Abstract
The sodium chloride co-transporter (NCC) is the primary target of thiazides diuretics, drugs used commonly for long-term hypertension therapy. Thiazides also completely reverse the signs of Familial Hyperkalemic Hypertension (FHHt), suggesting that the primary defect in FHHt is increased NCC activity. To test whether increased NCC abundance alone is sufficient to generate the FHHt phenotype, we generated NCC transgenic mice; surprisingly, these mice did not display an FHHt-like phenotype. Systolic blood pressures of NCC transgenic mice did not differ from those of wild type mice, even after dietary salt-loading. NCC transgenic mice also did not display hyperkalemia or hypercalciuria, even when challenged with dietary electrolyte manipulation. Administration of fludrocortisone to NCC transgenic mice, to stimulate NCC, resulted in an increase in systolic blood pressure equivalent to that of wild type mice (approximately 20 mmHg). Although total NCC abundance was increased in the transgenic animals, phosphorylated (activated) NCC was not, suggesting that the defect in FHHt involves either activation of ion transport pathways other than NCC, or else direct activation of NCC, in addition to an increase in NCC abundance.
Keywords: Hypertension, Hyperkalemia, Sodium Chloride Symporters, Thiazides, Mice, Transgenic
Introduction
Familial Hyperkalemic Hypertension (FHHt) is characterized by hyperkalemia, hypertension, metabolic acidosis, with normal glomerular filtration rate 1. Plasma renin levels are low, but plasma aldosterone is often in the normal range, but inappropriately low with respect to the observed high level of plasma potassium, a strong stimulus of aldosterone secretion 2, 3. Importantly, administration of thiazide diuretics, which inhibit the distal convoluted tubule (DCT)-specific sodium chloride cotransporter (NCC), is uniquely effective at ameliorating these abnormalities 2–4. FHHt is caused by mutations in two members of the With-No-lysine [K] (WNK) kinases, so-named due to the unusual positioning of the lysine involved in coordinating ATP 5. Deletion of part of the first intron of WNK1 increases its expression in leukocytes 2, and was proposed to be a gain-of-function mutation. Mice heterozygous for WNK1 display lower blood pressure than wild type controls, supporting the hypothesis that WNK1 acts to increase blood pressure 6. Missense mutations within the WNK4 gene also lead to FHHt 2. WNK4 strongly inhibits NCC activity in Xenopus oocytes, whereas mutant WNK4 stimulates it; WNK1 increases NCC activity both through suppression of WNK4 and by activating STE20-and-SPS1-related proline/alanine-rich kinase SPAK kinase 7. Subsequent studies have shown that WNK1 itself is inhibited by a kidney-specific isoform lacking the kinase domain (KS-WNK1) 8. Dysregulation of NCC activity has therefore been proposed to be the primary defect underlying FHHt 9, 10. In vitro studies, however, have revealed that the WNK kinases regulate a wide variety of ion channels and transporters besides NCC (reviewed in 11) resulting in controversy regarding the central role of NCC in the etiology of FHHt 12.
Two mouse models that closely resemble FHHt have been reported. In the first, transgenic mice expressing two copies of WNK4 with an FHHt-causing mutation, in addition to the two endogenous wild type alleles, were generated 9. These mice displayed an FHHt-like phenotype, including elevated blood pressure, hyperkalemia, hypercalciuria and hyperplasia of the distal convoluted tubule, the nephron segment to which NCC is restricted. Interestingly, mice expressing an additional copy of wild type WNK4 displayed an opposite phenotype. Another model was generated in which an FHHt-causing WNK4 mutation was knocked-in, and similarly, an FHHt phenotype was observed 10. In both cases, the FHHt phenotype was completely reversed by administration of thiazides 9, 10. Therefore, over-expression of NCC, achieved by other means, should be sufficient to cause an FHHt-like phenotype. The current experiments were designed to test this hypothesis.
Methods
Expanded methods are provided as supplementary information.
Generation of NCC transgenic mice
All procedures were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University (protocol number A858). To generate mice over-expressing NCC, a BAC clone containing the entire mouse NCC gene was obtained from CHORI. The closed circular BAC was purified using the Qiagen Large Construct kit and microinjected into (C57BL/6 X SJL)F2 mouse eggs and surgically transferred to recipients. Founders were crossed with C57BL/6 wild type mice and offspring of interbreeding of the resulting N2 generation were used in subsequent experiments. The numbers of animals used for each procedure are given in the results section.
