Abstract
Disinfection-by-products (DBPs) have long been a human health concern and many are known carcinogens and teratogens. Skin is exposed to DBPs in water through bathing and swimming; however, dermal uptake of many DBPs has not been characterized. The present studies were initiated to measure the permeation coefficients (Kp) for haloacetonitriles (HANs) and chloral hydrate (CH), important cytotoxic DBPs. The Kp values measured using fully hydrated dermatomed torso skin at 37 °C for the HANs ranged from 0.099 to 0.167 cm/hr, and was 0.0039 cm/hr for CH. Of the HANs, dibromoacetonitrile had the highest permeability while chloroacetonitrile had the lowest permeability and a direct relationship was observed between their Kp and their octanol/water partition coefficients (Kow). The Kps of the HANs were also approximately 30 times that of CH. The monthly dermal and ingestion doses of HANs and CH of an average American population were estimated using Monte Carlo simulations. The dermal doses of HANs ranged from 0.39 to 0.75 times that of their ingestion doses but only approximately 0.02 times their ingestion doses for CH, assuming that the Kp values determined are applicable to shorter water contact times. However, that ratio can vary markedly with chlorinated swimming pool exposures; with a range from 0.304 to 2.25 for HANs and 0.192 to 0.245 for CH. Dermal exposure to HANs and CH seems to be a significant route of exposure and should be considered when evaluating their total exposure during the routine usage of water for bathing and swimming.
INTRODUCTION
Activities such as showering, bathing and swimming are daily parts of life and can result in exposure to contaminants in water such as disinfection-by-products (DBPs). DBPs include a wide range of compounds such as trihalomethanes, haloacetic acids, haloacetonitriles (HANs) and haloketones (Krasner, et al., 1989). These compounds are formed by reactions of disinfectants such as chlorine with humic substances and other organic material commonly found in water (deLeer, et al., 1985, Kopfler, et al., 1985, Krasner, et al., 1989 and Stevens, et al., 1985).
Multi-route exposures to chloroform, the DBP with the highest concentration in both drinking and pool water, have been observed during showering, bathing and swimming (Aggazzotti et al., 1990, Erdinger et al., 2004, Gordon, et al., 1998, Jo et al., 1990a,b, Lindstrom, et al., 1997, Weisel et al., 1992, and Weisel and Jo, 1996). It has been estimated that the daily dermal dose of chloroform from showering is approximately that of the daily ingestion dose from consuming two liters of tap water (Jo et al., 1990). Brown, et al. (1984) estimated that an average of 64% of a total dose to lipophilic compounds in drinking water from showering is accounted for by dermal exposure and the rest from inhalation exposure. Dermal exposure to water from bathing is expected to be even greater than from showering (Brown, et al., 1984). Potential dermal exposures to HANs and CH have not been reported.
The present studies focus on the dermal absorption of the DBPs haloacetonitriles and chloral hydrate (CH). These compounds have been reported to be mutagenic and teratogenic (Bull et al., 1985, Daniel, et al., 1986, Lin et al., 1986, Nouraldeen and Ahmed, 1996, Smith et al., 1986, Smith et al., 1989, and Valencia et al., 1985) and thus represent a potential health concern. They typically reach levels as high as 24 µg /L within drinking water (Bull and Kopfler, 1991). Much higher concentrations of dichloroacetonitrile (DCAN) (265 µg/L) and CH (45 µg/L) have been measured in swimming pool water (World Health Organization, 2000).
Absorption of DBPs into the body is a function of their concentrations in bath and/or pool water, exposure durations, frequency of exposures, area of the skin exposed, and the skin’s permeation to the contaminants. In the present study the skin’s permeability is estimated from in vitro measurements of the permeability coefficient (Kp).
MATERIALS AND METHODS
Materials
Chloroacetonitrile (99+%) was purchased from Fluka (Milwaukee, MI), and dichloroacetonitrile (98+%) and trichloroacetonitrile (99%) from TCI America (Portland, OR). Bromochloroacetonitrile (90%) was from Fluorochem USA (West Columbia, SC), and chloral hydrate (CH) (99.7%) from Sigma (Bellefonte, PA). Tritiated water (3H2O, 1 mCi/ml) was purchased from American Radiolabeled Chemicals (St. Louis, MO).
Human cadaver skin tissue sections were purchased from the National Disease Research Interchange (NDRI) (Philadelphia, PA).
Side-bi-side diffusion cells and drive consoles were purchased from Crown Glass Company, Inc. (Somerville, NJ).
Skin preparation
Human cadaver skin was frozen prior to use, so no metabolic activity was expected. It was assumed that any solutes that passed through the skin did so by passive diffusion. Full thickness human cadaver skin sections from the torso were prepared by the removal of the subcutaneous tissue, leaving the dermis and epidermis. Some of the skin was dermatomed to a thickness between 0.25 and 0.28 mm. All skin sections were stored at −20° C, a process not expected to affect their permeability characteristics (Harrison et al., 1984).
Skin integrity
The physical integrity of a skin section was tested by determining its permeability (Kp) for tritiated water (Bronaugh et al., 1986). If the Kp was greater than 2.5 × 10−3 cm/hr or if >0.29% of the applied tritiated water penetrated the skin after a 20-min exposure, the skin was considered to be damaged and not used. The Kp values were measured using side-bi-side diffusion cells maintained at 37° C. One hundred and three hundred µl of 10 µCi/ml tritiated water was used to test the integrity of dermatomed skin and whole skin, respectively. At the end of each time point, 100 µl of the receptor solutions were pipetted into 3 ml of Fisher Scientific ScintiVerse scintillation fluid in 5-ml plastic scintillation vials. The vials were counted for radioactivity using a Packard TRI-CARB 2100TR liquid scintillation analyzer.
