Abstract
Introduction
Sublobar resection (SR) is commonly used for patients considered high-risk for lobectomy. Non-operative therapies are increasingly being reported for similar risk patients because of perceived lower morbidity. We report 30 and 90 day adverse events (AEs) from ACOSOG Z4032; a multicenter phase III study for high-risk stage I non-small cell lung cancer (NSCLC) patients.
Methods
Data from 222 evaluable patients randomized to SR (n=114) or SR with brachytherapy (SRB) (n=108) are reported. AEs were recorded using the Common Terminology Criteria for Adverse Events, Version 3.0 at 30 and 90 days post surgery. Risk factors (age, baseline DLCO%, and FEV1%, upper lobe versus lower lobe resections, performance status, surgery approach; VATS versus open and extent ; wedge versus segmentectomy) were analyzed using a multivariable logistic model for their impact on the incidence of Grade 3 (G3+) and higher AEs. Respiratory AEs were also specifically analyzed
Results
Median age, FEV1% and DLCO% were similar for the two treatment groups. There was no difference in the location of resection (upper versus lower lobe) or in the use of segmental or wedge resections. There were no differences between the groups with respect to “respiratory” G3+ (30 days: 14.9% vs. 19.4%; p=0.35; 0–90 days: 19.3% vs. 25%; p=0.31) and “any” G3+AEs (30 days: 25.4% vs. 30.6%; p=0.37; 0–90 days: 29.8% vs. 37%; p=0.25). Further analysis combined the two groups. Mortality occurred in 3 (1.4%) patients by 30 days and in 6 (2.7%) patients by 90 days. Four of the six deaths were felt to be attributable to surgery. When considered as continuous variables, FEV1% was associated with “any” grade 3 + AE at days 0–30 (p=0.03; OR=0.98), and days 0–90 (p=0.05; OR=0.98) respectively; and DLCO% was associated with “respiratory” grade 3+AE at days 0–30 (p=0.03; OR=0.97), and days 0–90 (p=0.05; OR=0.98) respectively. Segmental resection was associated with a higher incidence of any G3+ AE compared to wedge at days 0–30(40.3% versus 22.7%; OR=2.56; p<0.01) and days 0–90 (41.5% versus 29.7%; OR=1.96; p=0.04). The median FEV1% was 50% and the median DLCO% was 46%. Using these median values as potential cutpoints, only a DLCO% of less than 46% was significantly associated with an increased risk of “respiratory” and “any” grade 3+ AE for days 0–30, and 0–90.
Conclusions
In a multicenter setting, SRB was not associated with increased morbidity compared to SR alone. SR/SRB can be performed safely in high-risk patients with NSCLC with low 30 and 90 day mortality and acceptable morbidity. Segmental resection was associated with increased “any” G3+ AE, and DLCO% less than 46% was associated with “any” G3+AE as well as “respiratory” G3+ AE at both 30 and 90 days.
Introduction
Sublobar resection is usually reserved for patients with non-small cell lung cancer (NSCLC) who are considered high-risk for lobectomy. The principal reason for selective use in higher risk patients is the higher loco-regional recurrence rate after sublobar resection compared to lobectomy (1). One approach that may reduce the incidence of local recurrence is the addition of adjuvant brachytherapy (2–4). Z4032 is a prospective randomized clinical trial by the American College of Surgeons Oncology Group [ACOSOG] that compares sublobar resection with intraoperative brachytherapy [SRB] to sublobar resection alone [SR]. This study has recently completed accrual. The primary outcome of interest of the study is 2-year local control, and this endpoint will be reported when sufficient follow-up becomes available. The current report examines the incidence and severity of adverse events (AE) occurring at both 30 and 90 days following surgery from this multicenter randomized prospective study. This is of particular importance as non-operative therapies such as stereotactic body radiation therapy (SBRT) and radiofrequency ablation (RFA) are gaining increasing attention within the medical community, even for patients who are candidates for operation (4,5). The toxicity profiles of the various lung cancer therapies are important considerations when discussing treatment options with patients. We report the incidence and severity of adverse events after sublobar resection in the high-risk population selected for Z4032.
