Abstract
Transcranial magnetic stimulation (TMS) of the prefrontal cortex can cause changes in acute pain perception. Several weeks of daily left prefrontal TMS has been shown to treat depression. We recruited 20 patients with fibromyalgia, defined by American College of Rheumatology criteria, and randomized them to receive 4000 pulses at 10Hz TMS (n=10), or sham TMS (n=10) treatment for 10 sessions over 2 weeks along with their standard medications, which were fixed and stable for at least 4 weeks before starting sessions. Subjects recorded daily pain, mood, and activity. Blinded raters assessed pain, mood, functional status, and tender points weekly with the Brief Pain Inventory, Hamilton Depression Rating Scale, and Fibromyalgia Impact Questionnaire. No statistically significant differences between groups were observed. Patients who received active TMS had a mean 29% (statistically significant) reduction in pain symptoms in comparison to their baseline pain. Sham TMS participants had a 4% nonsignificant change in daily pain from their baseline pain. At 2 weeks after treatment, there was a significant improvement in depression symptoms in the active group compared to baseline. Pain reduction preceded antidepressant effects. TMS was well tolerated, with few side effects. Further studies that address study limitations are needed to determine whether daily prefrontal TMS may be an effective, durable, and clinically useful treatment for fibromyalgia symptoms.
Introduction
Fibromyalgia is a chronic pain syndrome characterized by pain above and below the waist bilaterally, axial skeletal pain, and 11 of 18 discrete tender points [49]. It affects 1-3% of the general population, and has a high comorbidity with depression [48]. The pathophysiology may involve sensitization of central pain processing [32; 50], dysfunctional pain inhibition [26] and abnormalities in cortical excitability [33]. There may be decreased rostral anterior cingulate activity in response to pain provocation, indicating an abnormality in the descending pain regulatory circuitry [26]. Increased insula activity, related to evoked pain processing, and increased connectivity with subgenual anterior cingulate cortex and DLPFC might represent other mechanisms leading to exaggerated pain perception [34]. Fibromyalgia may involve abnormally low corticospinal excitability that additionally correlates with depressive symptomatology [33]. TMS may be a reasonable method to modulate nociceptive circuitry in fibromyalgia.
Transcranial magnetic stimulation (TMS) is a brain intervention that modulates activity in discrete cortical regions and associated neural circuits through the noninvasive induction of intracerebral currents over several sessions of stimulation. Several studies support the notion that regular, high-frequency (5–30 Hz) stimulation increases cortical excitability (long term potentiation-like effect ((LTP)) whereas low-frequency (0.3–1 Hz) stimulation decreases cortical excitability (long term depression-like effect ((LTD)) [17; 24]. Thus TMS over motor cortex at high frequencies increases motor evoked potentials (MEP), a measure of cortical excitability, and low frequency TMS decreases MEP. With respect to mood, it is hypothesized that chronic repetitive stimulation of the prefrontal cortex initiates a cascade of events in the prefrontal cortex and in connected limbic regions [23]. Prefrontal TMS sends information to important mood-regulating regions including the cingulate gyrus, orbitofrontal cortex, insula and hippocampus, and may induce dopamine release in the caudate nucleus [23; 36; 44].
Fibromyalgia is associated with deficits in intracortical modulation [33]. Speculatively, repetitive transcranial magnetic stimulation (rTMS) may reduce fibromyalgia pain via enhancing intracortical modulation. Regardless of mechanisms, optimal TMS parameters and locations for fibromyalgia are not clear. Sampson, et al. [41] using low frequency rTMS over the right dorsal lateral prefrontal cortex (RDLPFC) showed a reduction in pain in 4 patients with fibromyalgia. This was a secondary analysis from a larger study, thus limiting conclusions. In a replication study by Carretero, et al. [15], the results were negative, however subjects were given 400 fewer pulses per session and thus may have received subtherapeutic dosing [15; 43]. Passard, et al. [35] studied high frequency rTMS in motor cortex and found a reduction in fibromyalgia pain that remained significant for two weeks.
To our knowledge there are no studies of high frequency rTMS over the left DLPFC for fibromyalgia, despite growing evidence that high frequency rTMS over the left DLPFC is able to acutely treat depression [29; 42; 22]. rTMS may modify overlapping mood and fibromyalgia pain regulation circuitry, particularly with stimulation of left DLPFC. We hypothesized that patients with fibromyalgia might experience a clinically significant reduction in daily pain if we were to administer rTMS in a manner similar to rTMS antidepressant protocols.
