Abstract
Background
Cardiovascular disease (CVD) and cognitive impairment are common in dialysis patients. Given the proposed role of microvascular disease on cognitive function, particularly cognitive domains that incorporate executive functions, we hypothesized that prevalent systemic CVD would be associated with worse cognitive performance in hemodialysis patients.
Design
Cross-sectional cohort
Setting and Participants
200 maintenance hemodialysis patients without prior stroke from 5 Boston-area hemodialysis units
Predictor
CVD, defined by history of coronary disease or peripheral vascular disease
Outcome
Performance on a detailed neurocognitive battery. Primary analyses quantified cognitive performance using principal components analysis to reduce cognitive tests to a processing speed/executive function domain and a memory domain. Multivariable linear regression models adjusted for age, sex, education, race and other clinical and demographic characteristics.
Results
Mean (SD) age of participants was 62 (18) years and 75 (38%) had CVD. Individuals with CVD were older, more likely to be men, diabetic, and current or former smokers. In adjusted models, individuals with CVD performed 0.50 standard deviations worse (p<0.001) on tests assessing processing speed/executive function, while there was no difference in performance on tests of memory. Similar results were seen when assessing individual tests, with performance on the block design, digit symbol coding and Trail Making Tests A and B significantly associated with CVD in age, sex, education and race-adjusted analyses and approaching toward significance in fully adjusted models.
Limitations
CVD ascertainment dependent on patient recall and dialysis unit documentation. No brain imaging.
Conclusions
The presence of CVD is associated with worse cognitive performance on tests of processing speed and executive functioning in hemodialysis patients and identifies a high risk population for greater difficulty with complex tasks.
Introduction
Cardiovascular disease (CVD) and cognitive impairment are both prevalent in patients with chronic kidney disease. Cardiovascular disease is the leading cause of death among dialysis patients, with 10 to 50-fold higher rates of CVD mortality at any given age.1 While this increase in mortality may represent more rapid development and progression of CVD in dialysis patients, this increased risk also is a manifestation of the increased incidence and prevalence of CVD among people with chronic kidney disease not yet on dialysis.2 Similarly, cognitive impairment, ranging from mild impairment to overt dementia, is common in individuals with all stages of kidney disease3–11 and may be most prominent in those with concurrent vascular disease.12 Mild cognitive impairment may affect as many as 64% of dialysis patients,13–17 and is an independent risk factor for mortality in this population.18
There are multiple potential causes of cognitive impairment in dialysis patients, ranging from pathologies common in the general population, such as Alzheimer's disease and vascular disease, to conditions specific to dialysis, such as metabolic derangements and aluminum toxicity. Given the lack of an association between cognition and dialysis adequacy in our population,19 and the finding that cognitive performance appears similar just before dialysis versus on an intra-dialytic day,20 it is less likely that cognitive functioning is due primarily to `uremia' per se. We propose that cognitive impairment in dialysis patients may be a manifestation of small vessel cerebrovascular disease that is prevalent in individuals during all stages of CKD.3,10,21–23 In support of this statement are data from the general population demonstrating that cognitive impairment secondary to cerebrovascular disease may be a chronic process resulting from a cardiovascular disease milieu24–25 leading to multiple subclinical vascular insults26 that initially manifest as subtle cognitive impairment, particularly in executive function domains.27 Executive functions broadly encompass processes responsible for planning, abstract thinking and cognitive flexibility, all of which are of critical importance for individuals managing a complex chronic illness.
In the current study, we examine the relationship between clinically recognized CVD and cognitive function in hemodialysis patients. Given the high prevalence of vascular disease in hemodialysis patients, the interrelationship between large and small vessel vascular disease, and the proposed role of microvascular disease on cognitive function, particularly cognitive domains that incorporate attention, processing and executive functions, we hypothesized that prevalent systemic cardiovascular disease would be associated with worse cognitive performance in these domains.
Methods
Participants
Patients receiving chronic in-center hemodialysis at 5 Dialysis Clinic Inc. (DCI) units in the greater Boston area were evaluated for the Cognition and Dialysis Study. Reflecting the nature of the cognitive battery, eligibility criteria included English fluency as well as sufficient visual and hearing acuity to complete cognitive testing. To minimize cognitive testing floor effects, those individuals with Mini-Mental State Exam (MMSE) ≤10 were excluded from the study; other exclusion criteria were advanced dementia based on provider history, confusion, non-access related hospitalization within 1 month, receipt of hemodialysis for less than 1 month, and single pool Kt/V <1.0. Demographic data were obtained through participant report, medical charts, and the DCI database. Patients were queried about personal history of myocardial infarction and coronary revascularization (which were used to define coronary disease) and intermittent claudication and peripheral vascular disease (which were used to define peripheral vascular disease). Additionally, DCI electronic medical records and paper records were reviewed for a history of these conditions with specific focus on problem lists, hospital discharge summaries, cardiac testing results and procedure results. CVD was defined as history of either coronary disease or peripheral vascular disease. Similarly, stroke was defined by patient history or documentation in the patient's electronic or paper chart. Transient ischemic attack was not ascertained. The Tufts Institutional Review Board approved the study and all participants signed informed consent and research authorization forms.
