Abstract
The matricellular protein Thrombospondin (TSP)-1 is induced following tissue injury and may regulate reparative responses by activating TGF-β, by suppressing angiogenesis, and by modulating inflammation and matrix metabolism. We hypothesized that endogenous TSP-1 may be involved in the pathogenesis of cardiac remodeling in the pressure-overloaded heart. Myocardial TSP-1 expression was increased in a mouse model of pressure overload due to transverse aortic constriction. TSP-1 -/- mice exhibited increased early hypertrophy and enhanced late dilation in response to pressure overload. Pressure-overloaded TSP-1 null mice had intense degenerative cardiomyocyte changes, exhibiting more extensive sarcomeric loss and sarcolemmal disruption when compared with wildtype hearts. Accentuated hypertrophy and cardiomyocyte injury in TSP-1 -/- hearts was accompanied by increased myofibroblast density. However, despite a two-fold higher infiltration of the cardiac interstitium with myofibroblasts, pressure-overloaded TSP-1 null hearts did not exhibit significantly increased collagen content when compared with wildtype hearts. The disproportionately low collagen content in TSP-1 null hearts was due to infiltration with abundant, but functionally defective, fibroblasts that exhibited impaired myofibroblast differentiation and reduced collagen expression in comparison to wildtype fibroblasts. Impaired myofibroblast activation in TSP-1 null hearts was associated with reduced Smad2 phosphorylation reflecting defective TGF-β signaling. Moreover, TSP-1 null hearts had increased myocardial Matrix Metalloproteinase (MMP)-3 expression and enhanced MMP-9 activation following pressure overload. TSP-1 upregulation in the pressure-overloaded heart critically regulates fibroblast phenotype and matrix remodeling by activating TGF-β signaling and by promoting matrix preservation, thus preventing chamber dilation.
Keywords: fibrosis, matricellular proteins, cardiac remodeling, thrombospondin-1, myofibroblast, transforming growth factor-β1, collagen
INTRODUCTION
Pressure overload induces cardiac remodeling through effects on both cardiomyocytes and cardiac interstitial cells. Hypertrophy and apoptosis of cardiac myocytes play an important role in the development of heart failure in the pressure-overloaded heart 1, 2. On the other hand, fibroblast activation is a hallmark of the cardiomyopathic process following pressure overload, and results in accentuated deposition of collagen in the cardiac interstitium, increasing myocardial stiffness and inducing diastolic dysfunction 3. Beyond its effects on the cellular elements of the myocardium, pressure overload also markedly alters the composition of the extracellular matrix network 4. It is becoming increasingly appreciated that extracellular matrix proteins not only determine the mechanical properties of the ventricle, but also play an important role in modulating cellular responses in the remodeling myocardium 5. Cardiac injury induces expression of the matricellular proteins 6, a family of extracellular matrix proteins that, unlike collagen or elastin, do not serve a structural role, but modulate cell:cell and cell:matrix interactions 7. Several members of the matricellular family, (including tenascin-C, osteopontin, periostin, Secreted protein, acidic cysteine-rich/SPARC and the thrombospondins) have been implicated in cardiac remodeling, either by maintaining the integrity of the extracellular matrix 8, or by inducing phenotypic alterations on cardiomyocytes and fibroblasts 9, 10.
Thrombospondin (TSP)-1 is the archetypal matricellular protein, a 450kD homotrimeric protein with multiple functional domains 11. TSP-1 is upregulated in injured tissues and is capable of regulating wound healing and tissue remodeling by modulating a variety of cellular functions essential to the reparative process 12. First, TSP-1 functions as an essential activator of TGF-β signaling 13 through an association with the latent complex that results in a conformational rearrangement in the Latency-Associated Peptide (LAP) and renders the TGF-β dimer biologically active 14, 15. Second, TSP-1 exerts potent angiostatic actions through direct effects on endothelial cells that result in inhibition of proliferation and increased apoptosis 16, 17. Third, TSP-1 directly inhibits inflammation through CD47-mediated interactions 18. Fourth, TSP-1 modulates matrix metabolism by inhibiting MMP activation. We, and others, have reported important effects of TSP-1 in cardiac injury and repair. In healing myocardial infarction, TSP-1 is selectively deposited in the border zone and may serve as a protective “barrier” that protects the non-infarcted myocardium from extension of the inflammatory infiltrate by locally activating anti-inflammatory signals 19, 20. Moreover, in a rat model of diabetic cardiomyopathy exacerbated by abdominal aortic coarctation, TSP-1 expression was upregulated, and administration of a peptide antagonist of TSP-1-dependent TGF-β activation prevented the development of cardiac fibrosis 21. Although these findings suggest that TSP-1 may modulate myocardial inflammation and repair, its role in cardiac remodeling due to pressure overload has not been investigated.
Our study examines for the first time the role of TSP-1 in the development of the fibrotic cardiomyopathy associated with pressure overload. We report that TSP-1 is markedly upregulated in the myocardial interstitial space following experimental transverse aortic constriction (TAC). TSP-1 gene disruption resulted in adverse cardiac remodeling of the pressure-overloaded heart, associated with infiltration of the myocardium with abundant, but dysfunctional, fibroblasts that exhibited defective myofibroblast transdifferentiation and decreased matrix-synthetic capacity. Accumulation of functionally impaired fibroblasts in the remodeling myocardium was associated with defective activation of TGF-β/Smad3 signaling and enhanced MMP activity. Our findings suggest that TSP-1 deposition in the extracellular matrix of the pressure-overloaded heart, activates TGF-β-mediated signaling in reparative cardiac fibroblasts and preserves the extracellular matrix, affording protection from adverse remodeling.