Methods
Subjects
The Medical University of South Carolina Institutional Review Board approved this study and all patients provided written informed consent before inclusion in the study. Twenty subjects, naive to TMS, met American College of Rheumatology criteria for fibromyalgia enrolled and completed the study. Patients were excluded if they were taking medications known to increase the risk of TMS-induced seizures (e.g., theophylline, Ritalin, high dose thyroid supplementation), if they had medication changes within the 4 weeks of starting the trial or during the trial, or if they had pacemakers, epilepsy, recent head trauma, stroke, bipolar disorder or schizophrenia. Subjects could enroll with or without a history of major depressive disorder, but the depression could not be the main reason for their functional impairment or study enrollment. Rather subjects were recruited solely for fibromyalgia pain. Subjects were screened with clinical exam, Structured Clinical Interview for DSM-IV [21], and the TMS Adult Safety Screen [27]. Subjects were recruited through the Medical University of South Carolina (MUSC) Rheumatology clinics and local newspaper. All data collection was completed in the MUSC Brain Stimulation Laboratory.
Clinical assessment
Questionnaires for assessing fibromyalgia pain, fatigue, and depression were completed at baseline, day 5 and day 10 of treatment vs. sham, and once weekly for 2 weeks after treatment or sham, over the course of one month. The Hamilton Depression Rating Scale (HDRS) [25], Brief Pain Inventory (BPI) [19], and Fibromyalgia Impact Questionnaire (FIQ) [14] were used. Subjects completed a 9-item daily pain diary at bedtime to track pain, mood, activity, and sleep symptoms 1 week before TMS intervention as a baseline pain report and daily through the four weeks of the trial. The daily pain rating was an a priori primary outcome variable. The subjects were asked to record at the end of the day (at bedtime if possible) symptoms on a numeric rating scale (0=no pain at all to 10=worst pain imaginable) and were told they could rate in non-integer increments. Subjects specifically were asked to report average pain rating, pain at its worst, pain at its least, activity level, depression, number of times pain medications were taken, number of hours of sleep (night before), and how unpleasant pain was experienced.
Subjects also had a laboratory pain assessment, serum measurement of cytokines, and a central visual analog scale pain and mood rating, the results of which will be presented separately.
Transcranial magnetic stimulation procedure and design
Resting motor threshold (rMT) was determined using a NeoPulse Neotonus® Model 3600 (with a solid focal coil) TMS machine by starting with 80% of the machine output and 1 Hz stimulus frequency. The coil was positioned over the area of the skull roughly corresponding to the motor cortex and then systematically moved and adjusted until each pulse results in isolated movement of the right thumb at rest (Abductor Pollicis Brevis; APB muscle). The machine output was adjusted to the lowest intensity that reliably produces thumb movement using PEST [11]. As the left prefrontal cortex was the cortical target, a mark was made 6 cm anterior to the motor cortex target. This may be reasonably accurate with respect to locating Brodman’s Area 9, given that standard “5 cm rule” compared with image-guided TMS coil positioning for DLPFC can be approximately 1 or 2 cm posterior to target location at least 30% of the time [1; 22]. During active and sham stimulation, the TMS coil was aligned in a parasaggital orientation, 6 cm from the area that produced right APB muscle movement for rMT testing. Patients were randomly assigned (random generator software developed by Dr. Borckardt in the Brain Stimulation Laboratory using Real Studio 5 on the Macintosh Platform) to receive 10-sessions (5 per week for 2 weeks) of active or sham rTMS. Subjects were randomized before baseline measures were collected. A co-investigator, not directly involved in ratings or treatment, released treatment condition to the TMS operator. Thus TMS operators were not blind to treatment condition, however they were not involved in ratings. A masked continuous rater (CR) assessed patients at baseline, the end of each treatment week, and the two follow-up weeks. Importantly, the CR did not administer the TMS, minimizing chances of unmasking due to events during the TMS treatment session. The length of treatment and the number of pulses on the head was the same for all subjects; whether they receive active or sham. The same stimulation frequency was used for all active subjects (chosen as a priori stimulation based on studies showing antidepressant and antinociceptive effects): 10 Hertz - Pulse train duration (on time) 5 seconds, Power (intensity) level 120% of resting motor threshold, Inter-train interval (off time) 10 seconds (15 second cycle time). Additionally, stimulation-train duration and inter-stimulus intervals were determined such that they are in compliance with current published rTMS safety guidelines [39].
Both Active and sham groups received the same treatment sessions 5x per week
80 trains × 15 sec = 4000 pulses per session, 5 × per week= 20,000 pulses per week, × 2 weeks = 40,000 pulses. Time - 1200 sec = 20 minutes/ session, all days.