Methods
Eligible patients for this study, included patients with stage I lung cancers 3cm or less in maximum diameter [i.e. stage IA or the subset of stage IB with visceral pleural involvement] on pre-operative CT scan. Patients were defined as high-risk for lobectomy if they met at least one major criterion or two minor criteria as described in Table 1. In addition to meeting these criteria, patients had to be evaluated by am ACOSOG-approved thoracic surgeon and considered either to not be a candidate for lobectomy, or to be too high-risk for any form of pulmonary resection. Patients considered medically inoperable (but who met these criteria) were usually referred for non-operative therapies such as RFA or SBRT. We did not record the details of screened patients who met the major and minor criteria described above, who were not offered participation in this study. In order to confirm that patients did not have nodal involvement, all suspicious lymph nodes seen on PET or CT scan required biopsy by mediastinoscopy, endobronchial ultrasound, or sampling at the time of resection. Sublobar resection included wedge or segmental resection, and could be performed by video-assisted thoracic surgery (VATS) or thoracotomy. Two methods of brachytherapy were allowed (6, 7). The method used was at the discretion of the treating surgeon. In the first technique, polyglactin sutures containing 125I seeds [Oncura, Princeton, NJ] were placed parallel to and 5 mm away from the staple line on each side of the resection margin. The suture strands were fixed to the lung surface with several 3.0 silk or polyglactin sutures placed 1–2cm apart. With the second brachytherapy technique a polyglycolic mesh implant is created during the procedure. The same 125I suture strands were woven into a piece of vicryl mesh. The strands were placed at 1cm intervals. The mesh is then sutured over the staple line. The dosimetry goal of the brachytherapy was to deliver 100 Gy at a 5–7mm along the central axis of the resection margin.
Table 1.
Major and Minor Eligibility Criteria for Z4032 trial*
| Major Criteria | SR** (N=114) |
SRB** (N=108) |
|
|---|---|---|---|
| 1. | FEV1 ≤ 50% predicted | 67 (58.8%) | 49 (45.4%) |
| 2. | DLCO ≤ 50% predicted | 72 (63.2%) | 74 (68.5%) |
| Minor Criteria | |||
| 1. | Age ≥75 | 43 (37.7%) | 42 (38.9%) |
| 2. | FEV1 51–60% predicted | 18 (15.8%) | 25 (23.1%) |
| 3. | DLCO 51–60% predicted | 19 (16.7%) | 19 (17.6%) |
| 4. | Pulmonary hypertension (defined as a pulmonary artery systolic pressure greater than 40mmHg) as estimated by echocardiography or right heart catheterization | 4 (3.5%) | 1 (0.9%) |
| 5. | Poor left ventricular function (defined as an ejection fraction of 40% or less) | 9 (7.9%) | 3 (2.8%) |
| 6. | Resting or Exercise Arterial pO2 ≤ 55 mm Hg or SpO2 ≤ 88% | 5 (4.4%) | 6 (5.6%) |
| 7. | pCO2 > 45 mm Hg | 3 (2.6%) | 3 (2.8%) |
| 8. | Modified Medical Research Council (MMRC) Dyspnea Scale ≥ 3. | 31 (27.2%) | 17 (15.7%) |
Eligible patients must have met either 1 Major or 2 Minor Criteria
One patient may have multiple criteria
AE were recorded using the Common Terminology Criteria (CTC) for Adverse Events Version 3.0 (8). The CTC is a broad classification of AE with several defined categories. Within each category, AE’s are listed and accompanied by a description of severity (Grade). Grade 1 is mild, Grade 2 moderate, Grade 3 severe, Grade 4 life-threatening or disabling AE and Grade 5 is death related to the AE.
AE were analyzed at 0–30 days and then again at 0–90 days. For the purpose of this report, we limit discussion to grade 3 and higher (3+) AE. Since this group was considered high-risk primarily on the basis of lung function, two groups of AE were studied: “any AE” or “respiratory AE”, where “respiratory AE” included adult respiratory distress syndrome (ARDS), aspiration, bronchospasm, bronchostenosis, dyspnea, hypoxia, pleural effusion, pneumonitis, chest tube drainage or leak, prolonged intubation, pulmonary-other, and pneumonia as defined by the CTC.
All patients provided written informed consent before trial enrollment in accordance with applicable guidelines. At each participating site, Institutional Review Board approval was obtained in accord with an assurance filed with and approved by the United States Department of Health and Human Services.