Experimental procedure
The skin was thawed at room temperature in phosphate buffered saline solution and placed between two DC-100B side-bi-side diffusion cells. Both cells were maintained at 37° C and completely mixed using Teflon-coated magnetic stirring bars spun at 600 rpm. The area of skin exposed in the diffusion cells was 0.636 cm2. The skin was immersed between two fluid filled solutions, the receptor, a phosphate buffered saline solution at a pH of 7.4 was selected to have the same ionic strength and pH value present in blood, though it did not contain organic compounds so did not have the same lipophilic nature as blood, and the donor water containing the target DBP representing the bath water.
Steady-state experiments were conducted for 12 hr using both dermatomed and whole skin with donor cell concentrations of 0.8 g/L for HANs and CH. The receptor solution was sampled and completely replaced with a fresh solution at least every hour for the steady-state experiments. A 1L bottle was filled and the fluid continually circulated through the donor diffusion cell in a closed system for the steady-state experiments to ensure a constant donor concentration. The receptor and donor cells were sealed to minimize the evaporation of solutes.
Sample analysis
The HANs and CH in both the donor and receptor solutions were extracted with methyl-tert-butyl-ether and analyzed using US EPA Method 551.1 with iodoacetonitrile (98%) as an internal standard (Aldrich, Milwaukee, WI) (US EPA, 1995). The extracts were injected into an HP5890 gas chromatography fitted with a 60 m Restek Rtx-624 capillary column (0.25 mm i.d., 1.4 µm film thickness) (Bellefonte, PA), and an electron capture detector. Seven point calibration curves were prepared with standards in 35 ml buffered water solutions (pH = 4.5 to 5.5) that were extracted in an identical manner to samples. Analysis of the standards was based on the peak area ratio of the target compound to the internal standard. External standards were also analyzed to evaluate the stability of the system.
Data analysis
The skin’s permeability was measured in vitro as the permeability coefficient (Kp) using Fick’s law assuming steady-state conditions and a proportionality between the measured flux and water concentration in the donor cell. Fick’s law is valid under steady-state conditions and reflects an “infinite dose” model that describes skin permeation (Franz, et al., 1993) provided that the flux and donor concentrations remain constant. The main advantage of calculating the Kp value is that it remains independent of concentration and time and can be applied to many exposure conditions (Poet and McDougal, 2002).
Test Statistics
Student’s t-tests were used to determine whether there was a statistical difference between the Kp values determined at steady-state conditions for full thickness and dermatomed skin permeability.
Monte Carlo Simulations of Exposure and Dose
The potential distributions of ingestion and dermal doses from drinking and pool water to the general population was estimated using Monte Carlo simulations run using Crystal Ball Pro version 4.0 software (SAS Institute Inc.). The variables included in the dose calculations were: body size; ingestion rates of drinking water; exposure durations for bathing, showering and swimming; and drinking water concentrations (Tables 1–3).
TABLE 1.
Body weight, surface area and ingestion (all normal distributions) assumptions made for Monte Carlo calculations of population dermal exposures.
| Body weight (kg) a | Total surface area (m2) (excluding the head) b |
Total surface area (m2) c |
Drinking water ingestion rate (mL/day) d |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 10% | 90% | 50% | 10% | 90% | 50% | 10% | 90% | Mean | 10% | 90% | |
| Girlse | 35.2 | 27.1 | 45.6 | 0.976 | 0.846 | 1.14 | 1.1 | 0.954 | 1.29 | 811 | 278 | 1540 |
| Boyse | 34.8 | 26.9 | 44.8 | 0.967 | 0.851 | 1.14 | 1.09 | 0.959 | 1.29 | 914 | 294 | 1860 |
| Womenf | 65.4 | 50.3 | 84.4 | 1.58 | 1.38 | 1.87 | 1.69 | 1.49 | 1.98 | 1300 | 551 | 2150 |
| Menf | 78.1 | 62.3 | 95.7 | 1.81 | 1.6 | 2.06 | 1.94 | 1.72 | 2.2 | 1460 | 582 | 2490 |
National Center for Health Statistics (1987).
Data used for bath exposure from U.S. EPA (1985). The head surface area for children was calculated to be on average 11.3 % of the body.
Data used for pool exposure from U.S. EPA (1985).
The data are for children 1–19 years old.
Applied to adults >20 years old.
TABLE 3.
Drinking and pool water concentrations used in Monte Carlo simulations to estimate population dermal ingestion exposures.
| Compounds | Drinking water concentrations (µg/L) b |
Pool water concentrations (µg/L) c | ||||
|---|---|---|---|---|---|---|
| Mean | Range a | Standard deviation |
Mean | Range | Pool type | |
| CAN | 0.0926 | ND-0.9 | 0.199 | ----- | ----- | ----- |
| DCAN | 1.64 | ND-19 | 2.83 | 45 | ----- | Outdoor |
| BCAN | 0.86 | ND-3.2 | 0.862 | ----- | ----- | ----- |
| DBAN | 0.671 | ND-4 | 0.928 | 2.5 | 0.0-16 | Outdoor |
| CH | 2.51 | ND-26 | 3.93 | 265 | ----- | Indoor |
ND was entered as a value of 0 when defining assumptions in Monte Carlo.
Drinking water concentrations were obtained from Weinberg, et al. (2002)- a logarithmic scale was assumed.