Neuropsychiatric Assessment
Subjects were administered a battery of cognitive tests by research assistants following training and direct observation by the study neuropsychologist (Dr. Scott); to maintain quality and inter-rater reliability, testing was observed by the study neuropsychologist at 3–6 month intervals. To limit subject fatigue, all testing was completed during the 1st hour of hemodialysis. The neuropsychological battery included well validated and commonly used cognitive tests that possess high inter- and intra-rater reliability and have established age, gender, and education-matched normative scores. Tests performed included the MMSE,28 the Wechsler Memory Scale-III (WMS-III) Word List Learning Subtest,29 the Wechsler Adult Intelligence Scale-III (WAIS-III) Block Design and Digit Symbol-Coding Subtests,29 and Trail Making Tests A and B (Table 1).30 The overall battery assesses a broad range of functioning including global ability, supraspan learning, auditory retention, visual retention, attention/mental processing speed, visual construction/fluid reasoning, and motor speed.
Table 1.
Components of the neuropsychiatric battery and orthogonal rotated component pattern for principal components score generation
| Function Assessed | Cognitive Test/Subtest | Scoring | Test Description | Rotated Executive Score Loading | Rotated Memory Score Loading |
|---|---|---|---|---|---|
| Cognitive Screen | Mini-Mental State Exam | Number Correct | Thirty-point questionnaire sampling basic abilities; memory-focused | N/a | N/a |
| Supraspan Learning, Working Memory & Word Recall | Immediate Recall (Trial 1 only) | Number Correct | Subjects asked to recall list of 12 words immediately after hearing | 0.300 | 0.701 |
| Immediate Recall (Trials 1 to 4) | Sum of Correct in Trials 1 to 4 | Trial 1 word list repeated four times, with subject asked to recall after each repetition | 0.166 | 0.870 | |
| Short Delayed Recall | Number Correct | Subjects asked to recall list of 12 words after an interference task | 0.241 | 0.858 | |
| Delayed Recall | Number Correct | Subjects asked to recall list of 12 words after 25 to 35 minute delay | 0.243 | 0.841 | |
| Delayed Recognition | Number Correct | Subjects asked to recognize the 12 words previously presented from list of 24 words | 0.321 | 0.624 | |
| Construction & Fluid Reasoning | Block Design | Number completed | Subjects are required to reproduce depicted patterns using a set of colored blocks | 0.755 | 0.231 |
| Attention, Processing Speed, & Executive Function | Digit Symbol-Coding | Number of copied symbols in 2 min | Decoding symbols matching it to a digit provided in an answer key | 0.712 | 0.462 |
| Trail Making Test A | Time to Completion | “Connect-the-dots” for a consecutive number sequence from 1 to 25 | −0.852 | −0.099 | |
| Trail Making Test B | Time to Completion | “Connect-the-dots” alternating between numbers (1 to 13) and letters (A to L) | −0.737 | −0.419 |
Score loadings represent the correlations between the subtests and the two retained scores following orthogonal rotation.
Statistical Analysis
All analyses were performed using SAS, version 9.1 (SAS Institute, http://www.sas.com) and all hypothesis tests were 2-sided. Baseline characteristics of eligible dialysis patients who consented and did not consent to participate were compared using chi-square tests, t-tests and ANOVA as appropriate. Similarly, baseline characteristics of participants with and without CVD were also compared.
Primary Outcomes based on Principal Component Analysis
For 14 individuals with missing scores on one cognitive test (up to two scores if derived from the same test), multiple imputation was performed, incorporating linear regression models based on the results of the available cognitive tests; these results were incorporated into the data for principal components analysis.31 As it is likely that within patient covariance in cognitive test performance would not substantially differ by stroke status and to facilitate secondary analyses that include stroke patients, all patients were included in the generation of principal component scores. We confirmed this assumption by generating a second set of principal component scores in the subpopulation without stroke, finding the results nearly identical. Principal component analysis (PCA) with varimax rotation was used as a data reduction technique to derive composite scores for separate cognitive domains.32–33 Two principal components with eigenvalues greater than 2 were obtained, and the resulting component scores were subsequently used for primary statistical analyses. Using this methodology, all component scores have a mean of 0 and standard deviation of 1.33 The first component was interpreted to reflect executive functioning, attention and processing speed (referred to as executive function within the results section), with Trails A and B, Block Design, and Digit Symbol-Coding tests contributing significantly; the second component was primarily comprised of Word List Learning Recall and Recognition and was interpreted to reflect memory (Table 1). After exclusion of individuals with a history of known stroke, simple linear regression was used to assess the relationship between CVD and the principal components. Parsimonious models adjusted for age, sex, race, and education, while fully adjusted models further accounted for any variables with a p-value <0.20 in the parsimonious models, with the exception that, in cases with high collinearity among covariates (history of hypertension with systolic and diastolic blood pressure and history of diabetes with primary cause kidney disease), the variable most significant in extended models would be retained. This results in different covariates in each fully adjusted model.