Statistical Analysis
Chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables were used to compare the baseline patient characteristics between the SR and the SRB arms. We compared the two treatment arms for the incidence of any Grade 3 or higher AE, and any grade 3 or higher respiratory AE using a Fisher’s exact test. Risk factors for AEs (age, baseline DLCO% and FEV1% considered as continuous variables, upper lobe versus lower lobe resection, and performance status) were analyzed using a multivariable logistic model for any grade 3+ and grade 3+ respiratory AEs at 0–30 days and 0–90 days. In addition to these factors, surgery extent (wedge vs. segmentectomy) and type (thoracotomy vs. VATS) were also considered for any grade 3+ AEs outcomes at 0–30 and 0–90 days. Odds ratios (OR) and 95% CIs are reported, where the OR estimates for a continuous covariate correspond to a one unit increase. Optimal cutpoints were therefore explored to define the high-risk versus low-risk categories for the baseline DLCO% and FEV1% using data dependent methods (mean, or median) and outcome based approaches (graphical diagnostic plots; and the minimum p-value approach)(9). Subsequently, univariable logistic regression models using the categorized DLCO% and FEV1% were explored. In addition, the entry criteria used to define high risk subset were explored further by first analyzing patients eligible by at least 2 minor criteria and secondly, by including patients eligible with at least FEV1% and DLCO% as minor criteria as risk factors in univariable logistic regression models for all the endpoints considered. P-values ≤ 0.05 were considered statistically significant.
Results
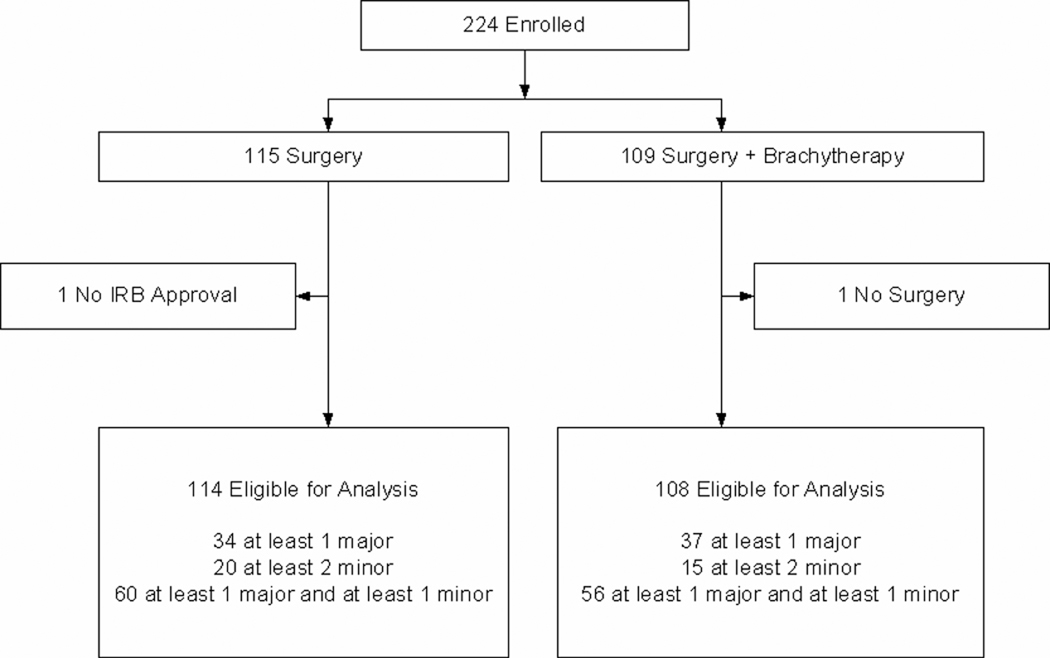
Z4032 met its target accrual and was permanently closed to enrollment on January 22, 2010. A total of 224 patients were registered. One patient from the SR arm had the intervention at a non-IRB approved hospital and was deemed not evaluable. One patient randomized to the SRB group did not have surgery and was also not evaluable. A total of 222 patients (SR =114 and SRB =108 patients) were eligible for analysis (see figure 1).
Figure 1.
Patient Consort Diagram
Table 2 depicts the patient characteristics for the two groups. There were no significant differences between the two intervention groups in baseline characteristics, except ASA class, for which a higher percentage of SR patients were classified as ASA III/IV. The median age, FEV1% and DLCO% were similar for the two groups.