TMS Sham Design
This study began before the OPT-TMS trial was fully functional and the sham system employed here was less developed than the OPT-TMS sham system. A specially designed sham TMS coil was used for all sham conditions that produces auditory signals identical to active coils but is shielded so that actual stimulation does not occur, however subjects do experience sensory stimulation that is difficult to distinguish from real TMS [12]. A portable electrical stimulus generator (Epix VT; Empi, St. Paul, MN; USA) powered by a 9-volt battery was used to delivery a constant stimulus (150 pulses per second) to a custom developed switch-box that blocked the continuous electrical stimulus from reaching the participant. Two flat Thymapad ® Stimulus Electrodes (Somatics, LLC; Lake Bluff, IL, USA) were attached from the switch box to the subject’s forehead directly underneath the TMS coil. A Bayonet Neill-Concelman (BNC) cable connected the TMS machine to the switch-box and every time the TMS machine delivered a pulse, a Transistor–transistor logic (TTL) signal was sent via the BNC cable to the switch-box. Upon receiving the TTL pulse, the switch box opened a gate for ~250 μs, allowing the electrical stimulus through to the subject’s scalp. Thus, participants experienced a brief (~250 μs) electrical pulse every time the sham TMS coil clicked. The intensity of the stimulus was adjustable at the electrical generator (1 to 60 mA) and the time that the gate was let open after each TTL trigger was adjustable on the switch-box as well.
Titrating the Sham System
Subjects were administered 1-second-trains of real TMS over the prefrontal cortex (10 Hz) at 80%, 100%, and 120% of rMT (randomly ordered) and they rated the painfulness of each sensation using a numeric rating scale (0=no pain at all to 10=worst pain imaginable). These ratings were recorded on the clinical research form for future reference. Next, the sham TMS coil was placed over the subject’s prefrontal cortex and the sham system was set to deliver electrical stimuli starting at 1mA (in sync with the audible TMS pulses at 10Hz) in trains lasting 1-second. Subjects were asked to rate the painfulness of each 1-second train using the same numeric rating scale. The intensity of the electrical pulses were adjusted and a PEST algorithm was used to match the subjective pain rating of the electrical stimulation to the rating of the real TMS at 100%. A minimum of 30 seconds elapsed between all of the 1-second pulse trains. The entire sham titration procedure took approximately 5 minutes to complete.
Sham assessment
Each subject was asked if they could determine whether they had received sham or real TMS and why they believed they received one condition versus the other. This was assessed at the end of every session for week 1 and the last session of weeks 2,3, and 4.
Data was stored electronically with the assistance of MUSC South Carolina Clinical and Translational Research Institute (formerly the General Clinical Research Center). Data stored on paper was located in a locked room in the Brain Stimulation Laboratory.
Statistical Analysis
To lower Type II errors while maximizing feasibility for the study with reasonable sample size for the pilot trial [8], we employed 10 subjects in the treatment arm and 10 subjects in the control arm (per personal consultation with Paul Nietert and Barbara Tilley). For the primary outcome measure (daily pain-on-average ratings), hierarchical linear modeling was used (proc mixed in SAS; Singer, 1999). Participants’ individual intercepts were entered into the model as random effects at level-1 and the model covariance structure was set to lag-1 autoregressive [AR(1)] to appropriately handle serial dependence associated with this time-series data. For evaluation of FIQ scores, mean number of tender points, BPI functional impairment scores and HDRS scores, 3X2 mixed models were run (SPSS 17) examining the interaction of time (baseline, end of treatment week-1, end of treatment week-2) and group (real TMS, sham TMS). Analysis of follow-up data were conducted using paired t-tests examining follow-up scores relative to baseline.
Results
Subjects
20 patients meeting the inclusion criteria were included in the study with 10 in the active treatment and 10 in the sham arm between December 2007 and January 2010. All 20 subjects were included for analysis. A CONSORT e-flowchart is accessible online version of PAIN®. Two subjects complained of a headache after the first treatment. No subjects dropped out of the study. Of the twenty subjects, only two had confidence they had received TMS, but only due to subjective symptom improvement (both were in the treatment arm). Otherwise subjects could not distinguish whether they had received real or sham TMS. The TMS administrators were not blinded to the sham or active TMS. A continuous rater remained blinded for the entirety of study for all measures except tender points. A separate, blinded continuous rater with expertise in tender point measurement became unavailable after the thirteenth subject. A replacement rater with expertise in tender point measures remained blinded to all measures but knew the initial treatment arm assignment for the last seven subjects. The demographic and clinical characteristics of the two groups of patients were similar (Table 1).
Table 1.
Demographic and clinical characteristics of patients
| Real | Sham | Total | ||
|---|---|---|---|---|
| Sex | %Female | 90% | 78% | 84% |
| Age | Mean Yrs | 54.20(8.28) | 51.67(18.19) | 53.00(13.53) |
| Duration of Illness | Mean Yrs | 12.10(7.75) | 10.10(12.81) | 11.10(10.36) |
| Antidepressants | % | 90% | 90% | 90% |
| Anticonvulsants | % | 20% | 10% | 25% |
| Opiate | % | 20% | 20% | 20% |
| NSAIDS | % | 30% | 20% | 25% |
| Benzos | % | 30% | 40% | 35% |
| Muscle Relaxants | % | 0% | 20% | 10% |
| Total # Meds | Mean | 1.90(1.66) | 1.78(0.83) | 1.84(1.30) |
The data represents gender, mean age, and duration (with standard deviations) of fibromyalgia by treatment arm. It includes the percentage of patients, by treatment arm, which were prescribed medication at unchanged doses before and during the research trial.
