Abstract
Recent genetic studies of human hair disorders have suggested a critical role of lysophosphatidic acid (LPA) signalling in hair follicle development, mediated by an LPA-producing enzyme, phosphatidic acid-selective phospholipase A1α (PA-PLA1α, also known as LIPH), and a recently identified LPA receptor, P2Y5 (also known as LPA6). However, the underlying molecular mechanism is unknown. Here, we show that epidermal growth factor receptor (EGFR) signalling underlies LPA-induced hair follicle development. PA-PLA1α-deficient mice generated in this study exhibited wavy hairs due to the aberrant formation of the inner root sheath (IRS) in hair follicles, which resembled mutant mice defective in tumour necrosis factor α converting enzyme (TACE), transforming growth factor α (TGFα) and EGFR. PA-PLA1α was co-localized with TACE, TGFα and tyrosine-phosphorylated EGFR in the IRS. In PA-PLA1α-deficient hair follicles, cleaved TGFα and tyrosine-phosphorylated EGFR, as well as LPA, were significantly reduced. LPA, P2Y5 agonists and recombinant PA-PLA1α enzyme induced P2Y5- and TACE-mediated ectodomain shedding of TGFα through G12/13 pathway and consequent EGFR transactivation in vitro. These data demonstrate that a PA-PLA1α–LPA–P2Y5 axis regulates differentiation and maturation of hair follicles via a TACE–TGFα–EGFR pathway, thus underscoring the physiological importance of LPA-induced EGFR transactivation.
Keywords: EGFR transactivation, hair follicle, lysophosphatidic acid (LPA), transforming growth factor α (TGFα), tumour necrosis factor α converting enzyme (TACE)
Introduction
Lysophosphatidic acid (1- or 2-acyl-lysophosphatidic acid; LPA) is a bioactive lipid with numerous biological functions. LPA exerts most of its functions through G protein-coupled receptors (GPCRs) (Anliker and Chun, 2004; Moolenaar et al, 2004). So far, six LPA-specific GPCRs belonging to either the endothelial cell differentiation gene family (LPA1,2,3) or P2Y family (LPA4,5,6) have been identified (Anliker and Chun, 2004; Ishii et al, 2009; Chun et al, 2010). These LPA receptors, by coupling with different G proteins and having distinct expression patterns, play important roles in various physiological and pathophysiological conditions (Aoki et al, 2008; Skoura and Hla, 2009; Choi et al, 2010).
Extracellular LPA production occurs at least in two distinct pathways (Aoki et al, 2008). In biological fluids such as serum, a soluble enzyme called autotaxin (ATX) hydrolyzes lysophospholipids with its lysophospholipase D activity and produces LPA (Aoki et al, 2002). In cellular membranes, membrane-associated enzymes called phosphatidic acid (PA)-selective phospholipase A1α (PA-PLA1α, also known as mPA-PLA1α and LIPH) (Sonoda et al, 2002) and PA-PLA1β (also known as mPA-PLA1β and LIPI) (Hiramatsu et al, 2003) hydrolyze PA and produce 2-acyl-LPA (with acyl chain at the sn-2 position of glycerol). While the functions of ATX have been well characterized in angiogenesis (Tanaka et al, 2006; van Meeteren et al, 2006), brain development (Koike et al, 2009), cancer metastasis (Mills and Moolenaar, 2003) and lymphocyte traffic (Kanda et al, 2008), the physiological roles of the latter enzymes have remained unclear until recently.
Kazantseva et al (2006) reported that homozygous mutation in the PA-PLA1α gene was found to cause a congenital hair disorder in human (termed LAH2). Affected individuals were characterized by hereditary woolly hair and/or sparse hair (Kazantseva et al, 2006; Shimomura et al, 2009). Interestingly, homozygous mutations in the P2Y5 (also known as P2RY5) gene were found to be responsible for another congenital hair disorder (termed LAH3) (Pasternack et al, 2008; Shimomura et al, 2008). The P2Y5 gene encodes an orphan GPCR sharing 56% amino-acid identity with LPA4, which is presently the closest known homologue of P2Y5. Accordingly, Yanagida et al (2009) showed that LPA behaved as a ligand for P2Y5 and thus named P2Y5 as LPA6. Thus, we postulated that PA-PLA1α hydrolyzes PA on the plasma membrane and provides 2-acyl-LPA for P2Y5, which leads to hair follicle formation (Figure 1A). The idea that PA-PLA1α and P2Y5 have cooperative roles in hair follicle formation is also supported by observations that (i) both PA-PLA1α and P2Y5 are highly expressed in hair follicles, especially in epithelial cells (Kazantseva et al, 2006; Shimomura et al, 2008) and (ii) affected individuals with LAH2 and LAH3 are clinically indistinguishable. It is likely that PA-PLA1α activates P2Y5 through production of LPA. However, how LPA-P2Y5 signalling regulates hair follicle formation remains to be determined. In this study, to obtain clues about the molecular mechanism underlying LPA-mediated hair follicle formation, we generated and analysed PA-PLA1α-deficient (PA-PLA1α−/−) mice and found that epidermal growth factor receptor (EGFR) signalling underlies LPA-mediated hair follicle formation.