Table 2.
Baseline Patient Characteristics
| SR (N=114) |
SRB (N=108) |
p value* | |
|---|---|---|---|
| Age | 0.37** | ||
| Median (Range) | 70 (49–85) | 72 (50–87) | |
| Sex | 0.72 | ||
| Female | 65 (57%) | 59 (55%) | |
| Male | 49 (43%) | 49 (45%) | |
| Ethnicity | 0.16 | ||
| Hispanic or Latino | 0 (0%) | 2 (1.9%) | |
| Not Hispanic or Latino | 104 (91%) | 91 (84%) | |
| Unknown | 10 (9%) | 15 (14%) | |
| Race | 0.42 | ||
| White | 107 (93.9%) | 103 (95.4%) | |
| Black or African American | 7 (6.1%) | 4 (3.7%) | |
| Unknown | 0 (0%) | 1 (0.9%) | |
| Baseline Performance Status | 0.56 | ||
| 0 | 20 (17.5%) | 25 (23.1%) | |
| 1 | 66 (57.9%) | 60 (55.6%) | |
| 2 | 28 (24.6%) | 23 (21.3%) | |
| T Stage | 0.12 | ||
| T1 | 114 (100%) | 104 (96.3%) | |
| T2 | 0 (0%) | 3 (2.8%) | |
| T3 | 0 (0%) | 1 (0.9%) | |
| M Stage | NA | ||
| M0 | 114 (100%) | 108 (100%) | |
| N Stage | 0.37 | ||
| N0 | 113 (99.1%) | 107 (99.1%) | |
| N1 | 0 (0%) | 1 (0.9%) | |
| N2 | 1 (0.9%) | 0 (0%) | |
| Surgery in Upper Lobe | 0.07 | ||
| No | 38 (33.3%) | 49 (45.4%) | |
| Yes | 76 (66.7%) | 59 (54.6%) | |
| Surgery Type | |||
| VATS | 79 (69.3%) | 65 (60.2%) | 0.16 |
| Thoracotomy | 35 (30.7%) | 43 (39.8%) | |
| Surgery extent | 0.29 | ||
| Segmentectomy | 38 (33.3%) | 29 (26.9%) | |
| Wedge Resection | 76 (66.7%) | 79 (73.1%) | |
| ASA class (III/IV vs. I/II) on surgery day*** | 0.02 | ||
| I/II | 10 (8.8%) | 21 (19.8%) | |
| III/IV | 104 (91.2%) | 85 (80.2%) | |
| Baseline DLCO% | 0.36** | ||
| N | 111 | 106 | |
| Median (Range) | 47 (18–97) | 44 (8–137) | |
| Baseline FEV1% | 0.25** | ||
| N | 114 | 107 | |
| Median (Range) | 48 (22–117) | 53 (25–110) |
SR= Sublobar Resection; SRB=Sublobar Resection with Intraoperative Brachytherapy
Chi-square test;
Wilcoxon rank sum test;
Excludes 2 SRB with missing data
30 Day and 90 Day Mortality: SR versus SRB
There were no significant differences between the SR and SRB groups with respect to 30-day and 90-day mortality (grade 5 AE). In total 3 (1.4%) deaths occurred within 30 days. One (0.9%) was from the SR group and was due to a cardiopulmonary arrest. In the SRB group, two deaths (1.9%) occurred, one in a patient who suffered from a cerebrovascular accident, and the second from a pulmonary embolus. By 90 days an additional 3 deaths (total 6; 2.7%) had occurred. Two (1.8%) were from the SR group and included cancer progression in the one patient found to have N2 disease on pathological staging, and a fatal cardiac event in the second patient. One death (0.9%) occurred in the SRB group from sepsis. Four of the six deaths occurring by 90 days were felt to be attributable to the surgery performed.
30-day and 90 day AE; SR versus SRB
Median length of stay was 5 days for each group (p=0.33). At 0–30 days, grade 3+ AE occurred in 29 (25.4%) SR compared to 33 (30.6%) SRB patients (p=0.37). Grade 4+ AE were also similar, occurring in 8 (7%) SR compared to 8 (7.4%) SRB patients (p=0.90). In terms of respiratory AE, grade 3+ AE and grade 4+ AE occurred in 17 (14.9%) and 21 (19.4%); and 4 (3.5%) and 4 (3.7%) in SR and SRB arms, respectively (p=0.35; p=0.93). The most common grade 3+ AE (defined as occurring in 5% or more patients within each arm) were 9 (4.1%) hemorrhage (3 in SR 6 in SRB); 19 (8.6%) dyspnea (11 in SR. 8 in SRB) and 14 (6.3%) hypoxia (5 in SR and 9 in SRB).