METHODS
(A detailed description of the methods is provided in the online supplement, please see http://hyper.ahajournals.org).
Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Baylor College of Medicine and Albert Einstein College of Medicine Institutional Review Boards. Male and female, wildtype (WT) and TSP-1 null 13 C57/BL6 mice from our colony were genotyped using established protocols and underwent TAC as previously described 22. Functional assessment of the pressure-oveloaded mouse heart was performed using echocardiography. At the end of the experiment, the heart was excised, fixed in zinc-formalin, and embedded in paraffin for histological studies, or frozen for RNA or protein isolation. Animals used for histology underwent 3, 7, and 28 days of banding (n=8/group). Additional groups of mice were used for RNA and protein extraction after 3 (n=7) or 7 days (n=7) of banding, for isolation and flow cytometric analysis of cardiac fibroblasts (WT, n=10; TSP-1 -/-, n=6) and for assessment of collagen content using a biochemical hydroxyproline assay (WT, n=9; KO, n=12). As a control, a “sham” operation without aortic constriction was performed on age-matched mice (histology n=6, RNA n=6, protein n=6). The effects of TSP-1 loss on the inflammatory and fibrotic response were studied using immunohistochemistry, quantitative histology, qPCR, ribonuclease protection assays, and western blotting. Effects on apoptosis of cardiomyocytes and interstitial cells were investigated using TUNEL staining combined with Wheat Germ Agglutinin (WGA) lectin fluorescence to outline the surface of cardiomyocytes. The consequences of TSP-1 deficiency on TGF-β signaling were studied through assessment of Smad2 phosphorylation using western blotting. Effects on matrix metabolism were assessed using zymography whereas collagen content was measured with a hydroxyproline biochemical assay. The role of TSP-1 in modulating fibroblast phenotype and function in the pressure-overloaded myocardium was studied using isolated cardiac fibroblasts and flow cytometry on cells harvested from the myocardium 23.
RESULTS
1. TSP-1 is upregulated in the pressure-overloaded myocardium
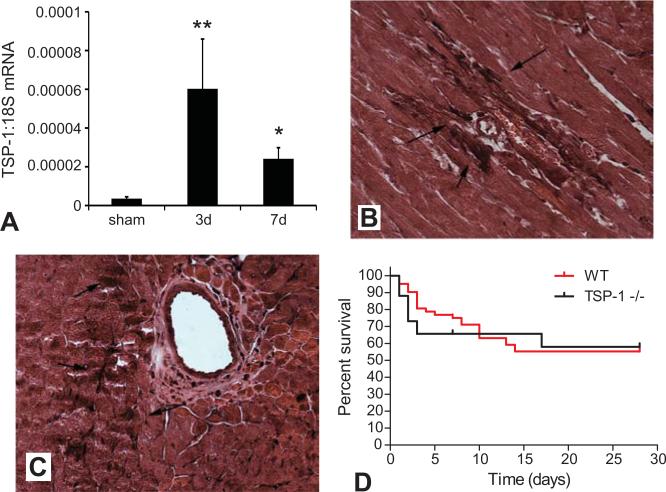
Using qPCR we demonstrated that TSP-1 mRNA expression was markedly induced in the pressure-overloaded heart after 3-7 days of TAC (Figure 1A). Immunohistochemical staining demonstrated that TSP-1 protein was primarily localized in the cardiac interstitial and perivascular space after 7-28 days of TAC (Figure 1B-C). TSP-1 immunoreactivity was absent in control mouse myocardium.
Figure 1.
TSP-1 upregulation in the pressure-overloaded heart. A. Quantitative rt-PCR demonstrated that TSP-1 mRNA was markedly induced in the pressure overloaded myocardium after 3-7 days of TAC (*p<0.01 vs. sham; *p<0.05 vs. sham). B-C: Immunohistochemical localization of TSP-1 protein in the myocardium after 7 (B) and 28 days (C) of TAC. TSP-1 was predominantly localized in the perivascular and interstitial space (arrows). Sections were counterstained with eosin. D. Kaplan-Meier curves demonstrating survival rates of WT and TSP-1 null mice undergoing pressure overload protocols. TSP-1 null mice and WT animals showed comparable mortality after 28 days of TAC (pNS), but had lower survival rates during the first 3 days of pressure overload (p=0.05).
2. TSP-1 null mice exhibit increased early mortality following TAC
TSP-1 null and WT mice exhibited comparable mortality after TAC (survival after 28 days TSP-1 -/- 57.9% vs. WT 55.3%, pNS) (Figure 1D). However, TSP-1 null mice had significantly increased early mortality over the first 3 days after TAC (% survival after 3 days TSP-1 -/-65.7 % vs. WT 80.3% p=0.05).