The HAN and CH concentrations were obtained from Baudisch, et al. (1997) - a logarithmic scale was assumed.
No data available, assumed to be zero
Equations that consider non steady-state conditions were used to estimate in vivo internal dermal doses from bath exposures to better reflect real life exposures (Bunge and McDougal 1999). Equation 1 was used when the exposure time is ≤ 2.4 × the lag time.
| Equation 1 |
Equation 2 was used when the exposure time is ≥ 2.4× the lag time.
| Equation 2 |
Where,
Min = cumulative mass into the stratum corneum (SC) during an exposure period (µg/kg×day)
A = area of skin exposed for each subject as shown in Table 1 (cm2)
C = the applied concentration of the chemical being examined in water (µg/L)
Kp = the steady-state permeability coefficient of the SC for the chemical within water (cm/hr) based on steady-state conditions for dermatomed skin
t = duration of an exposure (min/day)
Rsc/w = the equilibrium partition coefficient between the SC and the chemical being examined within water (0.71 log Kow)
Kow = octanol-water partition coefficient
L = the thickness of the stratum corneum (10 µm)
Wt = weight of the individual (kg)
Estimated monthly dermal doses were compared to estimated monthly ingestion doses in Monte Carlo simulations using Equation 3, adapted from Jo et al., (1990b):
| Equation 3 |
Where,
Ae = absorption efficiency of the gastrointestinal tract (assumed to be 100 % to represent a maximal possible dose)
C = water concentration (µg/L)
I = water ingested/day in Table 1 (L/day)
Wt = weight of the individual (kg)
Probability distributions from the US EPA's Exposure Factors Handbook were used as inputs to perform global sensitivity analysis of the exposures for different subpopulations (girls, boys, women, and men). Sensitivity analysis was performed using the Fourier Amplitude Sensitivity Test (FAST) using the Simlab toolbox (http://simlab.jrc.cec.eu.int/). For each combination of chemical and subpopulation combination, 20,000 samples were used to estimate the first order sensitivities.
RESULTS AND DISCUSSION
The stratum corneum is the skin’s primary barrier against the environment. Therefore, for our experiments, the dermis was removed when skin tissue was used for in vitro permeation studies. However, full thickness Kp values were also measured and compared with the Kp values of dermatomed skin. Whole skin studies are useful in situations where dermatoming or using other methods to separate the epidermis from the dermis is ineffective such as when course hair leaves holes in the epidermis when separated from the dermis.
Quantification of Kp and lag time
Six 12-hr steady-state experiments (3 dermatomed and 3 whole skin samples) were used to obtain Kp values and lag times for the HANs and CH. (Table 4). The Kp values for CAN, DCAN, BCAN and DBCN for dermatomed skin ranged from 0.099 to 0.167 cm/hr, and the value for whole skin ranged from 0.04 to 0.048 cm/hr. The Kp values for CH were 0.0039 cm/hr for dermatomed skin and 0.0017 cm/hr for whole skin. Only a range of Kp values could be determined for trichloroacetonitrile (TCAN), from 0.039 to 0.14 cm/hr using dermatomed skin and 0.0011 to 0.0072 cm/hr using whole skin. One caveat to applying these Kp values to calculating the dose from dermal contact is even though the procedures used are the accepted method for measuring skin permeability there may be a difference in the absolute permeability value between hydrated skin as used in the in-vitro experiments and non-hydrated skin that would result from baths and showers of typical durations. It is expected that rank order of absorption would be similar.
TABLE 4.
Permeability coefficients (cm/hr) and lag times of DBPs determined at steady-state conditions.
| Compounds | Dermatomed skin | Whole skin | ||
|---|---|---|---|---|
| Kp (cm/hr)a | Lag time (min)b | Kp (cm/hr)a | Lag time (min)a | |
| CAN | 0.0990± 0.00840 | 6.14± 0.500 | 0.0440± 0.000970 | 90.0± 18.6 |
| DCAN | 0.146± 0.00780 | 6.15± 0.470 | 0.0400± 0.00190 | 113.±25. |
| TCAN c | 0.0390–0.140 | 0.00110-0.00720 | ||
| BCAN | 0.155± 0.0100 | 6.43± 0.370 | 0.0440± 0.00370 | 129±30.6 |
| DBAN | 0.167± 0.0130 | 6.84± 0.450 | 0.0480± 0.00440 | 157.±43. |
| CH | 0.00390± 0.000470 | −1.72± 11.9 | 0.00170± 0.0000960 | 208.±52. |
Obtained from the slope of data from 1 to 12 hr with both diffusion cells at 37±1° C (n=3).
Obtained from the slope of data from 0.167 to 1 hr with both diffusion cells at 37±1° C (n=3).
The Kp for TCAN is reported as a range, and the lag times are not reported because of donor concentration changes (shown in Table 2) causing deviations in steady-state conditions.
The donor concentrations used to calculate Kp were the average of the values measured immediately before and after the steady-state exposures. Significant TCAN donor concentration losses of 45–82% were observed from the initial concentration of 0.47±0.06 g/L, measured at the start of the experiments (Table 5). The percent difference between the pre- and post-concentration levels in the donor cells for the remaining compounds were within expected experimental variations of ± 20% (Table 5).
TABLE 5.