Western blotting and immunofluorescence
Animals were killed by CO2 asphyxiation, and kidneys harvested. Homogenized samples were separated on a 4–12% NuPage Bis-Tris Gel (Novex; Invitrogen Corp.) and transferred to PVDF paper. Following antibody incubation, detection was performed using the Western Lightning kit (Perkin Elmer) according to the manufacturer’s protocol. For immunofluorescence on kidney sections, mice were anesthetized with ketamine/xylazine/acepromazine 50/5/0.5mg/kg and perfusion fixed with 4% paraformaldehyde via the abdominal aorta. Kidneys were frozen, and 7μm sections prepared on a cryostat. Standard procedures were used for immuostaining, using 5% fat-free milk in PBS as block. Primary antibodies against NCC and NCC phosphorylated at threonine-53 were developed in our laboratory (see Figure S1 at http://hyper.ahajournals.org for validation of anti-phospho-p-53-NCC antibody); all other antibodies were purchased.
Blood pressure measurements
Blood pressure was measured in male mice aged 3–4 months by tail-cuff, using a Coda 6 tail-cuff apparatus (Kent Scientific). This method is recommended for high throughput studies in mice, including initial characterization of genetically modifications, has been extensively validated and over the physiological range gives values similar to radiotelemetry 13.
Plasma and urine chemistry
Blood was collected by cardiac puncture, and analyzed immediately with an iSTAT blood chemistry analyzer (Abbott Labratories). For dietary K+ manipulation, blood was collected from the lateral saphenous vein into heparinized tubes and centrifuged at 2,000g for 5 minutes at room temperature. Plasma [Mg2+] was determined using a colorimetric assay (Pointe Scientific). Plasma [Na+] and [K+] were determined using a Model 2655-10 flame photometer (Cole-Parmer). Urinary calcium and creatinine were measured by colorimetric assays (Pointe Scientific) on spot urine samples. Plasma aldosterone was measured by ELISA, according to the manufacturer’s protocol (IBL America); the plasma renin concentration assay is described in the supplemental information at http://hyper.ahajournals.org.
Dietary manipulations
All diets used were obtained from Harlan Teklad: TD07309 (0.8% K+, control diet for K+ studies), TD07278 (5% K+), TD96208 (0.49% NaCl, control diet for NaCl studies), TD92012 (8% NaCl). Mice were placed on each diet for 10 days.
Results
Generation of NCC transgenic mice
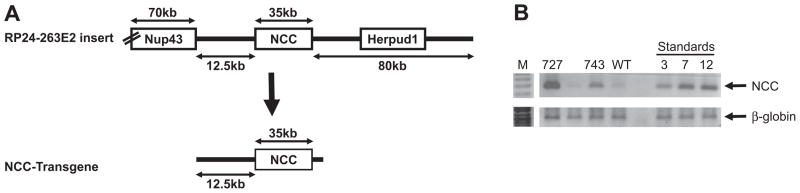
To generate mice over-expressing NCC, a BAC clone containing the entire mouse NCC gene, modified by recombineering to remove other genes, was used (Figure 1A). Twelve founders were identified, and semi-quantitative PCR using primers targeting the NCC gene confirmed that the mice carried additional copies of the NCC gene (Figure 1B). A single transgene integration site was confirmed by FISH analysis (Figure S2, please see http://hyper.ahajournals.org). Two lines were selected for further analysis, line 727 which carries >20 copies of the transgene, and line 743, which carries 5 copies (Figure 1B). Line 727 was not characterized extensively, due to 80% perinatal mortality which was most likely due to integration site of the transgene (see below, and Figure S2 (please see http://hyper.ahajournals.org)), and hereafter “NCC transgenics” will refer to line 743, which has 5 copies of the transgene integrated. A single transgene integration site in line 743 was confirmed by FISH analysis (Figure S2, please see http://hyper.ahajournals.org).
Figure 1. Generation of NCC transgenic mice.
(A) BAC clone RP-24-263E2 was modified by recombineering in E. coli, to remove Herpud1 and the region of Nup43 extending to its 3′ untranslated region (UTR). The resulting NCC transgene contains 12.5kb of sequence upstream of the NCC transcription start, and the 3′ UTR of NCC (vector sequence is not shown). (B) Semi-quantitative PCR on genomic DNA extracted from the tails of potential Founders identified lines 727 and 743 as containing >12 and 5 copies of the NCC transgene; amplification of the β-globin gene confirmed equal template input.