3. TSP-1 null mice have increased hypertrophy and dilation following pressure overload
In the absence of injury, WT and TSP-1 null mice had comparable cardiac dimensions, function, and Left Ventricular (LV) mass (Table 1). After 7 days of TAC, WT mouse hearts exhibited a marked increase in LV mass, associated with reduced Left Ventricular End-Diastolic Diameter (LVEDD) indicating the development of concentric left ventricular hypertrophy. At the same timepoint, TSP-1 null mice had accentuated hypertrophic remodeling exhibiting significantly higher LV mass and a trend for a higher LVEDD in comparison to WT animals (Table 1). After 28 days of TAC, LV mass was comparable between TSP-1 -/- and WT mice; however, TSP-1 null animals exhibited significantly higher LVEDD when compared to WT mice (p<0.05) (Table 1). Thus, TSP-1 deficiency was associated with increased early hypertrophy and enhanced late dilation in response to pressure overload. Left ventricular fractional shortening (LVFS) was comparable between WT and TSP-1 -/- mice at all timepoints examined, suggesting that the absence of TSP-1 did not affect the development of systolic dysfunction in the pressure overloaded heart.
Table 1.
Echocardiographic analysis of systolic function and chamber dimensions
| Functional parameter | Pre | 7d | Pre | 28d | ||||
|---|---|---|---|---|---|---|---|---|
| WT | -/- | WT | -/- | WT | -/- | WT | -/- | |
| LVEDD (mm) | 3.85+0.12 | 3.75+0.09 | 3.23+0.19* | 3.60+0.15 p=0.12 vs. WT |
3.72+0.09 | 3.89+0.08 | 3.79+0.15 (pNS) | 4.24+0.13* P<0.05 vs. WT |
| LVESD (mm) | 2.49+0.27 | 2.30+0.11 | 2.36+0.23 | 2.49+0.30 | 2.29+0.12 | 2.35+0.15 | 3.20+0.19* | 3.54+0.17** P=0.14 vs. WT |
| LVFS | 0.36+0.05 | 0.39+0.02 | 0.27+0.04 | 0.32+0.06 | 0.39+0.02 | 0.40+0.03 | 0.16+0.02* | 0.17+0.03* |
| LVM (mg) | 68+2.79 | 79.7+3.7 | 104.9+6.7* | 134.1+10.8 P<0.05 vs. WT |
77.4+4.5 | 79.8+4.9 | 181.5+14.5* | 193.2+12.0* |
p<0.01 vs. corresponding pre
4. Effects of TSP-1 absence on cardiomyocyte alterations and apoptosis in the pressure overloaded heart
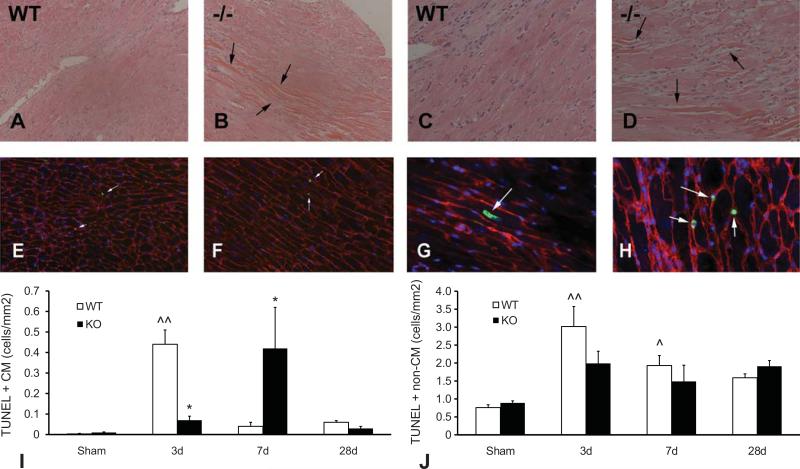
In order to examine the basis for enhanced cardiac remodeling following TAC in the absence of TSP-1, we compared the pathologic alterations in WT and TSP-1 null pressure-overloaded hearts. In both WT and TSP-1 null mice, TAC resulted in widening of the cardiac interstitium and focal cardiomyocyte degeneration. TSP-1 null animals had more intense cardiomyocyte changes following 3-7 days of TAC in comparison to WT animals, exhibiting more extensive sarcomeric loss and sarcolemmal disruption (Figure 2A-D). Combined TUNEL/WGA lectin staining (Figure 2E-H) was used to identify apoptotic cardiomyocytes in the pressure-overloaded heart. In both WT and TSP-1 null mice, the majority of apoptotic cells in the pressure overloaded myocardium were non-cardiomyocytes; apoptotic cardiomyocytes were rare. In WT hearts the density of apoptotic cardiomyocytes peaked after 3 days of TAC; in contrast, TSP-1 null animals had a delayed peak in the number of apoptotic cells after 7 days of TAC (Figure 2I). The number of apoptotic interstitial cells was comparable between WT and TSP-1 null hearts at all timepoints examined (Figure 2J).
Figure 2.