Chemical properties and donor concentration changes for the six steady-state experiments (3 dermatomed and 3 whole skin samples).
| Chemical | Donor conc (g/L) |
% Difference of donor conc. between the start and end of experiment |
Boiling point a | Water solubility a | Log Kow |
|---|---|---|---|---|---|
| CAN | 0.79 ± 0.033 | −4.21 to 4.04 | 124-6 | 50–100 g/L at 21.5 °C | 0.45 b |
| DCAN | 0.77 ± 0.015 | −3.15 to 16.5 | 112-3 | 10–50 g/L at 21.5 °C | 1.34 c |
| TCAN | 0.47 ± 0.06 | 45.2 to 82.2 | 83–84 | <1 g/L at 21.5 °C | 2.09 b |
| BCAN | 0.79 ± 0.029 | −0.01 to 14.4 | 138 | Slightly soluble | 1.46 c |
| DBAN | 0.8 ± 0.066 | 1.71 to 16.1 | 67–69/24 mm | 5–10 g/L at 21.5 °C | 1.57 c |
| CH | 0.8 ± 0.036 | −4.67 to −0.686 | 97.5 | >=10 g/L at 20.5 °C | 0.99 b |
Values obtained on-line through http://www.chemfinder.com.
Experimental data from Hansch, et al., (1995)
By Crippen’s fragmentation: J. Chem. Inf. Comut. Sci., 27,21 (1987)
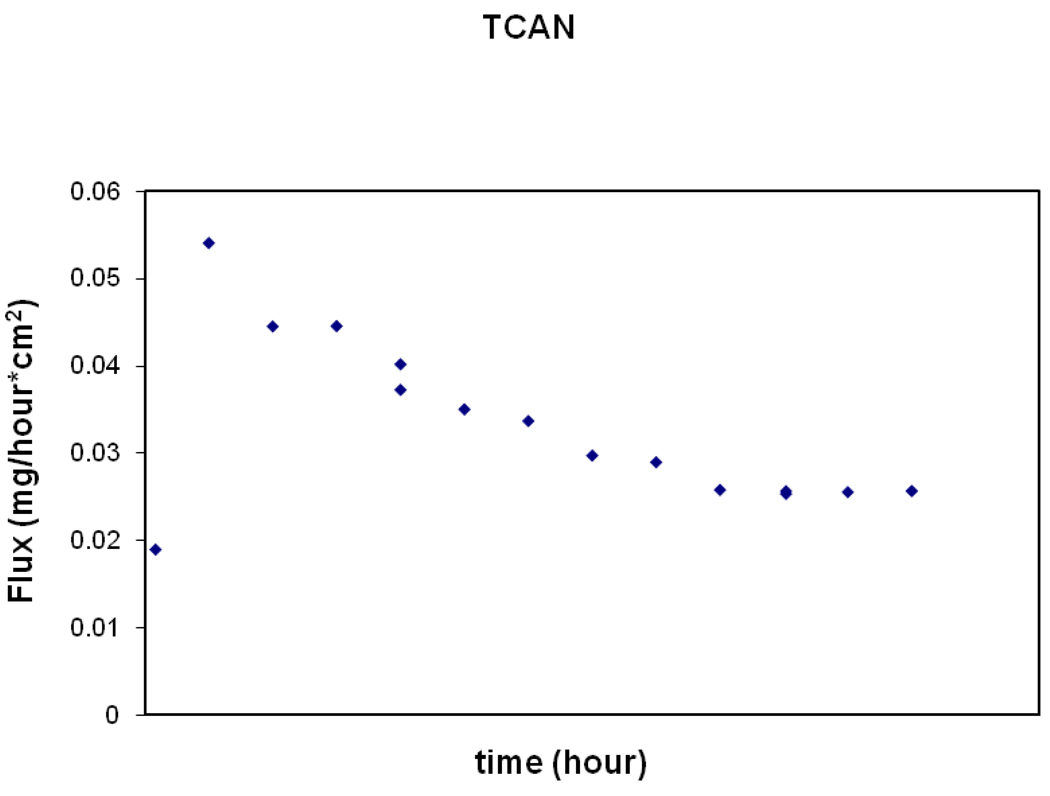
At steady-state conditions, both the flux and donor concentrations should remain constant to calculate Kp. If the donor concentration decreases, then a decreasing flux is expected. This is seen in Figure 1 for TCAN. Therefore, only a range of possible Kp values could be calculated using the initial and final donor concentrations (Table 4). A lag time could also not be determined for TCAN.
FIG. 1.
The decreasing flux over time through the skin for TCAN from a representative steady-state experiment using dermatomed skin.
The decreases in donor concentrations of TCAN is likely due to a degradation of TCAN over time as TCAN can be unstable at pH values outside of 4.5–5.5 and the donor solutions was maintained near neutral to slightly basic pH to reflect typical drinking and pool water conditions. Losses due to evaporation is unlikely since the same closed system was used for all HANs and no losses were observed for the other compounds
The minimum time for steady-state conditions to be reached has been suggested to be between 2.4 and 3 times the lag time (Crank, 1975). For the HANs for dermatomed skin, the mean lag times were between 6 and 7 min implying that the upper end estimate where steady-state can be reached is between approximately 14 min (2.4 × (6 min- the lag)) and 21 min (3 ×(7 min- the lag)). The mean lag time calculated for CH for the dermatomed skin was not different from zero, but the standard deviation was ±12 min suggesting that the maximum time required to reach steady-state for CH is no greater than 36 min. All compounds measured reached steady-state for the dermatomed skin within an hour. Therefore, the data points from 1 to 12 hours were used to calculate Kp. The permeation rate of whole skin is much lower than dermatomed skin. Thus it takes longer for the compounds to reach steady-state. The lag times were much longer in whole skin with the mean lag times ranging from 90 to 157 minutes for HANs and 208 minutes for CH. The time it takes to reach steady-state for HANs was between 216 and 471 minutes and 500 minutes for CH for whole skin.