At 0–90 days, grade 3+ AE occurred in 34 (29.8%) SR and 40(37%) SRB patients (p=0.25). Grade 4+ events were also similar (p=0.71) occurring in 10 (8.8%) of SR and 8 (7.4%) of the SRB patients. In terms of respiratory AE, grade 3+ AE and grade 4+ AE occurred in 22 (19.3%) and 27 (25%); and 6 (5.3%) and 4 (3.7%) in SR and SRB arms, respectively (p=0.31; p=0.58). The most common grade 3+ AE (defined as occurring in 5% or more patients within each arm) were the same as those been reported for day 0–30 days, with an exception of increasing reports of dyspnea occurring in 29 (13.1%); 14 in SR and 15 in SRB.
A total of 12 patients (9 SR, 3 SRB) had poor left ventricular function at study entry. There was no association between ASA class and poor left ventricular function (Fisher’s exact p=1.0). Logistic regression analysis considering ASA class and treatment arm as predictors of grade 3 or higher cardiovascular AEs showed no significant effect of ASA class (days 0–30: p=0.92; OR 0.92; 95% CI, 0.19 –4.45; days 0–90: p=0.94; OR 1.06; 95% CI, 0.22–5.02).
30-Day and 90-day AE; All patients
The two groups were therefore combined for further analyses. Patients eligible based on ‘any 2 minor criteria’ and ‘at least FEV1% and DLCO% as minor criteria were not significant predictors of AE outcomes. In a multi-variable model of FEV1% (considered as a continuous variable) without DLCO%, FEV1% was associated with any grade 3+ AE at days 0–30 (p=0.04; OR 0.98; 95% CI, 0.97–1.00); and also with respiratory grade 3+ AE at days 0–30 (p=0.04; OR 0.98; 95% CI, 0.96–1.00) and days 0–90 (p=0.03; OR 0.98; 95% CI, 0.96–1.00). Similarly, when DLCO% (as a continuous variable) was considered in a multi-variable model without FEV1%; DLCO% was associated with respiratory grade 3+ AE at days 0–30 (p=0.02; OR 0.97; 95% CI, 0.95–1.00) and days 0–90 (p=0.04; OR 0.98; 95% CI, 0.96–1.00).
Table 3 shows the multivariable model results when FEV1% and DLCO% were considered together as continuous variables. For days 0–30, and 0–90 only FEV1% was significantly associated with any grade 3 + AE.. DLCO% was significantly associated with respiratory grade 3+AE for days 0–30 and 0–90..
Table 3.
Results from multi-variable logistic regression models using FEV1% and DLCO% as continuous variables.