Daily Diary Pain-On-Average Ratings
The group (real versus sham rTMS) by phase (baseline, treatment-week-1, treatment-week-2) interaction was significant for daily pain-on-average ratings (F(2,444)=6.72, p=.001) (Table 2). No statistically significant differences in mean pain between groups were observed. Post-hoc analyses indicated no difference in pain ratings between groups during the baseline phase (t(18.1)=0.40, ns). Pain ratings decreased significantly during treatment-week-1 in the real TMS group compared to their baseline (t(143)=2.41, p=.017) but not in the sham group (t(164)=0.83, ns). Pain ratings were significantly lower in the real rTMS group during treatment-week-2 as well compared to baseline (t(1209)=3.48, p<.001), but were not different in the sham TMS group (t(1233)=1.20, ns). At 2-weeks follow-up, the gains were maintained relative to baseline in the real TMS group (t(873)=2.73, p=.006), but there was still no significant change from baseline observed in the sham group (t(891)=0.33, ns). In the treatment group, there was a mean 29% (statistically significant) reduction in average daily pain in comparison to their baseline pain. In the sham group, there was a 4% non-significant reduction in average daily pain in comparison to their baseline pain. Figure 1 shows the mean ratings for each phase of the study between groups.
Table 2.
Patient responses by treatment arm and measurement
| Baseline | Week-1 | Week-2 | Follow-Up-1 | Follow-Up-2 | ||
|---|---|---|---|---|---|---|
| Average Daily Pain | REAL | 5.60(1.85) | 4.90(1.89) | 3.99(1.90) | 4.19(1.90) | 4.41(1.95) |
| SHAM | 5.34(1.82) | 5.49(1.90) | 5.07(1.89) | 4.76(1.90) | 5.37(2.02) | |
| FIQ | REAL | 58.79(11.93) | 55.03(16.93) | 42.07(18.13) | 49.29(19.27) | 38.99(19.44) |
| SHAM | 54.38(13.96) | 51.22(15.96) | 51.50(17.32) | 43.47(16.10) | 47.93(14.70) | |
| HDRS | REAL | 21.80(7.79) | 17.30(8.27) | 16.10(8.19) | 15.80(8.74) | 14.10(9.42) |
| SHAM | 17.60(7.31) | 17.40(8.42) | 15.30(7.62) | 17.20(7.55) | 16.40(8.18) | |
| Tender Points | REAL | 13.20(2.53) | 11.40(3.60) | 9.44(4.53) | 10.33(5.79) | 10.40(4.77) |
| SHAM | 13.50(1.72) | 13.60(1.58) | 14.33(2.18) | 13.40(2.59) | 13.00(3.56) | |
| % ACR Criteria | REAL | 90% | 70% | 56% | 56% | 50% |
| SHAM | 100% | 100% | 100% | 80% | 70% | |
| BPI Functional Impairment | REAL | 5.57(2.58) | 4.66(2.72) | 3.46(2.19) | 4.08(2.23) | 3.60(2.18) |
| SHAM | 5.44(2.25) | 4.69(3.08) | 4.98(2.35) | 3.84(2.39) | 3.79(2.69) | |
The data represents mean scores and standard deviations for average daily pain, FIQ, HDRS, tender points, BPI, and percentage of patients that met ACR criteria for fibromyalgia during the five weekly assessments during the trial. ACR criteria tender points categorically require eleven of eighteen tender points for diagnosis.
Figure 1. Mean pain ratings by group over time.
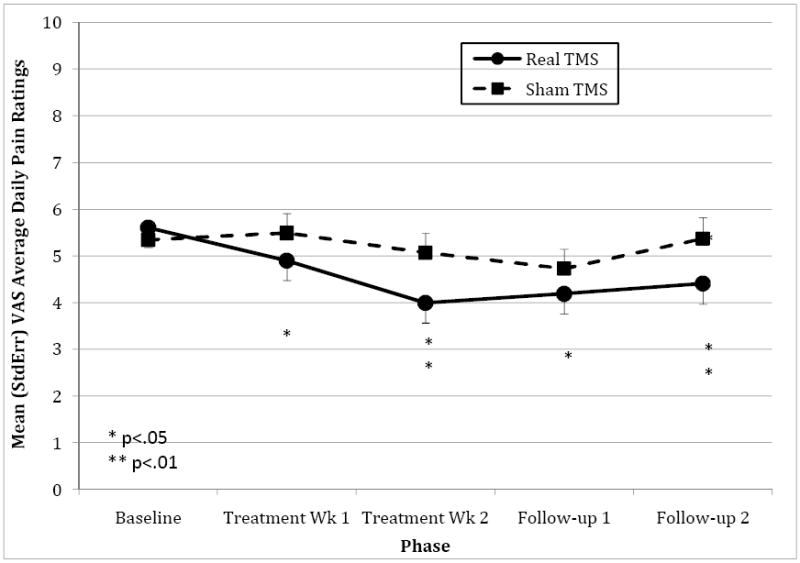
Mean daily pain ratings by treatment vs. sham TMS over treatment phases are illustrated. Pain was scored zero to ten with ten as the worst.