When all the data points were used for steady-state linear regression of cumulative dose (mg/cm2) vs. time, slight variations near the end of the experiments can drastically change the calculated lag times. This is the probable reason why the percent standard deviations in the whole skin were near 25% rather than the less than 10% calculated for the dermatomed skin. Very high variability may also be common when the lag times are very short as with CH in dermatomed skin. Data points from 0.167 to 1 hour were used to calculate the lag times from the dermatomed steady-state experiments as opposed to the 1 to 12 hour data points used for whole skin.
The stratum corneum (only about 10 to 40 µm thick) represents the most efficient barrier against hydrophilic compounds and the viable epidermis (about 100 µm thick), and the dermis underneath (about 10 to 40 µm thick) provides a barrier to lipophilic compounds (U.S. EPA, 1992). It is therefore not surprising that whole skin with the epidermis and dermis intact has lower Kp values and longer lag time values for the moderately lipophilic compounds HANs and CH compared to Kp values from dermatomed skin (Table 4). This decrease in Kp is more noticeable in the more lipophilic haloacetonitriles than with chloral hydrate, which is the least lipophilic of the compounds studied. Most absorbed compounds are quickly taken up into the bloodstream through a capillary network above the dermis. Thus, using dermatomed skin, where the dermis layer was cut away, provides a better estimate of the dermal absorption in viable skin than using whole skin since in vivo compounds do not need to transverse the dermis to enter the bloodstream.
Simulated Internal Dose Estimates for Haloacetonitriles and Chloral Hydrate
The monthly mean, median and range of ingestion doses across gender and for adults and children for the HANs and for CH based on the Monte Carlo Simulations are given in Table 6. Since a definitive Kp could not determined for TCAN it was not included in the simulations. It was assumed that all of the consumed drinking water was unfiltered tap water to provide an estimate of the maximum ingestion dose. The variation across compounds is a function of the different drinking water concentrations typically found for each compound in the distribution system. The potential mean, median and range of dermal doses for HANs and CH from the subset of the population that takes baths over a monthly period is given in Table 7. The variation in dermal dose across compounds is a function of both the water concentration and the lipophilic property of each compound. These calculations have the caveat that the Kp values calculated using the hydrated skin are applicable to exposures encountered during showering, bathing and swimming when the skin may not become fully hydrated. A sensitivity analysis of the Monte Carlo indicated that the most important input variables were the same across gender and age and for all compounds, which could be a function of using the actual concentration in the water delivered rather than changes in water concentration during use which would vary with volatility of the compounds. For ingestion, the key input variables were drinking water concentration followed by ingestion rate with a minor contribution by body weight. For bathing, the key variables were duration of bath and drinking water concentration. For swimming, the key variable was duration of swim with lag-time through the skin also important for chloral hydrate. It is likely that pool water concentration was not included since few concentrations were available in pool water so the actual range used under-represented the true range and for chloral hydrate and dichloroacetonitrile were single values, thus those could not be evaluated in the sensitivity analyses.
TABLE 6.
Estimated population drinking water ingestion dose distributions/month (from Monte Carlo simulations).
| Compounds | Ingestion Doses/month (µg/kg × month) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girls a | Boys a | Women | Men | |||||||||
| Mean | Median | Range | Mean | Median | Range | Mean | Median | Range | Mean | Median | Range | |
| CAN | 0.06 | 0.03 | 0–1.2 | 0.06 | 0.03 | 0.00- 0.74 |
0.05 | 0.02 | 0.00- 0.81 |
0.06 | 0.03 | 0.00- 1.7 |
| DCAN | 1.3 | 0.57 | 0.01- 26 |
1.4 | 0.59 | 0.02- 28. |
1.2 | 0.55 | 0.01- 23 |
1.2 | 0.58 | 0.01- 16. |
| BCAN | 0.62 | 0.42 | 0.03- 5.5 |
0.64 | 0.45 | 0.03- 6.4 |
0.56 | 0.40 | 0.03- 5.6 |
0.62 | 0.42 | 0.02- 5.1 |
| DBAN | 0.47 | 0.28 | 0.01- 6.7 |
0.49 | 0.30 | 0.01- 5.4 |
0.42 | 0.25 | 0.01- 3.4 |
0.46 | 0.29 | 0.01- 6.5 |
| CH | 1.9 | 1.00 | 0.02- 25 |
2.0 | 1.1 | 0.03- 25 |
1.8 | 0.91 | 0.04- 57. |
1.9 | 0.97 | 0.02- 24. |
For children aged 1–19 years.
TABLE 7.
Estimated dermal dose distributions/month from bathing (from Monte Carlo simulations) and the ratio of dermal to ingestion dose.