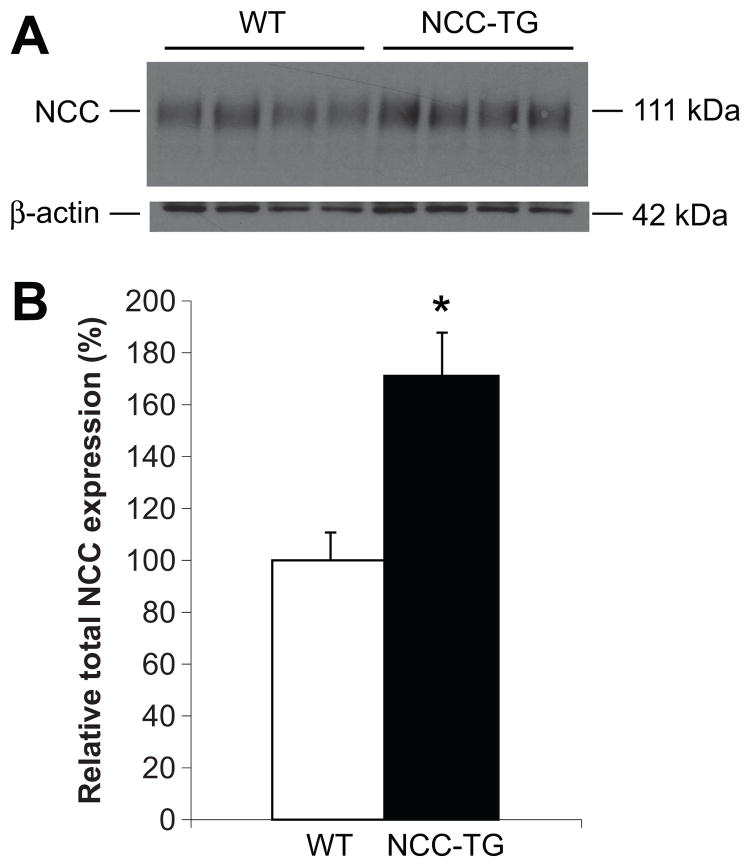
Western blotting revealed a 1.7-fold increase in total NCC expression in the kidneys of NCC transgenic mice (Figures 2A and 2B), similar to the increase observed in KS-WNK knockout mice 14. Expression levels of ENaC subunits, the sodium-hydrogen antiporter 3 NHE3, total and phospho-NKCC2, and the NCC regulatory protein SPAK, did not differ significantly between wild type and transgenic mice (Figure S3, please see http://hyper.ahajournals.org). Immunofluorescence on kidney sections, using an anti-NCC antibody, confirmed there was no ectopic expression of total NCC in the kidney (Figure 3C).
Figure 2. NCC transgenic mice display increased total NCC protein expression.
(A) Western blot analysis of whole kidney extracts from wild type (WT) and NCC transgenic (NCC-TG) mice was performed using antibodies against NCC and β-actin. (B) Densitometric quantitation was performed normalizing to β-actin, and expression of total NCC in NCC-TG (filled bars) relative to WT (open bars) mice was calculated. Wild type (WT), n=13; NCC transgenic (NCC-TG), n=16; values ± S.E.M., *p = 0.005. (C) Immunofluorescence showed that NCC expression is restricted to the DCT in NCC-TG mice.
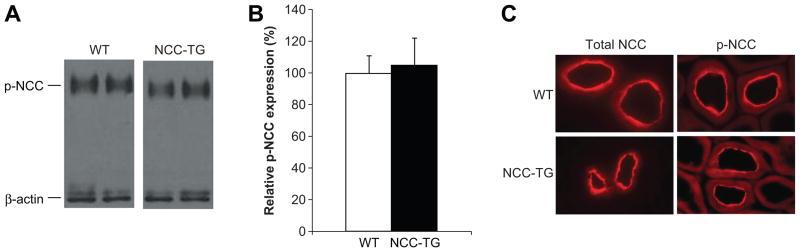
Figure 3. NCC phosphorylation and cellular distribution are not altered in NCC-TG mice.
(A) Western blot analysis of whole kidney extracts from wild type (WT) and NCC transgenic (NCC-TG) mice was performed using antibodies against phospho-NCC (T53) and β-actin. (B) Densitometric quantitation was performed normalizing to β-actin, and expression of phospho-NCC in NCC-TG (filled bars) relative to WT (open bars) mice was calculated. n=12 for each group; values ± S.E.M. (C) Immunofluorescence showed that in both WT and NCC-TG mice, total and phospho-NCC displayed expression at the apical membrane of the DCT.
Phosphorylation and cellular localization of NCC is not altered in NCC transgenic mice
WNK1 activates NCC indirectly, through phosphorylation and activation of SPAK kinase 15 which then phosphorylates NCC, a prerequisite for full transport activity 16, 17. Western blotting using an antibody that recognizes p-T53 NCC revealed that the abundance of phosphorylated NCC did not differ between wild type and NCC transgenics (Figure 3A and 3B), indicating that the ratio of phosphorylated to total NCC (Figure 2A and 2B) was reduced by 41% in the NCC transgenic animals. In addition to phosphorylation, for NCC to transport NaCl it must be localized to the apical membrane of the DCT. Over-expressed NCC could be mis-trafficked, and not reach the plasma membrane. Immunofluorescence, however, indicated that total and phospho-NCC cellular localization do not differ significantly between wild type and transgenic mice (Figure 3C), but could not clarify whether NCC expression in the luminal membrane was significantly different between genotypes.