Outcomes based on Individual Tests
For cognitive tests where population norms have been developed, raw scores were transformed into age-appropriate scaled scores,29 and, for Trails A and B, age, education and sex appropriate scaled T-scores.30 The scaled scores then were compared by baseline CVD status; however, raw scores were used for the PCA. Secondary analyses examined the individual elements of these components, using linear regression to evaluate the association between known cardiovascular disease history and specific tests; in these analyses, the raw scores on individual tests defined cognitive outcomes. Analyses performed with Trails B performance as an outcome used Tobit regression to censor for failure to complete the task within the allotted 5 minutes.34 In sensitivity analyses, individuals with stroke were included in the cohort and history of stroke was adjusted for in all models. Given prior findings in this cohort, we performed additional analyses adjusting for symptoms of depression, defined by a score on the Center for Epidemiologic Studies Depression Scale (CES-D) above 15.35 Finally, we performed additional analyses excluding individuals who may have been using a dialysis access arm to perform cognitive testing. In all analyses, non-linear relationships were assessed through testing risk factors quartiles and squared terms; non-linear relationships were not noted.
Results
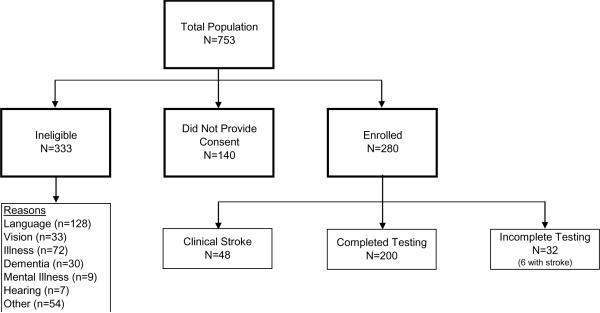
Of 753 hemodialysis patients screened, 420 were eligible for the study. There were 280 (66.7%) patients who consented and participated in cognitive testing, 248 of whom completed cognitive testing (Figure 1). Eligible patients not enrolled were similar to those enrolled across characteristics, including age, race, sex, primary cause of ESRD, history of CVD, except for slightly lower serum albumin among those who did not enroll (Table S1, available as online supplementary material). Individuals not included in principal components analysis due to incomplete cognitive testing were older (68.2 ± 13.6 versus 62.8 ± 17.1 years, p=0.09) but had a similar prevalence of comorbid conditions, including CVD (37.5% versus 41.1%, p=0.85), to those with complete or limited missing data. Following exclusion of 48 individuals with known stroke, 200 participants were evaluated in primary analyses, 75 (37.5%) of whom had a history of CVD, 62 (82.7%) of whom had known coronary disease and 41 (54.7%) of whom had known peripheral vascular disease. Those with CVD were significantly older, more likely to be men and have diabetes, and had significantly lower diastolic blood pressure, serum albumin and serum phosphorus (Table 2).
Figure 1.
Derivation of the study cohort. Incomplete testing indicates those who did not complete 2 or more tests used for generation of the principal components.
Table 2.
Characteristics of participants with and without history of cardiovascular disease.