| Compounds | Girls a | Boys a | ||||||
|---|---|---|---|---|---|---|---|---|
| µg/kg × month | Dermal/Ingestion dose |
µg/kg × month | Dermal/Ingestion dose |
|||||
| Mean | Median | Range | Mean | Median | Range | |||
| CAN | 0.028 | 0.013 | 0.00–0.35 | 0.46 | 0.028 | 0.013 | 0.00–0.50 | 0.46 |
| DCAN | 0.78 | 0.37 | 0.0077–11. | 0.62 | 0.80 | 0.38 | 0.0077–14. | 0.57 |
| BCAN | 0.42 | 0.30 | 0.010–3.1 | 0.67 | 0.42 | 0.29 | 0.010–3.0 | 0.67 |
| DBAN | 0.35 | 0.21 | 0.0029–2.9 | 0.78 | 0.36 | 0.22 | 0.0023–3.2 | 0.72 |
| CH | 0.037 | 0.021 | 0.00046–0.54 | 0.019 | 0.037 | 0.021 | 0.00–0.53 | 0.020 |
| Compounds | Womenb | Menb | ||||||
| µg/kg × month |
Dermal/Ingestion dose |
µg/kg × month |
Dermal/Ingestion dose |
|||||
| Mean | Median | Range | Mean | Median | Range | |||
| CAN | 0.024 | 0.013 | 0.00–0.45 | 0.49 | 0.023 | 0.011 | 0.00–0.32 | 0.39 |
| DCAN | 0.69 | 0.33 | 0.0077–16. | 0.60 | 0.66 | 0.31 | 0.0077–12. | 0.54 |
| BCAN | 0.37 | 0.27 | 0.0078–3.1 | 0.66 | 0.35 | 0.26 | 0.0078–2.1 | 0.57 |
| DBAN | 0.31 | 0.18 | 0.0029–2.9 | 0.74 | 0.30 | 0.18 | 0.0029–3.0 | 0.65 |
| CH | 0.032 | 0.019 | 0.00–0.44 | 0.018 | 0.031 | 0.017 | 0.00–0.49 | 0.016 |
children aged 1–19 years.
Adults from a typical population aged ≥20 years.
The dermal doses are 0.39 to 0.747 times the monthly ingestion doses for the HANs and 0.0160 to 0.0195 times the ingestion dose for CH. The additional dermal dose for the subset of the population that swims in pools over a monthly period from pool water is given in Table 8. Data on pool water concentration was available for only DCAN, DBAN and CH. The DCAN and DBAN mean pool derived dermal doses are 0.304 to 2.25 times the monthly ingestion doses and for CH 0.192 to 0.245 times the monthly ingestion dose. Since the dermal doses are a function of the drinking water concentrations and the Kp of each DBP, the higher water concentration of CH, when compared to the HANs, does not result in a high dermal dose for CH due to its low Kp value (0.0039 (cm/hour)).
TABLE 8.
Estimated dermal pool dose distributions/month (from Monte Carlo simulations) and the ratio of dermal to ingestion dose.
| Compounds | Girls a | Boys a | ||||||
|---|---|---|---|---|---|---|---|---|
| µg/kg × month | Dermal/ Ingestion dose |
µg/kg × month | Dermal/ Ingestion dose |
|||||
| Mean | Median | Range | Mean | Median | Range | |||
| DCAN | 2.8 | 0.90 | 0.0033–19 | 2.3 | 2.9 | 0.93 | 0.033–21. | 2.1 |
| DBAN | 0.18 | 0.063 | 0.0029–0.95 | 0.37 | 0.18 | 0.063 | 0.0029–1.2 | 0.36 |
| CH | 0.47 | 0.16 | 0.0036–4.4 | 0.25 | 0.47 | 0.16 | 0.0041–4.0 | 0.24 |
| Compounds | Womenb | Menb | ||||||
| µg/kg × month |
Dermal/ Ingestion dose |
µg/kg × month |
Dermal/ Ingestion dose |
|||||
| Mean | Median | Range | Mean | Median | Range | |||
| DCAN | 2.4 | 0.81 | 0.036–14. | 2.0 | 2.3 | 0.77 | 0.039–12. | 1.9 |
| DBAN | 0.15 | 0.052 | 0.0029–1.1 | 0.35 | 0.14 | 0.049 | 0.0029–1.1 | 0.30 |
| CH | 0.39 | 0.13 | 0.0059–5.2 | 0.22 | 0.37 | 0.13 | 0.0059–3.9 | 0.19 |
children aged 1–19 years.
Adults from a typical population aged >20 years.
The dermal bath and pool water exposures for the children were higher than that of the adults on a per weight basis. This is due to children having a higher surface area per kilogram body mass than adults. A smaller individual will have a higher dose of DBPs per kilogram. This is also the reason for higher dermal doses per body mass of women compared to men and higher doses per body mass for boys compared to girls since girls 5–11 years of age are on average larger than boys of the same age.
The dermal doses are a significant fraction of the ingestion doses for the HANs. For a population that routinely swims in chlorinated swimming pools, the dermal dose during swimming is an important contributor to the total dose of these DBPs because of the order of magnitude higher pool water concentrations compared to drinking water levels. For DCAN, the dermal dose while swimming is greater than the dose received from other dermal contributions or ingestion of chlorinated drinking water. Therefore, estimating dermal absorption of HANs and CH from water is important since dermal contact is a potentially significant route of exposure due to their high Kp values. In addition, Kp in vitro data is essential in estimating potential internal doses of in vivo dermal exposures.
TABLE 2.
Dermal bath and pool water exposure duration and frequency assumptions (lognormal distribution) used in Monte Carlo calculations of a typical population.
| Bathing Duration (minutes/bath) a | Swimming Pool Visit Duration (minutes/month) a |
|||||
|---|---|---|---|---|---|---|
| 50%c | 10% | 90% | 50%c | 10% | 90% | |
|
Typical population b |
20 | 10 | 45 | 60 | 15 | 180 |
| Bathing Frequency (baths/day)a |
Swimming Pool Visit Frequency (visits/month)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1c | 2 | 3 | 1 | 2 | 3 | 20 | 30 | range | |
|
Typical populationb |
70.7% | 22.2% | 3.08% | 22.5% | 14.4% | 11.2% | 3.83% | 3.98% | 1–60 exposures |
Applied to both adults and children.