| Model Outcome | Predictors | Odds Ratio (95% Confidence Limits) |
P- value |
|---|---|---|---|
| Any Grade 3+ AE Day 0 to 30 | Upper Lobe: Yes vs. No | 0.68 (0.35, 1.30) | 0.24 |
| Age (continuous) | 1.03 (0.99, 1.07) | 0.18 | |
| Baseline DLCO% (continuous) | 0.98 (0.96, 1.00) | 0.09 | |
| Baseline FEV1% (continuous) | 0.98 (0.96, 1.00) | 0.03 | |
| PS: 2 vs. 0, 1 | 0.90 (0.42, 1.92) | 0.78 | |
| Surgery Type: Thoracotomy vs. VATS | 1.03 (0.53, 2.00) | 0.94 | |
| Surgery Extent: Wedge vs. Segmentectomy | 0.39 (0.20, 0.75) | <.01 | |
| Any Grade 3+ AE Day 0 to 90 | Upper Lobe: Yes vs. No | 0.75 (0.41, 1.39) | 0.37 |
| Age (continuous) | 1.01 (0.97, 1.04) | 0.72 | |
| Baseline DLCO% (continuous) | 0.99 (0.97, 1.01) | 0.25 | |
| Baseline FEV1% (continuous) | 0.98 (0.97, 1.00) | 0.05 | |
| PS: 2 vs. 0, 1 | 0.98 (0.48, 1.99) | 0.96 | |
| Surgery Type: Thoracotomy vs. VATS | 0.87 (0.46, 1.64) | 0.67 | |
| Surgery Extent: Wedge vs. Segmentectomy | 0.51 (0.27, 0.96) | 0.04 | |
| Grade 3+ Respiratory AE Day 0 to 30* | Upper Lobe: Yes vs. No | 0.58 (0.27, 1.23) | 0.16 |
| Age (continuous) | 1.01 (0.97, 1.06) | 0.52 | |
| Baseline DLCO% (continuous) | 0.97 (0.95, 1.00) | 0.03 | |
| Baseline FEV1% (continuous) | 0.98 (0.96, 1.00) | 0.07 | |
| PS: 2 vs. 0, 1 | 1.01 (0.42, 2.47) | 0.98 | |
| Grade 3+ Respiratory AE Day 0 to 90* | Upper Lobe: Yes vs. No | 0.59 (0.29, 1.18) | 0.14 |
| Age (continuous) | 1.00 (0.96, 1.04) | 0.97 | |
| Baseline DLCO% (continuous) | 0.98 (0.96, 1.00) | 0.05 | |
| Baseline FEV1% (continuous) | 0.98 (0.96, 1.00) | 0.06 | |
| PS: 2 vs. 0, 1 | 1.15 (0.52, 2.53) | 0.74 |
Surgery type/extent not considered due to limited number of events
In the case of any grade 3+ AE, in addition to the risk factors already considered; surgery extent and type were also explored. Surgery extent was significantly associated with any grade 3+ AE, with events occurring in patients undergoing segmentectomy compared to wedge resection at days 0–30 (segment=40.3%, wedge =22.7%) and days 0–90(segment=41.8%, wedge=29.7%)
The median FEV1% for all patients was 50% (compared to 55% for any grade 3+ and 53% for any respiratory grade 3+ using the outcome oriented approaches) and the median DLCO% for all patients was 46% (compared to 42% and 44% for any grade 3+ AE and grade 3+ respiratory AE). Thus, we report the results using the median values as potential cutpoints for defining high and low risk categories based on baseline FEV1% and DLCO% (table 4). An FEV1% of less than 50% was not associated with an increased risk of grade 3+ respiratory or any AE. However a DLCO% of less than 46% was significantly associated with an increased risk of “respiratory “and “any” grade 3+ AE for days 0–30, and 0–90 (see table 4). Of note, the results were similar when utilizing the other potential cutpoints for DLCO% and FEV1%.
Table 4.
Results from the logistic regression models using median DLCO% and FEV1% as Cutpoints
| Model Outcome | Predictors | Incidence of AE (%) |
Odds Ratio (95% Confidence Limits) |
P- value |
|---|---|---|---|---|
| Grade 3+ Respiratory AE Day 0 to 30 | DLCO%: <46 vs. >=46 | 11.3% vs 5.4% | 2.41 (1.14, 5.09) | 0.02 |
| Grade 3+ Respiratory AE Day 0 to 90 | DLCO%: <46 vs. >=46 | 14% vs 7.2% | 2.34 (1.19, 4.59) | 0.01 |
| Any Grade 3+ AE Day 0 to 30 | DLCO%: <46 vs. >=46 | 17.1% vs 10.4% | 2.01 (1.09, 3.68) | 0.02 |
| Any Grade 3+ AE Day 0 to 90 | DLCO%: <46 vs. >=46 | 19.4% vs 13.1% | 1.82 (1.03, 3.24) | 0.04 |
| Grade 3+ Respiratory AE Day 0 to 30 | FEV1%: <50 vs. >=50 | 9.5% vs 7.7% | 1.29 (0.64, 2.61) | 0.48 |
| Grade 3+ Respiratory AE Day 0 to 90 | FEV1%: <50 vs. >=50 | 12.6% vs 9.5% | 1.46 (0.77, 2.78) | 0.24 |
| Any Grade 3+ AE Day 0 to 30 | FEV1%: <50 vs. >=50 | 15.3% vs 12.6% | 1.31 (0.73, 2.36) | 0.37 |
| Any Grade 3+ AE Day 0 to 90 | FEV1%: <50 vs. >=50 | 18.5% vs 14.9% | 1.40 (0.80, 2.46) | 0.24 |
Discussion
Pulmonary resection is generally considered the gold standard for patients with early stage NSCLC. For patients with impaired pulmonary function, the risks of surgical resection are a significant concern. With the increasing availability of non-operative approaches it is imperative that physicians have a good understanding of the relative risks of the various therapies available. The CTC classification is a useful tool that can be used to compare procedure therapy related complications. This is the standard approach used in most oncological studies, but rarely used in surgical studies. The availability of a grading system is also helpful, as many complications commonly reported in surgical series, could be considered minor and less than the usual grade 3+ reported. One example is atrial fibrillation which was reported as 9.3% in one series of sublobar resection (10). By CTC criteria, atrial fibrillation would be considered grade 3 if this was incompletely controlled by medical therapy or required a device. An example of another complication, which may be over or under-reported, is atelectasis. Grade 2 atelectasis would include the use of bronchoscopy, suctioning or chest physiotherapy for control. Grade-3 atelectasis would require operative intervention such as a stent or laser. In the STS General Thoracic Database, “atelectasis requiring bronchoscopy” is classified as a complication without any consideration given to severity.