Over-expression of NCC does not alter basal blood chemistry values or body weight
There were no significant differences in plasma chemistry values between wild type and transgenics, including plasma [K+], which is typically elevated in FHHt (Table 1). Plasma renin concentration and aldosterone levels were determined and did not differ significantly, nor did body weights (Table 1). Significantly, line 727 which carries 20 copies of the transgene, did not display hyperkalemia or hypocalciuria (data not shown).
Table 1.
Plasma chemistry and body weights of wild type and NCC transgenic mice
| Parameter | Wild type, ± S.D., (n) | NCC transgenic ± S.D., (n) |
|---|---|---|
| Body weight (g) males | 31.6 ± 3.6 (10) | 28.9 ± 2.4 (6) |
| Body weight (g) females | 23.6 ± 1.9 (9) | 23.2 ± 2.6 (17) |
| Na+ (mmol/l) | 147.5 ± 1.86 (11) | 147.9 ± 3.08 (11) |
| K+ (mmol/l) | 3.85 ± 0.29 (11) | 3.85 ± 0.31 (11) |
| Mg2+ (mmol/l) | 2.15 ± 0.29 (11) | 2.20 ± 0.31 (11) |
| Cl− (mmol/l) | 107.3 ± 0.9 (11) | 107.8 ± 1.7 (11) |
| iCa2+ (mmol/l) | 1.22 ± 0.03 (11) | 1.25 ± 0.05 (11) |
| TCO2 (mmol/l) | 24.0 ± 2.8 (11) | 23.2 ± 2.0 (11) |
| Glucose (mg/dl) | 241 ± 37 (11) | 226 ± 45 (11) |
| BUN (mg/dl) | 21.8 ± 2.7 (11) | 22.6 ± 2.9 (11) |
| Creatinine (mg/dl) | 0.24 ± 0.07 (11) | 0.23 ± 0.05 (11) |
| Hematocrit | 0.42 ± 0.01 (11) | 0.42 ± 0.03 (11) |
| Plasma renin conc. (ng/ml/h) | 79 ± 36 (10) | 108 ± 39 (9) |
| Aldosterone (nM) | 0.79 ± 0.12 (18) | 0.90 ± 0.29 (18) |
For plasma values, 5 males and 6 females were used, except for renin activity (5 males and 5 females for wild type; 5 males and 4 females for transgenic) and aldosterone (9 of each gender per group).
Mice over-expressing NCC are not hypertensive
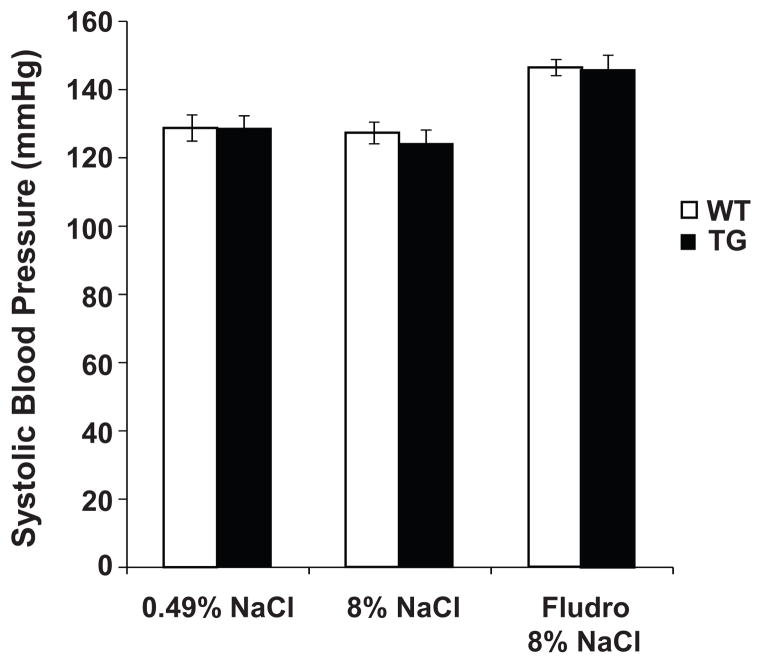
Systolic blood pressure values on a standard diet containing 0.49% NaCl did not differ between genotypes (Figure 4) on the standard diet, nor did heart rates (wild type 896 ± 14 versus NCC transgenic 918 ± 7, n=10). The mice were placed on a high NaCl (8% NaCl) diet for 10 days, which can elicit a hypertensive phenotype in mice normotensive on standard salt diets 18. Salt-loading did not lead to an increase in blood pressure in wild type or NCC transgenic mice, indicating that over-expression of NCC does not lead to salt-sensitive hypertension (Figure 4). The synthetic mineralocorticoid fludrocortisone has previously been shown to increase expression and activity of NCC 19, so to ensure full activation of NCC wild type and transgenic mice on the high NaCl diet were provided with fludrocortisone in their drinking water. Systolic blood pressure increased in both groups, but to a similar degree (19 mmHg in wild type and 22 mmHg in transgenics) (Figure 4). The absence of functional differences likely reflected the fact that the abundance of total and phospho-NCC did not differ, between wild type and transgenic animals (Figure S4, please see http://hyper.ahajournals.org). These data indicate that activation of NCC by fludrocortisone does not elicit a hypertensive phenotype in mice over-expressing NCC, at the message level, because NCC abundance at the protein level, and NCC activity are not enhanced. Immunofluorescence showed that the degree of phospho-NCC expression at the apical membrane was similar in both groups (data not shown), suggesting that activation of NCC regulatory pathways such as WNK/SPAK may be required to fully stimulate the over-expressed NCC.