| Total N=200 | Non CVD N=125 62.5% | CVD N=75 37.5% | P-value | |
|---|---|---|---|---|
| Age (years) | 61.5 ± 17.7 | 56.4 ± 18.3 | 69.9 ± 12.9 | <0.001 |
| Female (%) | 47.0 | 54.4 | 34.7 | 0.007 |
| African American (%) | 23.5 | 27.2 | 17.3 | 0.08 |
| Education (%) | ||||
| <12th grade | 9.0 | 6.4 | 13.3 | 0.05 |
| High school graduate | 59.5 | 58.4 | 61.3 | |
| 2+ Years college | 31.5 | 35.2 | 25.3 | |
| Medical History (%) | ||||
| Peripheral Vascular Disease | 20.5 | --- | 54.7 | --- |
| Coronary Artery Disease | 31.0 | --- | 82.7 | --- |
| Hypertension | 88.0 | 84.0 | 94.7 | 0.03 |
| Stroke | --- | --- | --- | --- |
| Diabetes | 46.5 | 36.8 | 62.7 | <0.001 |
| Heart Failure | 32.0 | 20.8 | 50.7 | <0.001 |
| Primary cause of ESRD (%) | ||||
| Diabetes | 35.5 | 24.8 | 53.3 | <0.001 |
| Glomerulonephritis | 19.5 | 23.2 | 13.3 | |
| Hypertension | 18.0 | 17.6 | 18.7 | |
| Other | 20.5 | 28.0 | 8.0 | |
| Unknown | 6.5 | 6.4 | 6.7 | |
| Smoking History (%) | ||||
| Never | 40.5 | 49.2 | 25.7 | 0.006 |
| Past | 52.1 | 45.0 | 64.3 | |
| Current | 7.4 | 5.8 | 10.0 | |
| Vascular Access (%) | ||||
| Fistula | 60.0 | 63.2 | 54.7 | 0.3 |
| Graft | 5.5 | 4.0 | 8.0 | |
| Catheter | 34.5 | 32.8 | 37.3 | |
| Systolic BP (mm Hg) | 143.4 ± 20.5 | 144.3 ± 20.6 | 141.9 ± 20.6 | 0.4 |
| Diastolic BP (mm Hg) | 74.4 ± 12.3 | 76.7 ± 12.6 | 70.6 ± 10.8 | <0.001 |
| Body Mass Index (kg/m2) | 28.0 ± 6.6 | 28.0 ± 6.9 | 27.9 ± 6.0 | 0.9 |
| Hematocrit (%) | 35.8 ± 3.3 | 35.6 ± 3.4 | 36.2 ± 3.2 | 0.2 |
| Serum Albumin (g/dL) | 3.8 ± 0.4 | 3.9 ± 0.3 | 3.8 ± 0.4 | 0.04 |
| Phosphorus (mg/dL) | 5.4 ± 1.5 | 5.6 ± 1.5 | 5.2± 1.4 | 0.04 |
| Dialysis Vintage (months) | 13.5 (6.4, 33.3) | 13.2 (5.5,29.7) | 14.5 (9.4,36.4) | 0.3 |
| PTH (pg/ml) | 285 ± 218 | 292 ± 230 | 274 ± 197 | 0.6 |
| spKt/V | 1.54 ± 0.27 | 1.53 ± 0.29 | 1.54 ± 0.22 | 0.9 |
Continuous data are mean ± standard deviation except vintage which is median (25th percentile, 75th percentile). CVD, cardiovascular disease; ESRD, End-Stage Renal Disease; BP, blood pressure; PTH, parathyroid hormone. To convert albumin to g/L, multiply by 10; to convert phosphorus to mmol/L multiply by 0.3229; and to convert PTH to ng/L multiply by 1.
The results of cognitive testing are presented in Table 3 and Table 4. Despite similar performance on the MMSE, a frequently performed screening test that may be of limited utility, particularly in vascular dementia, individuals with CVD performed significantly worse on tests assessing executive function, attention and speed (executive score) in univariate, parsimonious analyses adjusting for age, sex, race and education and extended analyses. In the extended models, individuals with CVD performed 0.50 standard deviations worse than those without CVD on these tests (p<0.001). For tests assessing memory and working memory (memory score), there was no difference by CVD status after parsimonious adjustment (p=0.58). When evaluating individual test performance, individuals with CVD had significantly lower standardized scores than those without CVD on cognitive tests that loaded onto the executive function component (Table 3). In univariate and parsimonious linear regression models, participants with CVD performed significantly worse on Block Design, Digit-Symbol Coding, and Trail Making Tests A and B (Table 4). Extended models demonstrated similar albeit attenuated findings, such that CVD was associated with significantly poorer performance on Block Design and trended to worse performance on Digit-Symbol Coding, Trails A and Trails B (Table 4).
Table 3.
Standardized scores on cognitive tests by CVD status.
| Cognitive Test | General Population Norm | Study Population N=200 | No CVD N=125 | CVD N=75 | p-value |
|---|---|---|---|---|---|
| Immediate Recall (Total) | 10 ± 3 | 7.9 ± 3.4 | 8.1 ± 3.1 | 7.8 ± 3.8 | 0.6 |
| Immediate Recall (Trial 1) | 10 ± 3 | 8.4 ± 3.2 | 8.3 ± 2.9 | 8.6 ± 3.6 | 0.7 |
| Delayed Recall | 10 ± 3 | 10.7 ± 2.6 | 10.5 ± 2.4 | 11.0 ± 3.0 | 0.3 |
| Recognition | 10 ± 3 | 9.6 ± 3.0 | 9.9 ± 2.9 | 9.2 ± 3.2 | 0.1 |
| Block Design | 10 ± 3 | 8.8 ± 2.7 | 9.2 ± 2.6 | 8.0 ± 2.7 | <0.001 |
| Digit Symbol Coding | 10 ± 3 | 7.0 ± 2.6 | 7.4 ± 2.7 | 6.3 ± 2.2 | 0.003 |
| Trails A | 50 ± 10 | 38.7 ± 9.1 | 40.2 ± 9.7 | 36.3 ± 7.6 | 0.002 |
| Trails B | 50 ± 10 | 37.9 ± 11.3 | 39.3 ± 12.1 | 35.6 ± 9.6 | 0.02 |
All tests except Trails A and B are standardized for age and reported as scaled scores centered at 10. Trails A and Trails B report T-scores standardized for age, sex and education and are centered at 50. Higher scores are consistent with better performance on all tests. P-values are for comparisons between those with and those without CVD Table 4. Test scores stratified by CVD status and the results of linear regression analyses showing the relationship of CVD status with cognitive testing results in 200 patients without stroke
Table 4.