Our series demonstrated an overall incidence of grade 3+ complications of 27.9% during the 0–30 day period. Specifically, grade 3 complications occurred in 19.4%, grade 4 in 5.9% and grade 5 (death) in 1.4%. These results provide a valuable benchmark for current day morbidity and mortality after sublobar resection in these high-risk patients. In contrast to these results, the recent Radiation Therapy and Oncology Group Phase 2 study of stereotactic body radiation therapy reported a 12.7% incidence of grade 3 AE, a 3.6% incidence of grade 4 AE and no grade 5 AE (11). Not surprisingly morbidity was less with this non-operative intervention, although oncological comparisons still need to be determined. A key factor to also consider is that the patient groups in the two studies may not be directly comparable. The best comparison between these two therapies would be a randomized study taking similar risk patients, and using similar definitions of outcome for the entire patient cohort. In fact this is being done by the American College of Surgeons Oncology Group who are partnering with the Radiation Therapy and Oncology Group in a phase III study that will compare SBRT to SR for high-risk patients, using the same eligibility criteria as Z4032. This Phase III study (Z4099) will be critical to help determine appropriate patient selection for operative or non-operative therapies.
This current report provides information on 90-day outcomes for our patients. Most surgical series and even the Society of Thoracic Surgeons Database only report outcomes to 30 days. This can potentially lead to under-reporting of poor outcomes in patients who have complications resulting in transfer to long-term care facilities and who sustain grade 5AE outside of the hospital. Our 90 day outcomes demonstrated a 2.7% mortality at 90 days, which is acceptable considering the greater than average risk of these surgical patients.
Bryant et al recently published 30 and 90-day outcomes from a large retrospective single institution series (12). Their series included 1,845 patients with variable operative risk, and included patients treated with both lobar and sublobar resections Discharge mortality was 3.1%. An additional 1% died after discharge, but within 30 days and an additional 2.5% died within 90 days. In patients who died before 30 days their most common cause of mortality was a pulmonary event with risk factors being older age, occurrence of a post-operative event, greater hospital length of stay and pneumonectomy. In patients who died between 30–90 days the most common cause of mortality was from natural causes, which included cancer progression.
ACOSOG previously reported morbidity and mortality in large randomized study comparing lymph node resection to lymph node dissection (13). In that study involving 1023 standard risk operable patients, toxicity grade was not reported. Complications occurred in 38% of patients and operative mortality was 1.37%. The most common complication was atrial arrythmias (14%). Respiratory complications occurred in 7% of the patients in that large series.
We are unable to determine risk factors for 90-day mortality, since only a few grade 5 events occurred in our series. However, we were able to evaluate the risk factors for grade 3+AE, both at 30 and at 90 days. Segmentectomy is generally considered to be preferable to wedge resection as a superior oncological procedure (14). Segmental resections however are more complex and in our study this is reflected in the higher incidence of any G3+AE at days 0–30 as well as 0–90.