Figure 4. NCC transgenic mice are normotensive.
Systolic blood pressure was measured in wild type (open bars) and NCC transgenic mice (filled bars) after 2 weeks on standard (0.49% NaCl) and high (8% NaCl) salt diets, and 2 weeks of high salt diet with fludrocortisone in drinking water (17mg/l). There were no significant differences in systolic blood pressure on standard or high salt diet. Fludrocortisone treatment led to a similar, significant increase in systolic blood pressure in both wild type and NCC transgenic mice.
NCC transgenic mice display normal responses to dietary electrolyte modification
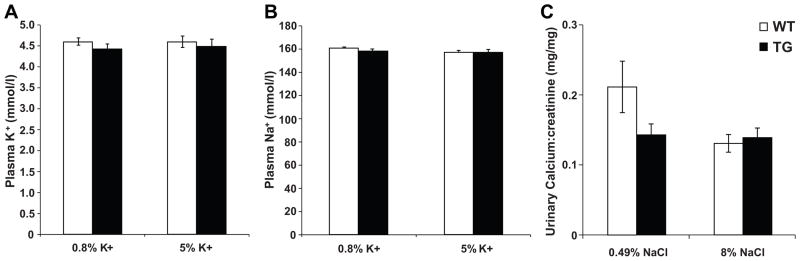
A distinctive characteristic of FHHt is the presence of hyperkalemia. On a control diet containing 0.8% potassium, NCC mice were normokalemic; increasing the dietary potassium level to 5% did not elicit hyperkalemia (Figure 5A). Similarly, NCC transgenic mice did not display a change in plasma sodium after 10 days on the high potassium diet (Figure 5B). Western blotting revealed that expression of total NCC was not altered by potassium loading in either genotype, but phospho-NCC trended to lower levels in wild type but not NCC transgenic mice (Table S1, please see http://hyper.ahajournals.org). In the distal convoluted tubule, sodium reabsorption is correlated inversely to calcium absorption 20. In FHHt, patients with mutations in WNK4 are hypercalciuric 21, whereas patients with mutations in WNK1 are normocalciuric 22. This suggests that mutant WNK4 may cause hypercalciuria by an interaction of WNK4 with a calcium channel or transporter. In contrast, inactivating mutations of NCC result in Gitleman Syndrome, characterized by hypochloremic metabolic alkalosis, hypokalemia and hypocalciuria. Analysis of spot urine samples revealed no significant difference in urinary calcium levels between NCC transgenic and wild type mice (Figure 5C). Furthermore, manipulation of the diet to provide 8% NaCl for 7 days did not induce hypercalciuria in either genotpye (Figure 5C). Salt-loading caused a trend toward lower expression of α-ENAC, but significantly increased expression of γ-ENAC in both wild type and NCC transgenic mice (Table S1, please see http://hyper.ahajournals.org); surprisingly, β-ENAC expression tended to increase in wild type mice, but decrease in NCC transgenic mice.
Figure 5. Normal electrolyte homeostasis in NCC transgenic mice.
(A) Plasma K+ and (B) plasma Na+ did not differ between wild type (open bars) and NCC transgenic mice (filled bars) on standard (0.8% K+) or high potassium (5% K+) diets. Values are means ± S.E.M., n=19–22. (C) Urinary calcium:creatinine, measured from spot urine collections, did not differ between wild type (open bars) and NCC transgenic mice (filled bars) on standard (0.49% NaCl) or high (8%) NaCl diets. Values are means ± S.E.M., n=18–22.
Responses of aldosterone secretion to both potassium- and sodium-loading did not differ between wild type and NCC transgenic mice, significantly increasing in response to high K+, and decreasing significantly in response to high NaCl (Figure S5). Similarly, no differences in plasma renin activities were observed between wild type and NCC transgenic mice following dietary manipulation (Figure S5, please see http://hyper.ahajournals.org).