Test scores stratified by CVD status and the results of linear regression analyses showing the relationship of CVD status with cognitive testing results in 200 patients without stroke
| Cognitive Test/Component | Test Scores | Univariate | Parsimonious | Extended | |||||
|---|---|---|---|---|---|---|---|---|---|
| No CVD | CVD | Beta (95% CI) | p-value | Beta (95% CI) | p-value | Beta (95% CI) | p-value | ||
| Executive Score* | 0.32 ± 0.92 | −0.42 ± 0.85 | −0.80 (−1.05, −0.55) | <0.001 | −0.52 (−0.77,−0.27) | <0.001 | −0.50 (−0.75, −0.25) | <0.001 | |
| Memory Score** | 0.15 ± 1.0 | −0.09 ± 1.07 | −0.24 (−1.04, 0.56) | 0.1 | 0.38 (−0.46, 1.22) | 0.3 | 0.34 (−0.48, 1.16) | 0.4 | |
| Mini-Mental State Exama | 26.9 ± 2.7 | 26.5 ± 3.0 | −0.4 (−1.2, 0.4) | 0.4 | 0.4 (−0.5, 1.2) | 0.4 | 0.3 (−0.5, 1.2) | 0.4 | |
| Immediate Recall (Total)b | 26.0 ± 7.3 | 22.9 ± 8.0 | −3.1 (−5.3, −0.9) | 0.006 | 1.2 (1.0, 1.4) | 0.2 | 1.0 (−1.0, 3.0) | 0.4 | |
| Immediate Recall (Trial 1)c | 4.1 ± 1.5 | 3.8 ± 1.8 | −0.3 (−0.8, 0.2) | 0.2 | 0.4 (−0.1, 0.9) | 0.09 | 0.5 (0.0, 0.9) | 0.06 | |
| Short Delayed Recalld | 5.4 ± 2.9 | 4.3 ± 3.2 | −1.2 (−2.0, −0.3) | 0.009 | 0.2 (−0.7, 1.0) | 0.7 | 0.2 (−0.6, 1.1) | 0.6 | |
| Delayed Recalle | 5.0 ± 2.8 | 4.1 ± 2.9 | −0.9 (−1.7, −0.1) | 0.03 | 0.3 (−0.5, 1.1) | 0.5 | 0.3 (−0.5, 1.0) | 0.5 | |
| Recognitionf | 21.6 ± 2.6 | 20.3 ± 3.3 | −1.2 (−2.1, −0.4) | 0.004 | −0.2 (−1.1, 0.6) | 0.6 | −0.3 (−1.1, 0.6) | 0.5 | |
| Block Designg | 30.1 ± 11.2 | 22.1 ± 9.1 | −8.0 (−10.9, −5.1) | <0.001 | −4.8 (−7.5, −2.1) | 0.001 | −4.7 (−7.4, −2.0) | 0.001 | |
| Digit Symbol Coding* | 47.0 ± 18.5 | 32.9 ± 12.2 | −14.1 (−18.8, −9.4) | <0.001 | −5.0 (−9.1, −0.9) | 0.02 | −4.1 (−8.4, 0.2) | 0.07 | |
| Trails Ah | 49.5 ± 26.6 | 70.2 ± 40.1 | 20.8 (11.6, 30.0) | <0.001 | 10.0 (0.4, 19.6) | 0.04 | 8.4 (−1.2, 18.0) | 0.09 | |
| Trails Bi | Completion | 122.5 ± 62.7 | 164.0 ± 61.9 | 70.2 (40.3, 100.0) | <0.001 | 30.5 (2.3, 58.6) | 0.03 | 26.4 (−2.1, 54.9) | 0.07 |
| % Non-Completion | 15.2% | 30.7% | |||||||
Results are mean ± standard deviation. Individual test results represent number correct except Trails A and B which are reported in seconds required to complete the task. Component scores by definition have a mean of 0 and standard deviation of 1 for the population. Negative coefficients indicate that CVD is associated with poorer performance on all tests except Trails A and B. Parsimonious models adjust for age, education, sex and race, while extended models additionally adjust for
cause of ESRD and BMI;
diabetes, BMI, diastolic blood pressure, Kt/V, phosphorus and vascular access;
cause of ESRD, BMI, Kt/V, and phosphorus;
diabetes, BMI, diastolic blood pressure, and Kt/V;
BMI, systolic blood pressure, PTH and Kt/V;
BMI, systolic blood pressure, Kt/V, and phosphorus;
BMI, diastolic blood pressure, Kt/V, and phosphorus;
diabetes, BMI, diastolic blood pressure, and Kt/V;
BMI;
Vintage and Kt/V; and
Cause of ESRD, BMI and Kt/V.