Effects of TSP-1 loss on cardiomyocyte injury and apoptosis in the pressure-overloaded myocardium. A-D. Hematoxylin-eosin staining shows histopathologic alterations after 7 days of TAC in WT (A, C) and TSP-1 null (B, D) hearts. Low (100X; A-B) and high (400X; C-D) magnification images demonstrate that TSP-1 null hearts (B, D) exhibited more intense alterations in cardiomyocyte morphology (including sarcomeric loss, hypereosinophilia and contraction band necrosis; arrows) than WT hearts. E-H: Apoptosis of cardiomycoytes and non-cardiomyocytes was quantitatively assessed using TUNEL staining (green - arrows), combined with WGA lectin histochemistry that outlines the surface of cardiomyocytes (red). The density of apoptotic cardiomyocytes (G – arrow) and interstitial cells (H – arrows) was quantitatively assessed in WT (E) and TSP-1 -/- (F) hearts after 3-28d of TAC. DAPI staining (blue) was used to label all nuclei in the murine myocardium. I-J: The majority of apoptotic cells were non-cardiomyocytes; TUNEL+ cardiomyocytes were rare. TSP-1 -/- mice had lower early cardiomyocyte apoptosis after 3 days of TAC, but exhibited a significantly higher apoptotic cardiomyocyte density after 7 days (*p<0.05 vs. WT) (I). The number of apoptotic non-cardiomyocytes was comparable between groups (J) (^p<0.05, ^^p<0.01 vs. sham).
5. Despite a marked increase in myofibroblast density, pressure-overloaded TSP-1 -/- hearts have no significant increase in collagen content
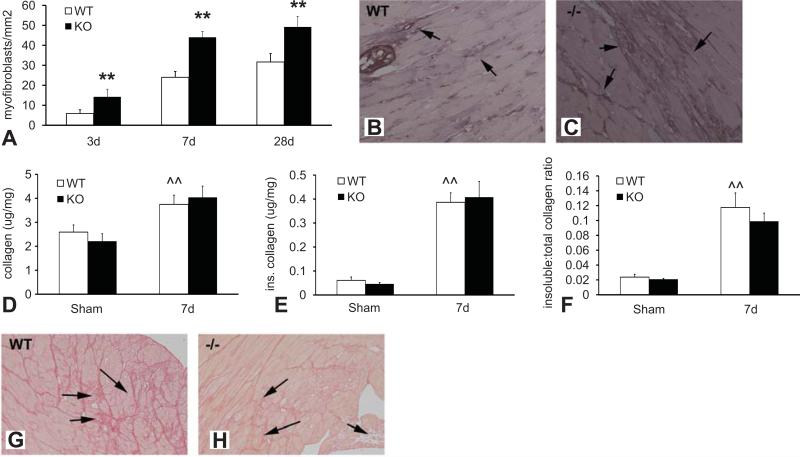
TSP-1 absence was associated with markedly increased myofibroblast density after 7-28 days of TAC (Figure 3A-C). Despite showing a two-fold higher myofibroblast infiltration, TSP-1 null animals had no significant increase in total, soluble, and insoluble collagen content after 7 days of TAC when compared to WT hearts (Figure 3).
Figure 3.
Although TSP-1 null pressure-overloaded hearts are infiltrated with abundant fibroblasts, collagen content is not significantly increased. A-C Myofibroblasts were identified in the pressure overloaded myocardium as spindle-shaped cells expressing α-SMA located outside the vascular media (arrows). Quantitative analysis demonstrated that myofibroblast density was significantly higher in TSP-1 -/- hearts after 3-28 days of TAC (**p<0.01 vs. WT). Representative images showing α-SMA staining to identify myofibroblasts in WT (B) and TSP-1 null (C) hearts after 7 days of pressure overload are shown. D-F. Cardiac pressure overload was associated with increased total and insoluble collagen content (measured with a hydroxyproline biochemical assay) and a higher ratio of insoluble:total collagen (^^p<0.01 vs. sham). Despite a markedly increased myofibroblast density, collagen content was not significantly higher in TSP-1 null hearts after 7 days of pressure overload (D). The amount of insoluble collagen (E) and the ratio of insoluble:total collagen (F) were comparable between TSP-1 -/- and WT animals. Sirius red staining showed that, while pressure overloaded WT hearts had interstitial deposition of dense collagen (arrows), TSP-1 -/- animals had more extensive cardiomyocyte loss, associated with replacement by a matrix network comprised of loose collagen (arrows).
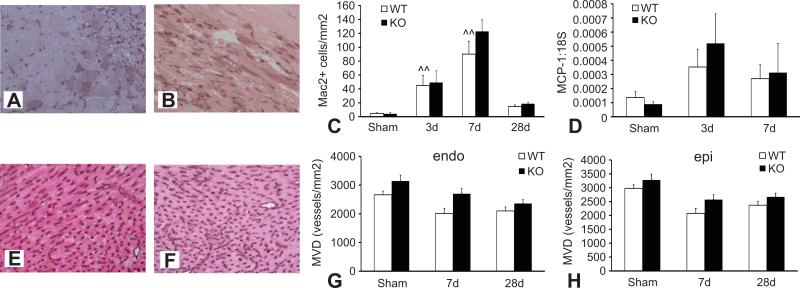
6. TSP-1 absence does not affect the inflammatory response in the pressure-overloaded heart
Because TSP-1 is an important modulator of the inflammatory response we examined whether increased cardiac remodeling in pressure-overloaded TSP-1 null mice is associated with increased expression of inflammatory mediators. TSP-1 null and WT animals showed no significant differences in the intensity and time course of the myocardial inflammatory response following TAC. RPA analysis demonstrated that expression of the pro-inflammatory cytokines Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-1β, and IL-6 was comparable between TSP-1 -/-and WT hearts after 3-7 days of TAC (Table 2). Mac-2 immunohistochemistry showed that macrophage density was comparable between WT and TSP-1 null mice after 3-28 days of TAC (Figure 4A-C). In addition, expression of the CC chemokine Monocyte chemoattractant Protein (MCP)-1, a critical regulator of mononuclear cell recruitment, was comparable between groups (Figure 4D).