Discussion
The current results add to an increasing body of evidence suggesting that increased NCC abundance alone is insufficient to cause hyperkalemia and hypertension. Initial studies in mice, coupled with clinical observations in humans, suggested that the predominant mechanism by which mutations in WNK kinases lead to FHHt is by increasing NCC abundance or trafficking to the apical membrane 9, 10. However, studies from many laboratories have indicated that WNK kinases regulate the activity of multiple transport proteins, as well as modulating paracellular permeability (reviewed in 11). We hypothesized that increased NCC abundance alone would be sufficient to causes the FHHt phenotype. Our data indicate that NCC transgenic mice, while having increased total NCC abundance, are not hypertensive (Figure 4); further, serum and urinary electrolyte levels are normal, under standard dietary conditions (Table 1 and Figure 5). In addition, none of the maneuvers initiated, including dietary electrolyte manipulation and fludrocortisone administration, resulted in any differences in blood pressure or electrolyte status, compared with wild type mice. This does not, however, indicate that an increased basal abundance of NCC is entirely without consequences. Following completion of this work, and reported elsewhere, we found that the calcineurin inhibitor, tacrolimus, increased blood pressure more in transgenic than in wild type mice 23.
While these results were surprising initially, two other studies obtained results that are highly consistent with ours. As noted above, KS-WNK1 is believed to be a dominant-negative regulator of WNK1, thereby suppressing the effects of WNK1 to stimulate NCC 8. KS-WNK1 deficient mice did not display an FHHt-like phenotype, despite a 1.8-fold increase in renal NCC expression levels, and despite hypertrophy of the DCT 14. Similar to our findings, these mice did not display overt hypertension, although there was a small increase in diastolic blood pressure. Furthermore, no hyperkalemia was observed in KS-WNK1 knockout mice, even when they were placed on a high potassium diet. In the second study, KS-WNK1 knockout mice were shown to be neither hypertensive, nor hyperkalemic, when consuming a normal diet, although mild hypertension and hyperkalemia developed, when challenged with high salt and high potassium intakes. In these mice, the abundance of NCC was increased 24, as in the present results. Hadchouel and colleagues observed a reduction in expression of both α- and γ-ENaC, which they proposed to compensate for increased NCC expression 14. In our studies, there were no differences in expression of ENaC isoforms on a standard diet, but following sodium loading NCC transgenic mice displayed a reduction in β-ENaC expression, which could be a compensatory mechanism in these mice. Our findings in wild type mice are the same as those obtained in salt-loaded rats by Song and colleagues 25. As noted, we observed a discrepant effect of salt loading on β-ENaC expression in NCC TG mice. Non-coordinate regulation of ENaC subunit expression in vivo (i.e. manipulations leading to changes in expression of only some subunits, or changes in expression of subunits in opposite directions) is frequently observed, and well-described 26.
In contrast to the mild phenotypes observed in these studies, other mouse models, in which FHHt mutants of WNK4 were over-expressed 9 or knocked-in 10, developed clear hyperkalemic hypertension, in association with increased NCC expression; the abundance of NCC increased by 2-fold in one model 10. One possible reason for the observed differences between the models results from differential effects on other transporters. Expression of ENaC is downregulated in KS-WNK1 knockout mice mice 14, whereas it is upregulated in mice over-expressing an FHHt mutant of WNK4 9. Taken together with our observations in NCC transgenic mice, these data suggest that WNKs must alter the activity of several transport proteins, to induce the FHHt phenotype.
Another possibility is that WNKs generate hyperkalemic hypertension by activating NCC via SPAK, in addition to increasing its abundance. In NCC transgenic mice, while total NCC expression was increased, there was no difference in the phospho-NCC level between wild type and transgenic mice (Figure 3). We reasoned that, by increasing NCC abundance and phosphorylation, fludrocortisone might uncover a difference between wild type and transgenic mice, but fludrocortisone increased blood pressure similarly in both groups. Notably, the phenotype of KS-WNK1 knockout mice, in which both total and phospho-NCC levels are elevated is intermediate between the phenotype observed in transgenic NCC mice and the FHHt-mutant WNK4 mice, with a mild increase in diastolic pressure under basal conditions 14. Thus, it seems unlikely that an increase in phospho-NCC alone would generate the complete FHHt phenotype.