The 48 individuals with a history of stroke were similar to those without stroke with the exception of a higher prevalence of CVD (56.3% versus 37.5%, p=0.02) (Table S1). In secondary analyses that included individuals with stroke, the association between CVD and cognitive performance was attenuated, although there was a significant difference in performance on the composite executive score (p=0.008, Table S2). Adjustment for symptoms of depression did not affect the relationship between CVD and cognitive performance (data not shown). Analyses excluding 25 participants where handedness was uncertain and 14 participants where they used their access arm during dialysis for cognitive tests revealed a robust analysis between CVD and performance on cognitive tests assessing executive functioning (β=−0.52, p=0.0004 in extended models).
Discussion
In chronic hemodialysis patients, we demonstrate that, despite similar performance on the MMSE, a history of cardiovascular disease in the absence of known stroke is independently associated with worse function on more detailed tests of cognition, particularly those assessing attention, processing speed and executive functions. As these cognitive domains are considered more consistent with subcortical brain pathology and are more closely linked with cardiovascular disease risk factors,36–37 worse performance in these domains may indicate underlying cerebrovascular disease in the absence of previous clinically recognized stroke.38–39
Dialysis patients are at high risk for both clinically recognized strokes and for subclinical cerebrovascular disease. Compared to the general population, there is a 3- to 9-times greater risk for hospitalization due to hemorrhagic and ischemic stroke among dialysis patients after adjustment for comorbidity.40 Subclinical cerebrovascular disease is also common in all stages of CKD and includes both previously unrecognized infarcts on cranial imaging that were not associated with a clinical syndrome consistent with an acute stroke23,41 as well as diffuse white matter changes and subcortical small-vessel disease.10,17,22–23,42 In the general population, the presence of silent infarcts predicts a range of long-term adverse outcomes including incident dementia, clinically evident strokes, and decline in physical and cognitive function;43–44 similarly, subcortical white matter abnormalities are associated with both cardiovascular disease risk factors and poorer performance on tests of processing speed and executive function.45 There are few data to date exploring the relationship between findings on brain imaging and cognitive function in dialysis patients. However, given the relationship in non-dialysis patients, we proposed that the presence of CVD in hemodialysis patients places them at an increased risk of subclinical infarcts and white matter disease that may predispose these individuals to cognitive impairment.46
Several recent studies have explored cognitive function and cognitive impairment in dialysis patients. Murray et al, examining a prevalent cohort of 338 hemodialysis patients, describe a high prevalence of cognitive impairment in maintenance hemodialysis patients as well as an association between prior stroke and cognitive impairment; however, they did not explore the relationship between prevalent CVD and cognitive performance.13 Similarly a recent evaluation of 383 participants in the Frequent Hemodialysis Network (FHN) Trials, comprised of a relatively healthy dialysis population, used the modified MMSE and Trails B, predominantly administered before a dialysis session, to assess cognitive performance in dialysis patients. In this study, there was a significant association between a history of stroke and failure to complete Trails B within 300 seconds.47 Systemic CVD was not reported. Similar to our study, both Murray et al and the FHN note more prominent impairment in tests assessing executive performance than global cognitive function and memory. The current study expands on these reports by excluding patients with stroke, and specifically evaluating the relationship between prevalent CVD and multiple cognitive domains in dialysis patients, suggesting that unrecognized cerebrovascular disease, which may accompany vascular disease affecting other systems, may mediate this relationship.