A 20-min bath exposure and a 60-min pool duration and a 1 bath/day assumption was made to calculate dermal doses from typical exposures.
ACKNOWLEDGMENT
Supported by the United States Environmental Protection Agency (US EPA) Research Foundation (#GR825953-01-0) and NIH grants ES005022 and AR055073. This presentation has not been subjected to US EPA review and therefore may not necessarily reflect the views of the Agency.
Footnotes
The authors would also wish to thank Thomas M. Mariano from the Environmental and Occupational Health Sciences Institute, Department of Environmental and Occupational Medicine and UMDNJ- Robert Wood Johnson Medical School, Piscataway, New Jersey for his valuable help with handling radiolabeled compounds, Dr. Sastry Isukapalli for his assistance in conducting the Monte Carlo Sensitivity Analysis, and the National Disease Research Interchange (NDRI) in Philadelphia, PA for providing the skin samples used in this study.
REFERENCES
- Aggazzotti G, Fantuzzi G, Tartoni PL. Predieri G. Plasma chloroform concentrations in swimmers using indoor swimming pools. Archives of environmental health. 1990;45:175–179. doi: 10.1080/00039896.1990.9936712. [DOI] [PubMed] [Google Scholar]
- Baudisch C, Pansch G, Prösch J, Puchert W. Determination of volatile halogenated hydrocarbons in chlorinated swimming pool water. Research report. Auβenstelle Schwerin: Landeshygieneinstitut Mecklenburg-Vorpommern (in German); 1997. [Google Scholar]
- Bronaugh RL, Stewart RF, Simon M. Methods for in vitro percutaneous absorption studies VII. Use of excised human skin. J. Pharm. Sci. 1986b;75(11):1094–1097. doi: 10.1002/jps.2600751115. [DOI] [PubMed] [Google Scholar]
- Brown HS, Bishop DR, Rowan CA. The role of skin absorption as a route of exposure for volatile organic compounds (VOCs) in drinking water. Am. J. Publ. Health. 1984;74(5):479–484. doi: 10.2105/ajph.74.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RJ, Kopfler FC. Health effects of disinfection by-products. Denver, CO: AWWA research foundation and American water works association; 1991. [Google Scholar]
- Bull RJ, Meier JR, Robinson M, Ringhand HP, Laurie RD, Stober J. A. Evaluation of mutagenic and carcinogenic properties of brominated and chlorinated acetonitriles: by-products of chlorination. Fundamental and Applied Toxicology. 1985;5(6 Pt 1):1065–1074. doi: 10.1016/0272-0590(85)90142-3. [DOI] [PubMed] [Google Scholar]
- Bunge AL, Cleek RL. A new method for estimating dermal absorption from chemical exposure: 2. Effect of molecular weight and octanol-water partitioning. Pharmaceutical Research. 1995;12(1):88–95. doi: 10.1023/a:1016242821610. [DOI] [PubMed] [Google Scholar]
- Chemfinder Ultra 5.0 CD. CS ChemOffice-Drawing, modeling and information. Cambridge, MA: http://wwwcamsci.com. [Google Scholar]
- Crank J. The mathematics of diffusion. London: Oxford University Press; 1975. pp. 46–53. [Google Scholar]
- Daniel FB, Schenck KM, Mattox JK, Lin EL, Haas DL, Pereira MA. Genotoxic properties of haloacetonitriles: drinking water by-products of chlorine disinfection. Fundamental and Applied Toxicology. 1986;6(3):447–453. doi: 10.1016/0272-0590(86)90218-6. [DOI] [PubMed] [Google Scholar]
- DeLeer EWB, Sinninghe Damst JS, de Galan L. Formation of aryl-chlorinated aromatic acids and precursors for chloroform in chlorination of humic acid. In: Jolley RL, Bull RG, Davis WP, Katz S, Roberts MH jr, Jacobs VA, editors. Water chlorination: chemistry, environmental impact and health effects. Vol.5. Chelsea, MI: Lewis Publishers, Inc; 1985. pp. 843–857. [Google Scholar]
- Erdinger L, Kuhn KP, Kirsch F, Feldhues R, Frobel T, Nohynek B, Gabrio T. Pathways of trihalomethane uptake in swimming pools. International Journal of Hygiene & Environmental Health. 2004;207:571–575. doi: 10.1078/1438-4639-00329. [DOI] [PubMed] [Google Scholar]
- Ershow AG, Cantor KP. Total water and tapwater intake in the United States: population-based estimates of quantities and sources. Life Sciences Research Office, Federation of American Societies for Experimental Biology; 1989. [Google Scholar]
- Franz TJ, Lehman PA, Franz SF, North-Root H, Demetrulia JL, Kelling CK, Moloney SJ, Getting SD. Percutaneous penetration of N-Nitrisodiethanolamine through human skin (in vitro): Comparison of finite and infinite dose applications from cosmetic vehicles. Fundam. and Appl. Toxicol. 1993;21:213–221. doi: 10.1006/faat.1993.1091. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Wallace LA, Callahan PJ, Kenny DV, Brinkman MC. Effect of water temperature on dermal exposure to chloroform. Environ. Health Perspect. 1998;106(6):337–345. doi: 10.1289/ehp.98106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Barry BW, Dugard PH. Effects of freezing on human skin permeability. Journal of Pharmacy and Pharmacology. 1984;36:261–262. doi: 10.1111/j.2042-7158.1984.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Physical chemical analysis of percutaneous absorption process. Journal Soc. Cosmet. Chem. 1960;11:85–97. [Google Scholar]