There are several possible explanations for the ability of thiazides to completely correct FHHt 27 that are not directly related to their inhibition of NCC. First, it is well-established that thiazide diuretics inhibit activity of carbonic anyhdrase in the proximal tubule 28. More recently, it has been shown that NCC knockout mice treated with thiazides still display a significant increase in urinary sodium output 29, showing that thiazides act on other sodium transport mechanisms. Finally, thiazide treatment leads to changes in kidney structure, including apoptosis and de-differentiation in the DCT1 30, where NCC is the predominant sodium entry pathway. These changes may result from reduced intracellular sodium concentration, or increased intracellular calcium levels. Atrophy of the DCT is also observed in NCC knockout mice 31, and transgenic mice over-expressing wild type WNK4, in which NCC activity is presumably lower 9. Therefore, it is possible that some of the effects of thiazides in patients with FHHt are secondary to structural changes in the distal nephron.
Surprisingly, salt-loading did not increase urinary calcium excretion in wild type mice. In humans, urinary calcium excretion is directly related to sodium intake 20. Furthermore, urinary calcium excretion was about five times higher in salt-loaded rats compared to controls drinking deionized water, irrespective of dietary calcium content 32. In their mouse model of FHHt, Lalioti and colleagues saw little change in urinary calcium excretion by wild type mice on an 8% NaCl diet, the same level of NaCl provided in our studies. These data suggest that renal calcium handling in mice may respond differently to salt-loading. Another explanation is that in the mouse studies animals were only provided with 8% NaCl for 10 days, while the rat studies involved 8 weeks of salt-loading 32.
Perspectives
These unanticipated data suggest that NCC over-expression alone is insufficient to induce hyperkalemic hypertension accompanied by hypercalciuria, and that dysregulation of NCC activity and/or of other other transporters/channels plays a significant role in the etiology of FHHt. In vitro, the WNK kinases have been shown to regulate the activities of a broad range of sodium and potassium transport mechanisms in the kidney (reviewed in 11). Therefore, it is likely that the WNK kinases play a broad role in ion homeostasis in normal physiology. Alternatively, the mouse may not precisely model human physiology and pathophysiology with regard to NCC function, since NCC knockout mice do not precisely mimic Gitelman Syndrome caused by inactivating mutations in the human NCC gene 33, 34. While the thiazides are extremely useful therapeutically (reviewed in 35), better knowledge of the mechanisms underlying sodium reabsorption by the distal nephron will enable the development of therapies for the treatment of presentations of hypertension with variations in electrolyte disturbance. The WNK kinases thus represent an important class of future drug targets in this regard.
Supplementary Material
Acknowledgments
The authors thank Nicole Desmarais for technical assistance with the animal studies, and Tom Roeschel for assistance with immunofluorescence.
Sources of Funding
This work was supported by grants from the NIH (Career development award K01 DK076617 to JM, 5T32DK067864-05 to JN, and DK51496 to DE). DE is also supported by a Merit Review from the Department of Veterans Affairs and a Grant-In-Aid from the American Heart Association.
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement
The authors have no conflicts of interest to disclose.
References
- 1.Farfel Z, Iaina A, Rosenthal T, Waks U, Shibolet S, Gafni J. Familial hyperpotassemia and hypertension accompanied by normal plasma aldosterone levels: Possible hereditary cell membrane defect. Arch Intern Med. 1978;138:1828–1832. [PubMed] [Google Scholar]
- 2.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in wnk kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 3.Achard JM, Disse-Nicodeme S, Fiquet-Kempf B, Jeunemaitre X. Phenotypic and genetic heterogeneity of familial hyperkalaemic hypertension (gordon syndrome) Clin Exp Pharmacol Physiol. 2001;28:1048–1052. doi: 10.1046/j.1440-1681.2001.03575.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type ii: Marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab. 2002;87:3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 5.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. Wnk1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain ii. J Biol Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 6.Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, Gupta A, Hansen G, Hu Y, Huang W, Jaing C, Key BW, Jr, Kipp P, Kohlhauff B, Ma ZQ, Markesich D, Payne R, Potter DG, Qian N, Shaw J, Schrick J, Shi ZZ, Sparks MJ, Van Sligtenhorst I, Vogel P, Walke W, Xu N, Zhu Q, Person C, Sands AT. Wnk1 kinase deficiency lowers blood pressure in mice: A gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci U S A. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang CL, Angell J, Mitchell R, Ellison DH. Wnk kinases regulate thiazide-sensitive na-cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of wnk1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol. 2006;290:F619–624. doi: 10.1152/ajprenal.00280.2005. [DOI] [PubMed] [Google Scholar]
