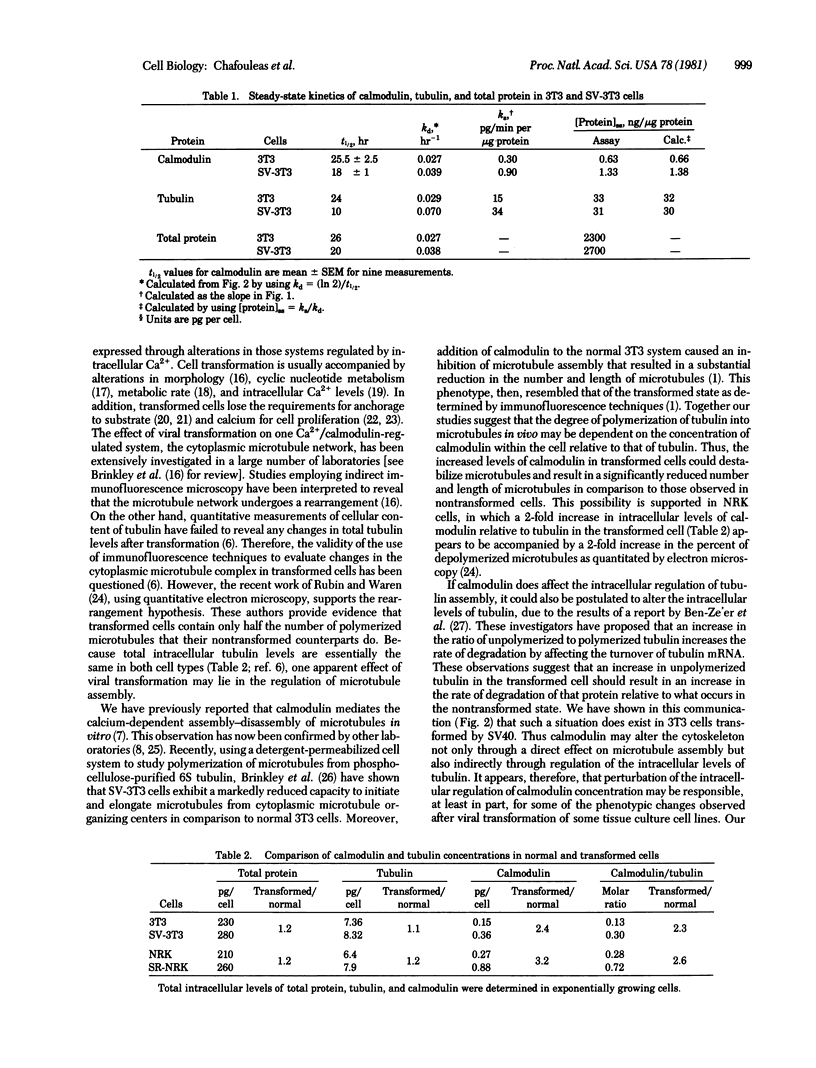
Transformation of mammalian tissue culture cells by oncogenic viruses results in a 2-fold increase in the intracellular concentration of calmodulin quantitated by radioimmunoassay. The two pairs of companion cell lines used in this study were the Swiss mouse 3T3/simian virus 40-transformed 3T3 cells and the normal rat kidney (NRK)/Rous sarcoma virus-transformed NRK cells. The increased intracellular levels of calmodulin in the transformed cells are due to a greater increase in the rate of synthesis (3-fold) relative to the change in the rate of degradation (1.4-fold). On the other hand, no increases were observed in tubulin levels as quantitated by a colchicine-binding assay. The lack of change in tubulin concentration was accounted for by a 2-fold increase in the rate of degradation that is compensated by a similar increase in the rate of synthesis. The consequence of such changes in both transformed cell types is a 2-fold increase in the calmodulin-to-tubulin protein ratio relative to that in their nontransformed counterparts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F. Different calcium requirements for proliferation of conditionally and unconditionally tumorigenic mouse cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1651–1654. doi: 10.1073/pnas.73.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K. Radioimmunoassay for tubulin: a quantitative comparison of the tubulin content of different established tissue culture cells and tissues. Cell. 1978 Aug;14(4):795–804. doi: 10.1016/0092-8674(78)90335-5. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Gidwitz S., Weber M. J., Storm D. R. Relationship between changes in the calcium dependent regulatory protein and adenylate cyclase during viral transformation. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1169–1177. doi: 10.1016/0006-291x(79)90240-7. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Nishida E., Kumagai H., Ohtsuki I., Sakai H. The interactions between calcium-dependent regulator protein of cyclic nucleotide phosphodiesterase and microtubule proteins. I. Effect of calcium-dependent regulator protein on the calcium sensitivity of microtubule assembly. J Biochem. 1979 May;85(5):1257–1266. [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979 Jan;16(1):11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Rubin R. W., Warren R. H. Organization of tubulin in normal and transformed rat kidney cells. J Cell Biol. 1979 Jul;82(1):103–113. doi: 10.1083/jcb.82.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierenga S. H., Whitfield J. F., Karasaki S. Loss of proliferative calcium dependence: simple in vitro indicator of tumorigenicity. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6069–6072. doi: 10.1073/pnas.75.12.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Studies on cells rendered neoplastic by polyoma virus: the problem of the presence of virus-related materials. Virology. 1962 Jan;16:41–51. doi: 10.1016/0042-6822(62)90200-3. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Waisman D. M. Calmodulin and its role in the second-messenger system. Curr Top Cell Regul. 1979;15:47–107. doi: 10.1016/b978-0-12-152815-7.50006-5. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Van Eldik L. J., Smith R. E., Vanaman T. C. Calcium-dependent regulatory protein of cyclic nucleotide metabolism in normal and transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2711–2715. doi: 10.1073/pnas.73.8.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]