Table 2.
Cytokine mRNA expression in the pressure-overloaded heart
| Ratio to L32 | Sham | 72h | 7days | |||
|---|---|---|---|---|---|---|
| WT | -/- | WT | -/- | WT | -/- | |
| TNF-α | 0.0022+0.0005 | 0.0013+0.0002 | 0.0085+0.0001* | 0.0055+0.0002* | 0.0023+0.0003 | 0.0023+0.0005 |
| IL-1β | 0.003+0.0007 | 0.0018+0.0003 | 0.01+0.001* | 0.01+0.002* | 0.0027+0.0005 | 0.0026+0.0003 |
| IL-6 | 0.007+0.001 | 0.004+0.0003 | 0.023+0.008* | 0.019+0.004* | 0.006+0.0008 | 0.01+0.0009 |
p<0.01 vs. corresponding sham.
Figure 4.
TSP-1 -/- and WT mice show comparable infiltration of the pressure-overloaded heart with macrophages and exhibit no significant difference in microvascular density. A-B: Mac2 immunohistochemistry was used to identify macrophages in WT (A) and TSP-1 null (B) pressure overloaded hearts. C: Quantitative analysis showed infiltration of the pressure-overloaded myocardium with Mac2+ cells after 3-7 days of TAC (^^p<0.01 vs. sham). The density of Mac2+ macrophages was comparable between WT and TSP-1 null animals at all timepoints examined. D: qPCR demonstrated that cardiac mRNA expression of the CC chemokine MCP-1, a potent mononuclear cell chemoattractant, was comparable between TSP-1 null and WT hearts. E-F: Vascular endothelial cells were identified in the mouse myocardium using staining for CD31. Microvascular density was compared between WT (E) and TSP-1 -/- animals (F). No statistically significant difference was observed in subendocardial (endo -G) and subepicardial (epi- H) microvascular density between WT and KO hearts after 7-28 days of TAC (pNS).
7. TSP-1 absence does not affect the angiogenic response in the pressure-overloaded myocardium
Because TSP-1 is a potent regulator of angiogenesis, we examined whether the altered response of TSP-1 -/- hearts to pressure overload may be due to changes in microvascular density. Cardiac microvessels were identified using staining with the endothelial marker CD31 (Figure 4E-F). TSP-1 null and WT animals showed no significant difference in microvascular density after 7-28 days of pressure overload (Figure 4G-H).
8. Impaired activation of TGF-β/Smad2/3 signaling in the pressure-overloaded heart
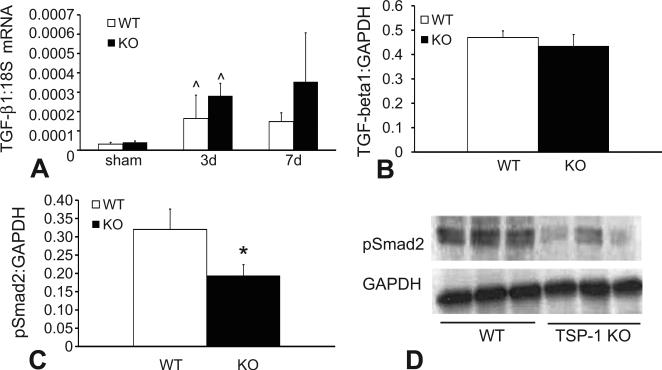
The TGF-β/Smad2/3 pathway plays an important role in the pathogenesis of cardiac fibrosis. Because TSP-1 is an essential activator of TGF-β, we examined the effects of TSP-1 gene disruption on TGF-β signaling in the pressure-overloaded heart. WT and TSP-1 null hearts exhibited comparable levels of TGF-β1 mRNA and protein expression following 3-7 days of TAC (Figure 5A-B). Despite showing comparable TGF-β1 levels, TSP-1 -/- animals had significantly reduced expression of p-Smad2 in the pressure-overloaded heart after 7 days of TAC suggesting an important role for TSP-1 in activation of pro-fibrotic TGF-β signaling (Figure 5C). In the absence of pressure overload sham WT and TSP-1 null hearts had comparable myocardial p-Smad2 expression (pNS).
Figure 5.
TSP-1 null mice exhibit impaired activation of TGF-β/Smad2 signaling. A: qPCR demonstrated that TGF-β1 mRNA was upregulated in pressure overloaded hearts after 3 days of TAC (^p<0.05 vs. sham). However, TGF-β1 mRNA (A) and protein (B) levels were comparable between TSP-1 null and WT animals. C. Activation of the Smad2/3 pathway was assessed using western blotting for p-Smad2. TSP-1 null hearts exhibited significantly lower Smad2 phosphorylation after 7 days of TAC than WT hearts (*p<0.05).