There are several limitations to our study. First, the cross-sectional design is unsuited for determining causality or the temporal relationship between cardiovascular disease and cognitive function. Additionally, there may be a survivor bias that obscures the importance of duration of kidney failure. A second important limitation was the lack of a gold standard for identifying the presence of cardiovascular disease, with reliance on patient history and chart abstraction. It is likely that incomplete ascertainment of CVD would bias to the null hypothesis, although incomplete ascertainment of stroke may bias in the opposite direction. Third, in the absence of imaging, we lack data on the presence of subclinical stroke or white matter hyperintensities. Future studies that incorporate neuroimaging will provide greater insight towards the underlying etiology of cognitive impairment in dialysis patients. Fourth, we performed cognitive testing during the dialysis procedure. While it is possible that this may affect performance on cognitive tests,20 most patient education and contact with medical practitioners occurs during dialysis, rendering cognitive function during the dialysis procedure itself critically important. Importantly, in the Frequent Hemodialysis Network Trials, rates of failure to complete Trails B were similar to those seen in our study (28.7% in FHN versus 21.0% in our study), suggesting that assessment during dialysis may not substantially affect performance on this test. Fifth, only block design retained statistical significance in fully adjusted models, while tests that similarly assessed attention, processing speed and executive function maintained similar magnitude of effect but no longer were associated with a p-value below 0.05. This likely reflects a relatively small sample size, albeit a substantial population given the extensive neurocognitive testing performed. However, because individual neurocognitive tests do not solely assess a single domain, we used a common data reduction technique in neurocognitive studies, principal components analysis, to maximize the domain specific information obtained from each individual cognitive test. Use of principal components analysis additionally reduces the risk of overinterpretation of associations with individual cognitive tests by taking into account the collinearity among components of the neurocognitive battery. Finally, given the cross-sectional nature of the study, it is unknown whether declines in cognitive function that may be associated with the hemodialysis procedure itself would differentially affect patients with and without CVD.
Our study has several strengths as well. We used a detailed neurocognitive evaluation that facilitated the identification of specifically affected cognitive domains, with the current population one of only two maintenance dialysis cohorts with such extensive cognitive evaluation.13 The utility of extensive testing is revealed by the lack of association between a simple cognitive screening tool (the MMSE) and CVD in our study. Second, we utilized principal components analysis as a data reduction technique to account for within patient between test variability.32 Additionally, we were able to enroll a substantial number of chronic hemodialysis patients with characteristics and distribution of causes of ESRD similar to those seen in the prevalent US dialysis population.48 Finally, exclusion criteria were few, and, overall, enrolled participants were similar to those who refused, further improving generalizability.
In summary, we have demonstrated an association between cardiovascular disease and worse performance on cognitive tests assessing executive function in dialysis patients. This relationship is consistent with the hypothesis that there is extensive microvascular pathology among maintenance dialysis patients affecting multiple vascular beds, including the brain. Critically, this study identifies dialysis patients with clinically evident CVD as a higher risk group for difficulty managing complex medication regimens and medical plans. Further research is required to assess longitudinal changes in cognitive performance among dialysis patients with CVD and to better describe the structural changes that occur in this vulnerable population.
Supplementary Material
Acknowledgements
We would like to acknowledge the tremendous assistance of Dialysis Clinic, Inc. and, in particular, the staff and patients at the five DCI units in the Boston area, without whose cooperation the study would not have been successful. Data contained in this manuscript were presented in poster form at Renal Week 2010 on November 19, 2010 in Denver, CO.
Support The study was funded through grants R21 DK068310, K23 DK71636, K24 DK078204 and R01 DK078204.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No authors have financial conflicts related to this manuscript. The results presented in this paper have not been published previously in whole or part, except in abstract format. An abstract based on the content of this manuscript was accepted as a poster the American Society of Nephrology Annual Meeting in Denver in 2010.
References
- 1.Sarnak MJ, Levey AS. Cardiovascular disease and chronic renal disease: a new paradigm. Am J Kidney Dis. 2000;35(4 Suppl 1):S117–31. doi: 10.1016/s0272-6386(00)70239-3. [DOI] [PubMed] [Google Scholar]
- 2.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48(3):392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis. 2008;52(2):216–226. doi: 10.1053/j.ajkd.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73(12):920–927. doi: 10.1212/WNL.0b013e3181b72629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009;24(8):2446–2452. doi: 10.1093/ndt/gfp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am J Epidemiol. 2010;171(3):277–286. doi: 10.1093/aje/kwp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 8.Kurella Tamura M, Wadley V, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52(2):227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008;56(11):2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009;53(3):438–447. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatri M, Nickolas T, Moon YP, et al. CKD associates with cognitive decline. J Am Soc Nephrol. 2009;20(11):2427–2432. doi: 10.1681/ASN.2008101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vupputuri S, Shoham DA, Hogan SL, Kshirsagar AV. Microalbuminuria, peripheral artery disease, and cognitive function. Kidney Int. 2008;73(3):341–346. doi: 10.1038/sj.ki.5002672. [DOI] [PubMed] [Google Scholar]