Fibromyalgia Impact Questionnaire
No statistically significant differences in FIQ scores between groups were observed. There was a significant main effect for time on FIQ scores (F(2,19)=8.33, p=.003). The time by group interaction was significant for total FIQ scores (F(2,17)=3.82, p=.043)(Table 2). Post-hoc analyses suggest no difference in baseline FIQ scores between groups (t(18)=.76, ns) and no change in FIQ scores from baseline to the end of treatment-week-1 in either group (t(9)=0.91, ns; t(9)=1.03, ns). However, by the end of treatment-week-2 participants in the real TMS group evidenced a significant decrease in FIQ scores (t(9)=4.09, p=.003), while participants in the sham group did not (t(9)=1.34, ns). Curiously, at 2-weeks follow-up, both groups evidenced significant improvement in FIQ scores relative to baseline (sham: t(9)=2.62, p=.028; real: t(9)=2.29, p=.048).
Brief Pain Inventory
There was a significant main effect for time on mean functional impairment due to pain (F(2,34)=5.69, p=.007) suggesting an overall improvement in the sample. However, the time by group interaction was not significant (F(2,34)=0.18, ns) (Table 2), suggesting no advantage for real TMS compared to sham in improving functional impairment due to pain.
Tender Point Evaluations
No main effect for time was observed on mean number of positive tender points (F(2,15)=1.47, ns). However, the time by group interaction was significant for mean positive tender points (F(2,15)=3.94, p=.042) (Table 2). Post hoc analyses indicate no difference between groups at baseline (t(18)=0.31, ns). There was a significant difference between groups at the end of treatment-week-2 (t(16)=2.92, p=.01) with those in the real TMS group exhibiting significantly fewer tender points than those in the sham group, but no other differences were observed.
HDRS Depression Scores
A main effect between groups was not observed. A main effect for time was observed on HDRS scores (F(2,17)=5.57, p=.014) (Table 2). The time by group interaction was not significant suggesting no treatment effect for TMS over time on mood (F(2,17)=2.32, ns). However, at 2-weeks follow-up, participants in the real TMS group evidenced a significant improvement in HDRS scores compared to baseline (t(9)=2.96, p=.016) whereas those in the sham group did not (t(9)=1.50, ns). The sham group had a non-significant drop by 1 point, while the treatment group had a 7-point decrease in the HDRS. Post-hoc cross-correlational analysis of daily pain and mood dairies revealed a decrease in pain on average one day before a reduction of depression in the treatment group.
Discussion
In a group of TMS naïve outpatients with fibromyalgia, we found that 10 sessions of high frequency left prefrontal TMS resulted in statistically significant reductions in daily pain over time in comparison to baseline mean pain in the treatment group. There were no significant reductions in pain over time in the sham group. However, there were no statistically significant reductions in mean pain between treatment versus sham groups in this pilot trial. Thus any conclusions of fibromyalgia pain reduction with TMS are premature until studies with larger sample sizes show differences between groups, not just pain reduction over time from baseline measures. There were few side effects and no dropouts. TMS was well tolerated. Subjects in both arms were similar across demographics. The pain reduction in the treatment arm was statistically different from baseline after day five of treatment and remained statistically different throughout the follow-up visits. Percent change in reduced pain was 29% at the end of 10 days of treatment over two weeks. Pharmaceutical clinical trials for fibromyalgia seek a 30% reduction in pain for clinical significance. The rapid onset of pain reduction in this pilot trial approximates that of pregabalin and duloxetine, although with markedly less side effects [31; 40].