9. TSP-1 null mice show increased MMP-3 expression and enhanced MMP-9 activity in the pressure-overloaded myocardium
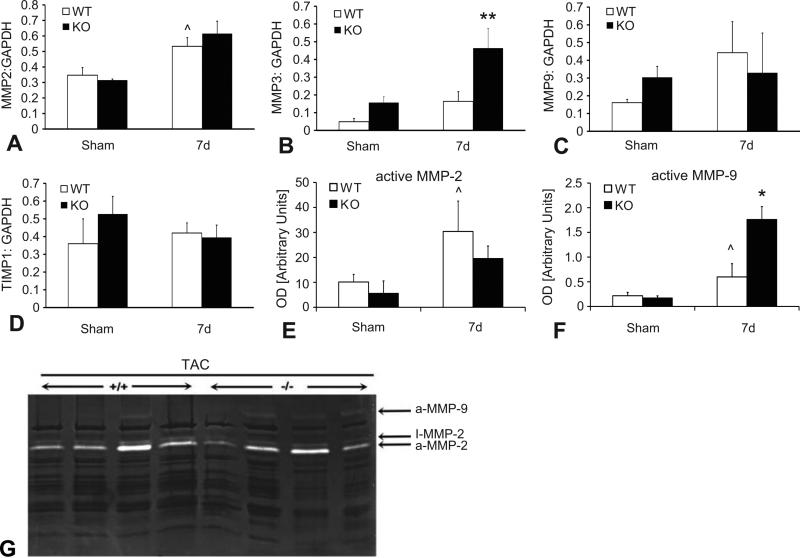
MMPs play an important role in cardiac remodeling and TSP-1 regulates MMP expression and activity both indirectly by activating TGF-β, and directly through inhibition of MMP-9 activity. Accordingly, we examined whether TSP-1 absence affected MMP expression in the pressure-overloaded heart. After 7 days of TAC, TSP-1 -/- animals exhibited markedly higher expression of MMP-3 protein in the pressure-overloaded myocardium in comparison to WT animals (Figure 6A). In contrast, MMP-2, MMP-9 and TIMP-1 protein levels were comparable between groups. In addition, zymographic assessment of MMP activity demonstrated that active MMP-9 levels were significantly higher in TSP-1 null hearts (Figure 6C, D). Active MMP-2 levels were comparable between groups (Figure 6B).
Figure 6.
TSP-1 null hearts have increased MMP-3 expression and enhanced MMP-9 activity after 7 days of pressure overload. A-D: Quantitative analysis of western blotting showed that MMP-3 protein levels were significantly higher in pressure-overloaded TSP-1 -/- hearts (**p<0.01 vs. corresponding WT); in contrast MMP-2, MMP-9 and TIMP-1 levels were comparable between groups (^p<0.05 vs. sham). B: Zymography showed significantly increased MMP-2 and MMP-9 activity in the pressure-overloaded myocardium after 7 days of TAC (^p<0.05 vs. sham). Active MMP-9 levels (aMMP-9) were significantly higher in TSP-1 hearts (C; *p<0.05 vs. WT), whereas MMP-2 activity (aMMP-2) was comparable between WT and -/- hearts (B). D. A Representative image of a zymogram comparing MMP activity in the pressure-overloaded myocardium between WT (+/+) and TSP-1 -/- mice after 7 days of TAC. Bands corresponding to active MMP-2 and active MMP-9 are identified.
10. TSP-1 null fibroblasts harvested from the pressure-overloaded heart exhibit impaired myofibroblast transdifferentiation and reduced collagen synthesis
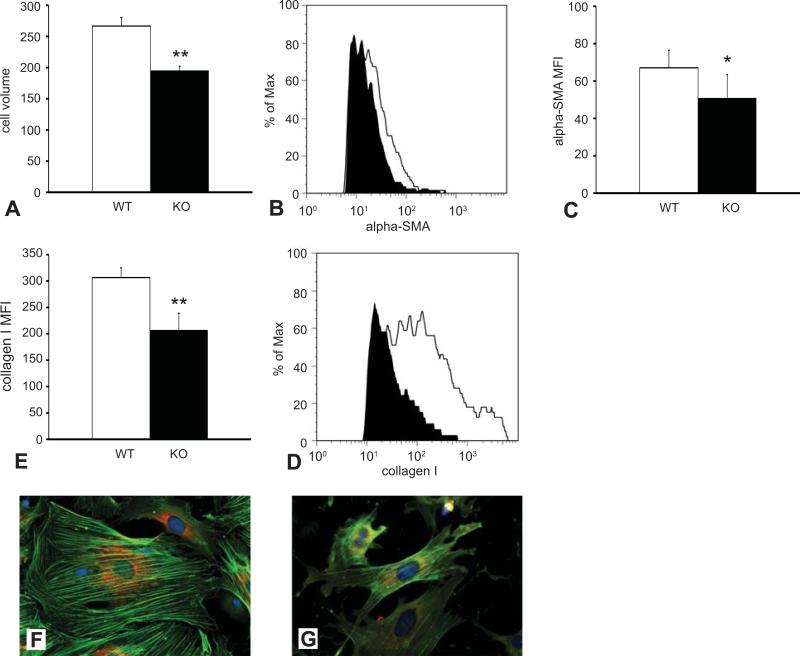
In the absence of TSP-1, cardiomyocyte injury in the pressure-overloaded heart is followed by replacement with abundant fibroblasts that produce disproportionately low amounts of collagen. We postulated that these findings may reflect functional impairment of TSP-1 null cardiac fibroblasts, In order to test this hypothesis we assessed type I collagen and α-SMA expression in myofibroblasts harvested from pressure-overloaded hearts after 7 days of TAC using two distinct methods. First, early passage myofibroblasts cultured from pressure-overloaded hearts underwent dual immunofluorescence for α-SMA and type I collagen. TSP-1 null myofibroblasts were smaller, exhibited a poorly-developed network of α-SMA+ myofilaments, and had reduced collagen type I staining in comparison to myofibroblasts harvested from WT hearts (Figure 7F-G). Second, cardiac cell suspensions were harvested from TSP-1 null and WT animals immediately after completion of a 7-day TAC protocol and were used for quantitative flow cytometric analysis (Figure 7A-E). Myofibroblasts were identified as α-SMA+/collagen I+ cells. Myofibroblasts isolated from pressure-overloaded TSP-1 null hearts had a significantly lower cell volume than cells isolated from WT hearts (Figure 7A). In addition, TSP-1 null cells exhibited significantly reduced mean fluorescent intensity for α-SMA (Figure 7B-C) and collagen I (Figure 7D-E). Thus, both immunofluorescence and flow cytometry experiments suggested that TSP-1 absence results in impaired myofibroblast transdifferentiation and activation following pressure overload.