- 9.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 10.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type ii: Generation and analysis of a wnk4(d561a/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 11.McCormick JA, Ellison DH. The wnks: Atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of romk by long and kidney-specific wnk1 isoforms. Proc Natl Acad Sci U S A. 2006;103:1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng M, Dipetrillo K. Non-invasive blood pressure measurement in mice. Methods in molecular biology (Clifton, NJ) 2009;573:45–55. doi: 10.1007/978-1-60761-247-6_3. [DOI] [PubMed] [Google Scholar]
- 14.Hadchouel J, Soukaseum C, Busst C, Zhou XO, Baudrie V, Zurrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X. Decreased enac expression compensates the increased ncc activity following inactivation of the kidney-specific isoform of wnk1 and prevents hypertension. Proc Natl Acad Sci U S A. 2010;107:18109–18114. doi: 10.1073/pnas.1006128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriguchi T, Urushiyama S, Hisamoto N, Iemura SI, Uchida S, Natsume T, Matsumoto K, Shibuya H. Wnk1 regulates phosphorylation of cation-chloride-coupled cotransporters via the ste20-related kinases, spak and osr1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 16.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive na+-cl− cotransporter by the wnk-regulated kinases spak and osr1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 17.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin ii signaling increases activity of the renal na-cl cotransporter through a wnk4-spak-dependent pathway. Proc Natl Acad Sci U S A. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111:1251–1258. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive na-cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman PA. Codependence of renal calcium and sodium transport. Annu Rev Physiol. 1998;60:179–197. doi: 10.1146/annurev.physiol.60.1.179. [DOI] [PubMed] [Google Scholar]
- 21.Mayan H, Munter G, Shaharabany M, Mouallem M, Pauzner R, Holtzman EJ, Farfel Z. Hypercalciuria in familial hyperkalemia and hypertension accompanies hyperkalemia and precedes hypertension: Description of a large family with the q565e wnk4 mutation. J Clin Endocrinol Metab. 2004;89:4025–4030. doi: 10.1210/jc.2004-0037. [DOI] [PubMed] [Google Scholar]
- 22.Achard JM, Warnock DG, Disse-Nicodeme S, Fiquet-Kempf B, Corvol P, Fournier A, Jeunemaitre X. Familial hyperkalemic hypertension: Phenotypic analysis in a large family with the wnk1 deletion mutation. Am J Med. 2003;114:495–498. doi: 10.1016/s0002-9343(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 23.Hoorn EJ, Walsh SB, McCormick JA, Furstenberg A, Yang C-L, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH. Calcineurin inhibitors activate the renal sodium chloride cotransporter to cause hypertension. Nat Medicine. 2011 doi: 10.1038/nm.2497. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL. Downregulation of ncc and nkcc2 cotransporters by kidney-specific wnk1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet. 2011;20:855–866. doi: 10.1093/hmg/ddq525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Hu X, Shi M, Knepper MA, Ecelbarger CA. Effects of dietary fat, nacl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am J Physiol Renal Physiol. 2004;287:F1204–1212. doi: 10.1152/ajprenal.00063.2004. [DOI] [PubMed] [Google Scholar]
- 26.Weisz OA, Johnson JP. Noncoordinate regulation of enac: Paradigm lost? Am J Physiol Renal Physiol. 2003;285:F833–842. doi: 10.1152/ajprenal.00088.2003. [DOI] [PubMed] [Google Scholar]
- 27.Gordon RD, Hodsman GP. The syndrome of hypertension and hyperkalaemia without renal failure: Long term correction by thiazide diuretic. Scott Med J. 1986;31:43–44. doi: 10.1177/003693308603100114. [DOI] [PubMed] [Google Scholar]
- 28.Velazquez H. Thiazide diuretics. Ren Physiol. 1987;10:184–197. [PubMed] [Google Scholar]
- 29.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The na+-dependent chloride-bicarbonate exchanger slc4a8 mediates an electroneutral na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loffing J, Loffing-Cueni D, Hegyi I, Kaplan MR, Hebert SC, Le Hir M, Kaissling B. Thiazide treatment of rats provokes apoptosis in distal tubule cells. Kidney Int. 1996;50:1180–1190. doi: 10.1038/ki.1996.426. [DOI] [PubMed] [Google Scholar]
- 31.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B. Altered renal distal tubule structure and renal na(+) and ca(2+) handling in a mouse model for gitelman’s syndrome. J Am Soc Nephrol. 2004;15:2276–2288. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 32.Saric M, Piasek M, Blanusa M, Kostial K, Ilich JZ. Sodium and calcium intakes and bone mass in rats revisited. Nutrition. 2005;21:609–614. doi: 10.1016/j.nut.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Morris RG, Hoorn EJ, Knepper MA. Hypokalemia in a mouse model of gitelman’s syndrome. Am J Physiol Renal Physiol. 2006;290:F1416–1420. doi: 10.1152/ajprenal.00421.2005. [DOI] [PubMed] [Google Scholar]
- 34.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE. Phenotype resembling gitelman’s syndrome in mice lacking the apical na+-cl− cotransporter of the distal convoluted tubule. J Biol Chem. 1998;273:29150–29155. doi: 10.1074/jbc.273.44.29150. [DOI] [PubMed] [Google Scholar]
- 35.Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361:2153–2164. doi: 10.1056/NEJMra0907219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.