While FEV1% was a significant predictor when considered as a continuous variable for any grade 3+ AE both at 30 and 90 days, with higher values being protective, it was not significant when considered as a categorical variable. A DLCO% <46% was predictive of any grade 3+ AE as well as respiratory grade 3+AE at both 30 and 90 days, suggesting that this might be a group that may have better outcomes with non-operative therapies. Clearly, the 95% CI for these OR estimates include 1.00, indicating that these results are suggestive but not definitive. This will be investigated further in Z4099.
DLCO has been demonstrated in other studies, to be an independent predictor of mortality even with otherwise normal spirometry. Ferguson et al reported results from an institutional database with 1046 patients (15). Within this series there were 558 patients without COPD. Postoperative predicted DLCO was the single strongest predictor of pulmonary morbidity and operative mortality in patients with and without COPD in their study.
Conclusions
In conclusion, this randomized prospective study demonstrates that there is no increase in morbidity or mortality with the addition of intraoperative brachytherapy to sublobar resection. Additionally, in a cohort of high-risk patients, sublobar resection (with or without brachytherapy) can be undertaken with low mortality and acceptable morbidity. Segmental resection was associated with higher “any” grade 3 or higher adverse events compared to wedge resection. Low pre-treatment DLCO% (< 46) was an adverse predictor of respiratory as well as “any” grade 3 and higher adverse events.
ACKNOWLEDGMENTS
We thank the ACOSOG staff, in particular the leadership of Heidi Nelson and David Ota for assistance in the development of this manuscript. We also thank all of the investigators and their site research teams. Finally, we wish to thank the brave patients with non-small cell lung cancer and their caregivers who participated in this study.
Supported by NCI U10 grant # CA076001, and by an additional Grant from Oncura, Princeton, NJ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ginsberg RJ, Rubenstein LV. Randomized trial of lobectomy versus limited resection for T1NO Non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 2.Fernando HC, Santos RS, Benfield JR, Grannis FW, Keenan RJ, Luketich JD, Close JM, Landreneau RA. Lobar and sublobar resection with and without brachytherapy for small stage Ia non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;129:261–267. doi: 10.1016/j.jtcvs.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Voynow G, Heron DE, Lin CJ, et al. Intraopeative (125)I vicryl mesh brachytherapy after sublobar resection for high-risk stage I non-small cell lung cancer. Brachytherapy. 2005;4:278–285. doi: 10.1016/j.brachy.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman RD, Park C, Kavanagh BD. The North American experience with stereotactic body radiation therapy in non-small cell lung cancer. J Thorac Oncol. 2007;2 Suppl 3:S101–S112. doi: 10.1097/JTO.0b013e318074e4fa. [DOI] [PubMed] [Google Scholar]
- 5.Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129:738–745. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]
- 6.Lee W, Daly BDT, DiPetrillo TA, et al. Limited resection for non-small cell lung cancer: Observed local control with implantation of I-125 brachytherapy seeds. Ann Thorac Surg. 2003;75:237–243. doi: 10.1016/s0003-4975(02)04098-5. [DOI] [PubMed] [Google Scholar]
- 7.d'Amato TA, Galloway M, Szydlowski G, et al. Intraoperative brachytherapy following thoracoscopic wedge resection of stage I lung cancer. Chest. 1998;114:1112–1115. doi: 10.1378/chest.114.4.1112. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. 2003. Jul, Common Terminology Criteria for Adverse Events (CTCAE) Version 3. [Google Scholar]
- 9.Williams BJ, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Technical Report. Division of Biostatistics, Mayo Clinic; 2006. Jun, Finding Optimal Cutpoints for Continuous Covariates with Binary and Time-to-event Outcomes; p. 79. [Google Scholar]
- 10.Shuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–933. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman R, Paulus R, Galvin J, et al. Sterotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant AS, Rudemiller K, Cerfolio RJ. The 30- Versus 90-Day Operative Mortality after Pulmonary Resection. Ann Thorac Surg. 2010;89:1717–1723. doi: 10.1016/j.athoracsur.2010.01.069. [DOI] [PubMed] [Google Scholar]
- 13.Allen MS, Darling GE, Pechet TTV, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: Initial results of the randomized prospective ACOSOG Z0030 Trial. AnnThorac Surg. 2006;81:1013–1020. doi: 10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 14.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14(8):2400–2405. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson MK, Vigneswaren WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85(4):1158–1164. doi: 10.1016/j.athoracsur.2007.12.071. [DOI] [PubMed] [Google Scholar]