Figure 7.
TSP-1 null myofibroblasts infiltrating the pressure overloaded heart are functionally impaired. A-D. Single cell suspensions were harvested from pressure-overloaded WT and TSP-1 null hearts after 7 days of TAC. Myofibroblasts were identified using flow cytometry as α-SMA+/collagen I+ cells. A: Myofibroblasts from TSP-1 null hearts were smaller that WT cells exhibiting a significantly lower cell volume (**p<0.01 vs. WT). B. A representative image shows the use of flow cytometry to assess α-SMA content in cardiac myofibroblasts from WT (white curve) and TSP-1 null hearts (black curve). C. Quantitative analysis shows that mean fluorescent intensity for α-SMA was significantly lower in myofibroblasts harvested from TSP-1 -/- hearts (*p<0.05 vs. WT). D. Representative image illustrating flow cytometric assessment of type I collagen content in α-SMA+/collagen I+ myofibroblasts from WT (white curve) and TSP-1 -/- (black curve) hearts. E: Quantitative analysis showed that TSP-1 KO cells had significantly lower expression of type I collagen (**p<0.01 vs. corresponding WT cells). F-G: Cardiac myofibroblasts were isolated from WT (F) and TSP-1 -/- (G) hearts after 7 days of TAC. Dual immunofluorescent staining combining labeling for α-SMA (green) and staining for collagen I (red) was performed. Confirming the findings obtained from flow cytometry of single cell suspensions (A-E), TSP-1 null myofibroblasts (G) were smaller, had a less developed network of α-SMA+ fibers and contained less collagen.
DISCUSSION
Matricellular proteins are a family of extracellular matrix proteins that do not provide structural support, but serve as modulators of cell:matrix interactions 7, transducing signals during development and following tissue injury. Increased expression of matricellular proteins is a hallmark of cardiac remodeling. Studies using genetically targeted mice have suggested that several members of the matricellular family play an important role in the pathogenesis of cardiac remodeling associated with pressure overload by modulating responses that may involve cardiomyocytes and fibroblasts. Osteopontin induction in the pressure-overloaded myocardium mediates cardiomyocyte hypertrophy through integrin-associated interactions without affecting development of fibrosis 24. SPARC signaling is essential for post-synthetic collagen processing and cross-linking, but does not affect cardiac hypertrophy; in its absence collagen deposition is reduced and myocardial diastolic stiffness is attenuated 25. Periostin contributes to the development of cardiac hypertrophy and fibrosis and activates fibroblast adhesion and gene expression 9. Members of the TSP family have been suggested to play a role in cardiac remodeling. TSP-2 expression appears to be essential for preservation of matrix integrity under conditions of stress; TSP-2 null animals exhibited a high incidence of cardiac rupture in a model of angiotensin II-mediated hypertrophy 8. TSP-1 is also upregulated in the pressure-overloaded myocardium 8, 26; however, its role in the pathogenesis of cardiac remodeling remains unknown. Our study demonstrates for the first time that TSP-1 is induced in the pressure-overloaded myocardium and may serve as a protective signal that prevents development of cardiac remodeling through effects on fibroblast function and matrix metabolism. TSP-1 absence was associated with increased early hypertrophy and accentuated cardiac injury. In response to pressure overload TSP-1 null hearts were infiltrated with abundant, but functionally-impaired fibroblasts that exhibited defective myofibroblast transdifferentiation and reduced collagen synthesis. Alterations in two distinct pathways appear to be responsible for defective fibroblast activation and increased matrix remodeling in the pressure-overloaded TSP-1 -/- heart. First, TSP-1 absence was associated with attenuated TGF-β/Smad2 signaling leading to an impairment in myofibroblast transdifferentiation and to reduced fibroblast-derived matrix-synthesis. Second, TSP-1 was involved in regulation of matrix metabolism; TSP-1 loss resulted in enhanced MMP-9 activity and accentuated MMP-3 expression in the pressure-overloaded heart.