Fibromyalgia Impact Questionnaire data suggest that the treatment arm experienced a significant reduction in pain compared to baseline at the end of week two of treatment. In follow up week one and two, treatment subjects continued to show a reduction in pain and improved function, however subjects that received sham additionally improved in these domains too. Overall there was no significant difference in follow up weeks one and two. It is unclear as to why sham subjects had robust improvements in these scales after, not during, sham treatment. Speculatively, subjects enrolled were not accustomed to repeat daily visits to the Brain Stimulation Lab that may have unexpectedly involved an element of behavioral activation along with a limited social engagement to the TMS device operator. These effects would occur in both arms, however the sham group received a noxious sham stimulation to simulate real TMS. When sham TMS subjects no longer received sham, they may have inadvertently recorded benefits of removing noxious stimuli along with some nonspecific benefits of behavioral activation, relative to baseline activity, needed to carry out the study. Other possibilities include delayed placebo effect, natural history, or regression to the mean. The BPI assesses pain comparisons over the past 24 hours and the FIQ measures symptom change over the past week. Both assessments possibly captured some non-specific effects but did not capture daily changes from baseline as the pain diary data could.
Secondary analyses of tender point changes are of interest. Subjects with real TMS showed a reduction in tender points in comparison to sham TMS subjects. The treatment sample mean number of tender points dropped below 11 points by the end of the session 10, technically reducing tender points below the categorical 11 of 18 tender points for a diagnosis of fibromyalgia. The tender point reduction was not seen in the placebo arm. Currently tender point counts are a topic of debate for research utility or as clinical marker of improvement in symptoms given the heterogeneity in the fibromyalgia population [46; 47]. In this sample, a reduction in tender points was pronounced, but potentially biased by an unblinded tender point rater later in the study.
In regards to depression, there was not a statistical difference in depression at the end of ten days of rTMS versus sham, however there was a statistical reduction in depression by HDRS in the follow up assessments in the treatment group compared to baseline. Post hoc analyses of daily pain and mood diaries revealed daily pain reduction occurred on average one day before any change in mood, supporting a change in pain before shifts in mood. Other trials have additionally seen reductions in TMS procedural pain and overall pain levels before antidepressant effects when stimulating at 10Hz, LDLPFC rTMS [6; 3].
Given antidepressant effects observed, it is relevant to note the evidence for high frequency rTMS antidepressant effects are not without ambiguity. A Cochrane review [38], analyzing TMS antidepressant trials until 2001, concluded there is no strong evidence for efficacy of transcranial magnetic stimulation for the treatment of depression. Small sample size, lower stimulation intensities, and shorter treatment courses were the primary methodological deficiencies in those trials. Recent meta-analyses [29; 42; 22] of subsequent rTMS antidepressant studies lend support to the FDA’s approval of high frequency rTMS for the treatment of unipolar, nonpsychotic, depression after failing to respond to one antidepressant. Any conclusions of antidepressant effects with TMS in patients with fibromyalgia are premature.
Fibromyalgia subjects have abnormalities in central pain modulation. They may have abnormalities in endogenous opioid systems [7] and enhanced spontaneous pain related to enhanced insula connectivity with default mode network circuitry [34]. Fibromyalgia subjects can have higher resting motor thresholds, motor evoked potentials, lower intracortical facilitation, and short intracortical inhibition suggesting abnormal intracortical modulation involving GABAergic and glutamatergic mechanisms [33]. Fibromyalgia subjects have been shown to have less rostral ACC activity with pain provocation [26], suggesting decreased activity of pain inhibition circuitry.
rTMS to DLPFC may reduce fibromyalgia pain via modulation of pain processing circuitry. Pain modulation circuitry may involve prefrontal cortex (PFC), anterior cingulate cortex, periaqueductal gray, and ventral medial medulla [37]. The PFC may be directly involved in placebo analgesia via release of endogenous opiods in these subcortical regions, and reducing pain transmission [45; 9; 10]. Placebo analgesia can be experimentally blunted with opiate antagonists [2]. Furthermore, the placebo response can be transiently blocked with low frequency TMS to bilateral DLPFC in healthy volunteers [28]. Speculatively, our study may be stimulating left DLPFC at high frequency and activating the same pain modulation circuitry without necessarily activating the psychological expectation. Might high frequency TMS in the DLPFC activate rostral ACC and pain control circuitry to facilitate the placebo response by endogenous opiod release? Fortunately, these are testable hypotheses.