- 13.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 14.Pereira AA, Weiner DE, Scott T, et al. Subcortical cognitive impairment in dialysis patients. Hemodial Int. 2007;11(3):309–14. doi: 10.1111/j.1542-4758.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 15.Pliskin NH, Yurk HM, Ho LT, Umans JG. Neurocognitive function in chronic hemodialysis patients. Kidney Int. 1996;49(5):1435–1440. doi: 10.1038/ki.1996.202. [DOI] [PubMed] [Google Scholar]
- 16.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30(1):41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 17.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134(1–2):83–88. doi: 10.1016/0022-510x(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 18.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56(4):693–703. doi: 10.1053/j.ajkd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Giang LM, Weiner DE, Agganis BT, et al. Cognitive Function and Dialysis Adequacy: No Clear Relationship. Am J Nephrol. 2011;33(1):33–38. doi: 10.1159/000322611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray AM, Pederson SL, Tupper DE, et al. Acute variation in cognitive function in hemodialysis patients: a cohort study with repeated measures. Am J Kidney Dis. 2007;50(2):270–278. doi: 10.1053/j.ajkd.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS) Stroke. 2007;38(12):3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim CD, Lee HJ, Kim DJ, et al. High prevalence of leukoaraiosis in cerebral magnetic resonance images of patients on peritoneal dialysis. Am J Kidney Dis. 2007;50(1):98–107. doi: 10.1053/j.ajkd.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Seliger SL, Longstreth WT, Jr., Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16(12):3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 24.Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Beiser A, Au R, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35(2):404–409. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- 25.Waldstein SR, Wendell CR. Neurocognitive function and cardiovascular disease. J Alzheimers Dis. 2010;20(3):833–842. doi: 10.3233/JAD-2010-091591. [DOI] [PubMed] [Google Scholar]
- 26.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1(7):426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 27.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277(10):813–7. [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler Adult Intelligence Scale-Third Edition (WAIS-III), Wechsler Memory Scale-Third Scale (WMS-III): Administrative and Scoring Manuals. Harcourt Brace and Company; San Antonio: 1997. [Google Scholar]
- 30.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources Inc; Odessa, FL: 1991. [Google Scholar]
- 31.Heyer NJ, Bittner AC, Jr., Echeverria D. Analyzing multivariate neurobehavioral outcomes in occupational studies: a comparison of approaches. Neurotoxicol Teratol. 1996;18(4):401–406. doi: 10.1016/0892-0362(96)00026-8. [DOI] [PubMed] [Google Scholar]
- 32.Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the Use of Exploratory Factor Analysis in Psychological Research. Psychological Methods. 1999;4(3):272–299. [Google Scholar]
- 33.Example 33.1 Principal Component Analysis. SAS Institute; Cary, NC: SAS/STAT(R) 9.22 User's Guide. [Google Scholar]
- 34.Tobin J. Estimation for relationships with limited dependent variables. Econometrica. 1958;26(1):24–36. [Google Scholar]
- 35.Agganis BT, Weiner DE, Giang LM, et al. Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2010;56(4):704–712. doi: 10.1053/j.ajkd.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oveisgharan S, Hachinski V. Hypertension, executive dysfunction, and progression to dementia: the canadian study of health and aging. Arch Neurol. 2010;67(2):187–192. doi: 10.1001/archneurol.2009.312. [DOI] [PubMed] [Google Scholar]
- 37.Ross GW, Petrovitch H, White LR, et al. Characterization of risk factors for vascular dementia: the Honolulu-Asia Aging Study. Neurology. 1999;53(2):337–343. doi: 10.1212/wnl.53.2.337. [DOI] [PubMed] [Google Scholar]
- 38.Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci. 2004;226(1–2):3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Roman GC. Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc. 2003;51(5 Suppl Dementia):S296–304. doi: 10.1046/j.1532-5415.5155.x. [DOI] [PubMed] [Google Scholar]
- 40.Seliger SL, Gillen DL, Longstreth WT, Jr., Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64(2):603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 41.Nakatani T, Naganuma T, Uchida J, et al. Silent cerebral infarction in hemodialysis patients. Am J Nephrol. 2003;23(2):86–90. doi: 10.1159/000068034. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Wada A, Isaka Y, Maki K, Inoue T, Fukuhara Y. Cerebral magnetic resonance T2 high intensities in end-stage renal disease. Stroke. 1997;28(12):2528–2531. doi: 10.1161/01.str.28.12.2528. [DOI] [PubMed] [Google Scholar]
- 43.Bernick C, Kuller L, Dulberg C, et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57(7):1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 44.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 45.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 46.Seliger SL, Sarnak MJ. Subclinical vascular disease of the brain in dialysis patients. Am J Kidney Dis. 2007;50(1):8–10. doi: 10.1053/j.ajkd.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 47.Tamura MK, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the frequent hemodialysis network trials. Clin J Am Soc Nephrol. 2010;5(8):1429–1438. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Am J Kidney Dis. 2010;55(Suppl 1):S1–S420. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.