In the cardiac interstitium extracellular matrix proteins not only form a structural framework that provides mechanical support, but also transduce signals that regulate cell survival, phenotype and function. TSP-1 limits adverse remodeling of the pressure-overloaded heart through modulatory effects on fibroblast function. Progressive cardiomyocyte loss triggers inflammatory and profibrotic pathways leading to infiltration of the pressure-overloaded myocardium with activated myofibroblasts 22. Although TSP-1 is known to exert direct anti-inflammatory actions through CD47 18, TSP-1 null and WT mice exhibited comparable levels of inflammatory signals in the pressure-overloaded myocardium (Table 2). However, TSP-1 absence significantly affected maturation and activation of myofibroblasts in the pressure-overloaded heart. Because of increased cardiomyocyte injury, TSP-1 null hearts exhibited markedly accentuated myofibroblast infiltration and more extensive areas of reparative fibrosis. However, despite the increased number of fibroblasts, TSP-1 null hearts showed no significant increase in collagen content following pressure overload (Figure 3) reflecting the functional impairment observed in TSP-1 null fibroblasts. Flow cytometry and immunofluorescence on cells harvested from the remodeling pressure-overloaded myocardium demonstrated that, in the absence of TSP-1, cardiac fibroblasts exhibited defective myofibroblast transdifferentiation and significantly reduced collagen synthesis (Figure 7). These defects were associated with a significant decrease in Smad2 phosphorylation suggesting that impaired fibroblast activation in the absence of TSP-1 may be due to defective TGF-β activation (Figure 5). TSP-1 is critically involved in TGF-β activation by binding to the LAP, inducing a conformational change in the latent complex that renders the TGF-β dimer biologically active. In addition, the LAP:TSP-1 interaction may occupy LAP molecules, preventing the generation of new inactive LAP:TGF-β small latent complexes since TSP-associated LAP does not confer latency on active TGF-β 15.
Why does impaired myofibroblast activation in TSP-1 null pressure-overloaded hearts contribute to adverse remodeling? The functional consequences of TSP-1 loss are probably due to the combination of defective myofibroblast function and accentuated matrix remodeling. In the absence of TSP-1, impaired TGF-β signaling in cardiac fibroblasts, results in decreased collagen deposition leading to formation of a matrix that provides inadequate mechanical support to the ventricle. In addition, accentuated MMP-3 expression and MMP-9 activity in TSP-1 -/- pressure overloaded hearts (Figure 6) may also contribute to adverse remodeling by promoting matrix degradation. Because MMP-3 is the most efficient MMP-9 activator identified to date 27, the increased MMP-9 activity observed in TSP-1 null pressure-overloaded hearts may be due to accentuated MMP-3 expression. Inhibitory effects of TSP-1 on MMP-9 activity have been previously suggested to play an important role in suppression of tumor growth 28. It should be noted that despite the increased matrix degradation and enhanced cardiomyocyte injury observed in TSP-1 null hearts following pressure overload, cardiac systolic function was preserved. This finding suggests that TSP-1 absence does not affect cardiomyocyte contractility, but may also reflect the compensatory effects of the increased left ventricular mass in TSP-1 -/- hearts.
PERSPECTIVES
The effects of TSP-1 in the remodeling heart are due to a combination of protective actions involving cardiac fibroblasts and the extracellular matrix. Pressure overload results in early MMP activation and matrix degradation; stimulation of a matrix-preserving fibroblast phenotype provides mechanical support to the myocardium preventing chamber dilation and adverse remodeling. TSP-1 induction in the cardiac interstitium may serve as a protective mechanism against cardiac remodeling that regulates the reparative properties of cardiac fibroblasts activating TGF-β signaling and inhibiting MMP activity, thus promoting matrix preservation..
Supplementary Material
Acknowledgments
SOURCES OF FUNDING: This work was supported by NIH grants R01 HL-76246 and R01 HL-85440 and by the Wilf Family Cardiovascular Research Institute. Dr Frangogiannis is supported by the Edmond J Safra/Republic National Bank of New York Chair in Cardiovascular Medicine.
Footnotes
DISCLOSURES: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Muraski JA, Fischer KM, Wu W, Cottage CT, Quijada P, Mason M, Din S, Gude N, Alvarez R, Jr., Rota M, Kajstura J, Wang Z, Schaefer E, Chen X, MacDonnel S, Magnuson N, Houser SR, Anversa P, Sussman MA. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci U S A. 2008;105:13889–13894. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anselmi A, Gaudino M, Baldi A, Vetrovec GW, Bussani R, Possati G, Abbate A. Role of apoptosis in pressure-overload cardiomyopathy. J Cardiovasc Med (Hagerstown) 2008;9:227–232. doi: 10.2459/JCM.0b013e328277f1d7. [DOI] [PubMed] [Google Scholar]
- 3.Weber KT, Jalil JE, Janicki JS, Pick R. Myocardial collagen remodeling in pressure overload hypertrophy. A case for interstitial heart disease. Am J Hypertens. 1989;2:931–940. doi: 10.1093/ajh/2.12.931. [DOI] [PubMed] [Google Scholar]
- 4.McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J Mol Cell Cardiol. 48:544–549. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res. 2004;64:24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 9.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka-Yoshida K. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 298:H1072–1078. doi: 10.1152/ajpheart.00255.2009. [DOI] [PubMed] [Google Scholar]
- 11.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 16.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 18.Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol. 2006;177:3534–3541. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 19.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 20.Chatila K, Ren G, Xia Y, Huebener P, Bujak M, Frangogiannis NG. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc Hematol Agents Med Chem. 2007;5:21–27. doi: 10.2174/187152507779315813. [DOI] [PubMed] [Google Scholar]
- 21.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–481. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension. 2004;44:826–831. doi: 10.1161/01.HYP.0000148458.03202.48. [DOI] [PubMed] [Google Scholar]
- 25.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–280. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SW, Chen YF. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.