There are several limitations to the study. As a pilot trial, the sample size is small, subjects are not matched by age or severity of symptoms, and subjects remained on medications for fibromyalgia pain. The treatment course was abbreviated (2 weeks instead of 4-6 weeks) in comparison to TMS depression clinical trials from whence the methods were derived. Between group differences were not statistically significant, rather differences were significant over time from baseline, respective to each group. Subsequent secondary functional outcome measures were limited statistically and clinically, which possibly is a function of limited therapeutic significance or premature termination of rTMS. Several pharmacotherapy clinical trials for fibromyalgia are similarly challenged with some secondary functional measures either not reaching statistical significance [5; 31] or modest clinical significance [18; 20; 40; 16; 30; 4; 13]. LDLPFC localization methodology did not use neuroimaging to confirm anatomic site of interest or account for inter-individual neuroanatomic differences. Although subjects were blinded to treatment condition, the TMS administrator was not blind to treatment condition and potentially introduced bias in subjects, effecting treatment response. The rater measuring tender points was not blind to treatment condition throughout the study, thus serving as another source of bias. A blinded, continuous rater was employed for all other measures. Subjects were not followed for months, as part of the study, and thus is it unclear how long the treatment effects lasted. Anecdotally, one subject contacted the research team requesting another course as her fibromyalgia pain had returned after an almost fibromyalgia pain free period of eleven months. A second had called noting approximately 6 months significant fibromyalgia pain relief. Future TMS fibromyalgia research can address several methodological issues with larger samples, double blinding, neuroanatomic localization, longer duration of treatment and observation to ascertain if there are actual treatment effects observed between TMS and sham groups. Future trials may also test for changes in cortical excitability, neuroanatomic changes in PFC, ACC and insula that may preclinically correlate with symptom improvement and be used as a biomarker.
Conclusion
This is the first published rTMS trial stimulating LDLPFC to assess for reductions in fibromyalgia pain. In total, the data lends inconclusive, but suggestive support to the hypothesis that high frequency rTMS at the LDLPFC, as an adjunct to pharmacotherapy, may reduce fibromyalgia pain. Further work is needed to determine if rTMS may have pain modulation effects for fibromyalgia in a larger clinical trial.
Acknowledgments
Funding for this pilot project, under Multidisciplinary Clinical Research Center grant P60 AR049459, was generously provided by the Office of the Provost and Vice-President for Research.
ClinicalTrials.gov Identifier: NCT00523302
Footnotes
Disclosures: A Neopulse machine, purchased by the BSL from previous studies, was employed for the TMS-fibromyalgia trial described in the manuscript. The equipment was not loaned and there was no industry involvement or sponsorship. Dr. George has received no compensation from any TMS manufacturer for the past 5 years, and has no equity in any device or pharmaceutical company. Following a competitive bid and request involving all TMS manufacturers, Neuronetics Inc (Malvern, PA) was selected and loaned the TMS devices, head-holders and coils for the OPT-TMS trial (not described in this manuscript.) Dr. George reports research grants in the past 5 years from Glaxo-Smith Kline, Jazz Pharmaceuticals, Brainsway, Cephos, and Force Protection. He has been an unpaid advisor to Brainsonix, Brainsway, Neuronetics, Neostim and Neosync (as they make products related to TMS), and a paid advisor to Jazz, Cyberonics, Neuropace, and Puretech ventures. The full amount of his advisory income has never been more than 10% of his university salary. MUSC has 2 patent applications in Dr. George’s name on combining TMS with MRI imaging.
All other authors have nothing to disclose and no conflicts of interest.
References
- 1.Ahdab R, Ayache SS, Brugieres P, Goujon C, Lefaucheur JP. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin. 2010;40(1):27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BS, Kavanagh K, Borckardt JJ, Nahas ZH, Kose S, Lisanby SH, McDonald WM, Avery D, Sackeim HA, George MS. Decreasing procedural pain over time of left prefrontal rTMS for depression: initial results from the open-label phase of a multi-site trial (OPT-TMS) Brain Stimul. 2009;2(2):88–92. doi: 10.1016/j.brs.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold LM, Gendreau RM, Palmer RH, Gendreau JF, Wang Y. Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(9):2745–2756. doi: 10.1002/art.27559. [DOI] [PubMed] [Google Scholar]
- 5.Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, Iyengar S, Goldstein DJ. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 6.Avery DH, Holtzheimer PE, Fawaz W, Russo J, Neumaier J, Dunner DL, Haynor DR, Claypoole KH, Wajdik C, Roy-Byrne P. Transcranial Magnetic Stimulation Reduces Pain in Patients With Major Depression: A Sham-Controlled Study. J Nerv Ment Dis. 2007;195(5):378–381. doi: 10.1097/NMD.0b013e31802f58d1. [DOI] [PubMed] [Google Scholar]
- 7.Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004;5(48):48. doi: 10.1186/1471-2474-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belle Gv. Statistical Rules of Thumb. New York: John Wiley & Sons; 2002. [Google Scholar]
- 9.Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, Asteggiano G. Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain. 2006;121(1-2):133–144. doi: 10.1016/j.pain.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1-2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Borckardt JJ, Nahas Z, Koola J, George MS. Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. J Ect. 2006;22(3):169–175. doi: 10.1097/01.yct.0000235923.52741.72. [DOI] [PubMed] [Google Scholar]