- Kopfler FC, Ringhand HP, Coleman WE, Meier JR. Reactions of chlorine in drinking water, with humic acids and in vivo. In: Bull RG, Davis WP, Katz S, Roberts MH Jr, Jacobs VA, editors. Water chlorination: chemistry, environmental impacts and health effects. Vol. 5. Chelsea, MI: Lewis publishers, Inc; 1985. pp. 161–173. Jolley, R.1. [Google Scholar]
- Krasner SW, McGuire MJ, Jacangelo JG, Patania NL, Reagen KM, Aieta EM. The occurrence of disinfection by-products in US drinking water. Journal of the American Water Works Association. 1989;81(8):41–53. [Google Scholar]
- Jo WK, Weisel CP, Lioy PJ. Routes of chloroform exposure and body burden from showering with chlorinated tap water. Risk Anal. 1990a;10(4):575–580. doi: 10.1111/j.1539-6924.1990.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Jo WK, Weisel CP, Lioy PJ. Chloroform exposure and the health risk associated with multiple uses of chlorinated tap water. Risk Anal. 1990b;10(4):581–585. doi: 10.1111/j.1539-6924.1990.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Lin EL, Daniel FB, Herren-Freund SL, Pereira MA. Haloacetonitriles: metabolism, genotoxicity, and tumor-initiating activity. Environmental Health Perspectives. 1986;69:67–71. doi: 10.1289/ehp.866967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Pleil JD, Berkoff DC. Alveolar breath sampling and analysis to assess trihalomethane exposure during competitive swimming training. Environmental Health Perspectives. 1997;105:636–642. doi: 10.1289/ehp.97105636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss GP, Dearden JC, Patel H, Cronin MTD. Quantitative structure-permeability relationships (QSPRs) for percutaneous absorption. Toxicology in Vitro. 2002;16(3):299–317. doi: 10.1016/s0887-2333(02)00003-6. [DOI] [PubMed] [Google Scholar]
- Nouraldeen AM, Ahmed AE. Studies on the mechanisms of haloacetonitrile-induced genotoxicity IV: In vitro interaction of haloacetonitriles with DNA. Toxicology in Vitro. 1996;10(1):17–26. doi: 10.1016/0887-2333(95)00100-x. [DOI] [PubMed] [Google Scholar]
- Potts RO, Guy RH. Predicting skin permeability. Pharmaceutical Research. 1992;9(5):663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- Poet TS, McDougal JN. Skin absorption and human risk assessment. Chemico-Biological Interactions. 2002;140(1):19–34. doi: 10.1016/s0009-2797(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Shah JC. Analysis of percutaneous permeation data: II evaluation of lag time method. Int. J. Pharm. 1994;109:283–290. [Google Scholar]
- Smith MK, Zenick H, George EL. Reproductive toxicology of disinfection by-products. Environmental Health Perspectives. 1986;69:177–182. doi: 10.1289/ehp.8669177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MK, Randall JL, Stober JA, Read EJ. Developmental toxicity of dichloroacetonitrile: a by-product of drinking water disinfection. Fundamental and Applied Toxicology. 1989;12(4):765–772. doi: 10.1016/0272-0590(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Stevens AA, Moore L, Dressman RC, Seeger DR. Disinfection chemistry in drinking water-overview of impacts on drinking water quality. In: Rice RG, editor. safe drinking water: the impacts of chemicals of a limited resources. Chelsea, MI: Lewis Publishers, inc; 1985. pp. 87–108. [Google Scholar]
- Tsang AM, Klepeis NE. Results tables from a detailed analysis of the National Human Activity Pattern Survey (NHAPS) response. Draft Report prepared for the U.S. Environmental Protection Agency by Lockheed Martin; 1996. Contract No. 68-W6-001, Delivery order No.13. [Google Scholar]
- US EPA. Dermal exposure assessment: principles and applications. Washington, DC: Office of Research and Development, Office of Health and Environmental Assessment; 1992. EPA/600/8-91/011B. [Google Scholar]
- US EPA. Method 551.1. Determination of chlorination disinfection byproducts, chlorinated solvents, and halogenated pesticides/herbicides in drinking water by liquid-liquid extraction and gas chromatography with electron-capture detection. Revision 1.0. Cincinnati, OH: National Exposure Research Laboratory, Office of Research and Development; 1995. [Google Scholar]
- Valencia R, Mason JM, Woodruff RC, Zimmering S. Chemical mutagenesis testing in Drosophila. III. Results of 48 coded compounds tested for the National Toxicology Program. Environmental Mutagenesis. 1985;7(3):325–348. doi: 10.1002/em.2860070309. [DOI] [PubMed] [Google Scholar]
- Weisel CP, Jo WK. Ingestion, inhalation and dermal exposure to chloroform and trichloroethene from tap water. Environmental Health Perspectives. 1996;104(1):48–51. doi: 10.1289/ehp.9610448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel CP, Jo WK, Lioy PJ. Utilization of Breath Analysis for Exposure and Dose Estimates of Chloroform. Journal of Exposure Analysis and Environmental Epidemiology. 1992;l(Suppl.):55–69. [Google Scholar]
- World Health Organization. Chapter 4. chemical hazards. Guidelines for safe recreational-water environments. 2000;Vol.2 swimming pools, spas and similar recreational-water environments. http://www.who.int/water_sanitation_health/Recreational_water/ recreall_ch4.pdf. [Google Scholar]