- 12.Borckardt JJ, Walker J, Branham RK, Rydin-Gray S, Hunter C, Beeson H, Reeves ST, Madan A, Sackeim H, George MS. Development and Evaluation of a Portable Sham TMS System. Brain stimulation. 2008;1(1):52–59. doi: 10.1016/j.brs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branco JC, Zachrisson O, Perrot S, Mainguy Y. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. J Rheumatol. 2010;37(4):851–859. doi: 10.3899/jrheum.090884. [DOI] [PubMed] [Google Scholar]
- 14.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 15.Carretero B, Martin MJ, Juan A, Pradana ML, Martin B, Carral M, Jimeno T, Pareja A, Montoya P, Aguirre I, Salva J, Roca M, Gili M, Garcia-Toro M. Low-Frequency Transcranial Magnetic Stimulation in Patients with Fibromyalgia and Major Depression. Pain Med. 2009;4:4. doi: 10.1111/j.1526-4637.2009.00625.x. [DOI] [PubMed] [Google Scholar]
- 16.Chappell AS, Bradley LA, Wiltse C, Detke MJ, D’Souza DN, Spaeth M. A six-month double-blind, placebo-controlled, randomized clinical trial of duloxetine for the treatment of fibromyalgia. Int J Gen Med. 2009;1:91–102. doi: 10.2147/ijgm.s3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R, Seitz RJ. Changing cortical excitability with low-frequency magnetic stimulation. Neurology. 2001;57(3):379–380. doi: 10.1212/wnl.57.3.379. [DOI] [PubMed] [Google Scholar]
- 18.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30(11):1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 20.Crofford LJ, Mease PJ, Simpson SL, Young JP, Jr, Martin SA, Haig GM, Sharma U. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalin. Pain. 2008;136(3):419–431. doi: 10.1016/j.pain.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 21.First MB, S R, Gibbon M, Williams JBW. Washington, D.C.: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- 22.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE, 3rd, Schwartz T, Sackeim HA. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 23.George MS, Wassermann EM. Rapid-rate transcranial magnetic stimulation and ECT. Convuls Ther. 1994;10(4):251–254. discussion 255-258. [PubMed] [Google Scholar]
- 24.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1-2):95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112(4):720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 28.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148(3):368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Lam RW, Chan P, Wilkins-Ho M, Yatham LN. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621–631. doi: 10.1177/070674370805300909. [DOI] [PubMed] [Google Scholar]
- 30.Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W, Palmer RH. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36(2):398–409. doi: 10.3899/jrheum.080734. [DOI] [PubMed] [Google Scholar]
- 31.Mease PJ, Russell IJ, Arnold LM, Florian H, Young JP, Jr, Martin SA, Sharma U. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol. 2008;35(3):502–514. [PubMed] [Google Scholar]
- 32.Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007;26(4):465–473. doi: 10.1007/s10067-006-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D. Alteration of cortical excitability in patients with fibromyalgia. Pain. 2010;149(3):495–500. doi: 10.1016/j.pain.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, Perrot S, Januel D, Bouhassira D. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130(Pt 10):2661–2670. doi: 10.1093/brain/awm189. [DOI] [PubMed] [Google Scholar]
- 36.Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14(8):1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 37.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia--imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Martin JosÈ L, Barbanoj JosÈ M, Schlaepfer TE, Clos Susana SC, PÈrez V, Kulisevsky J, Gironelli A. Cochrane Database of Systematic Reviews: Reviews 2001. 4. John Wiley & Sons, Ltd; Chichester, UK: 2001. Transcranial magnetic stimulation for treating depression. 101002/14651858CD003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009 doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell IJ, Mease PJ, Smith TR, Kajdasz DK, Wohlreich MM, Detke MJ, Walker DJ, Chappell AS, Arnold LM. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136(3):432–444. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Sampson SM, Rome JD, Rummans TA. Slow-frequency rTMS reduces fibromyalgia pain. Pain Med. 2006;7(2):115–118. doi: 10.1111/j.1526-4637.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 42.Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39(1):65–75. doi: 10.1017/S0033291708003462. [DOI] [PubMed] [Google Scholar]
- 43.Short EB, Borckardt JJ, George M, Beam W, Reeves S. Non-invasive brain stimulation approaches to fibromyalgia pain. Journal of Pain Management. 2009;2(3):47–63. [PMC free article] [PubMed] [Google Scholar]
- 44.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 46.Wilson HD, Robinson JP, Turk DC. Toward the identification of symptom patterns in people with fibromyalgia. Arthritis Rheum. 2009;61(4):527–534. doi: 10.1002/art.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson HD, Starz TW, Robinson JP, Turk DC. Heterogeneity within the fibromyalgia population: theoretical implications of variable tender point severity ratings. J Rheumatol. 2009;36(12):2795–2801. doi: 10.3899/jrheum.090432. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Glenn AM, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 50.Yunus MB. Fibromyalgia and Overlapping Disorders: The Unifying Concept of Central Sensitivity Syndromes. Semin Arthritis Rheum. 2007;10:10. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]