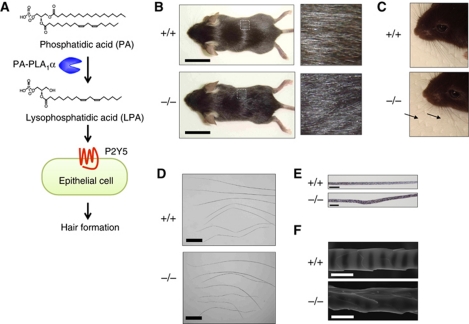
Figure 1.
Wavy hair phenotype of PA-PLA1α−/− mice. (A) Scheme of PA-PLA1α and P2Y5 pathway in hair formation, proposed by human genetic studies. Genetically, hereditary mutations in the PA-PLA1α gene (Kazantseva et al, 2006; Shimomura et al, 2009) and the P2Y5 gene (Pasternack et al, 2008; Shimomura et al, 2008) cause clinically indistinguishable hair disorders in human. Biochemically, PA-PLA1α is capable of hydrolyzing PA (Sonoda et al, 2002) and P2Y5 responds to LPA (Yanagida et al, 2009). It remained unclear whether LPA was actually involved in this pathway and, if so, how LPA signalling regulated hair formation. (B, C) Hair coats (B) and vibrissa hairs (C) of WT (+/+) and PA-PLA1α−/− (−/−) mice on postnatal day (P) 25. Note that hair coat of a WT mouse shows shiny appearance, whereas hair coat of a PA-PLA1α−/− mouse shows wavy and matted appearance. (D–F) Pelage hairs were observed under an optical microscope (D, E) and a scanning electron microscope (E). Compared with WT hairs, all of four hair types of PA-PLA1α −/− pelage hairs (inset from top: guard, awl, auchene and zigzag) show waviness (D). Note that PA-PLA1α−/− hair shows disorganized medulla and irregular melanin piles (E) and wavy cuticle (F). Scale bars, 2 cm (B), 500 μm (D), 20 μm (E) and 5 μm (F).
Results
PA-PLA1α−/− mice show wavy hair phenotype
To investigate the effect of PA-PLA1α deletion in mice, we disrupted the PA-PLA1α gene of murine embryonic stem cells by replacing exon 3, which encodes the active residue Ser154, with a neomycin resistance cassette (Supplementary Figure S1A). Deletion of exon 3 resulted in a frameshift mutation and no immunoreactive PA-PLA1α protein (Supplementary Figure S1C; Figures 3F and 4D). PA-PLA1α−/− mice were born with the expected Mendelian ratio from an intercross of heterozygous parents (+/+:+/−:−/−=86:205:84). PA-PLA1α−/− mice were distinguishable from wild-type (WT) or heterozygous littermates by aberrant hair characteristics including wavy vibrissa hair and a matted coat (Figure 1B and C). Pelage hairs from PA-PLA1α−/− mice showed waviness, disorganized medulla, irregular melanin piles and wavy cuticles (Figure 1D–F). The hair abnormalities were observed in all PA-PLA1α−/− mice (>300 animals observed) and, more importantly, in two PA-PLA1α−/− lines derived from independent ES clones (clones 1D-A1 and 4C-D3). Thus, in the following experiments, only the line derived from clone 1D-A1 was examined. The wavy hair phenotype was most apparent from 1 to 4 weeks of age and became less severe after the start of the anagen (growth) phase of the first hair cycle. Of note, these hair abnormalities of PA-PLA1α−/− mice resembled those of mutant mice defective in keratin genes specific to the inner root sheath (IRS), the layer surrounding the hair shaft (Figure 3E), of hair follicles (Kikkawa et al, 2003; Peters et al, 2003; Tanaka et al, 2007), as well as transforming growth factor α (TGFα)-related genes including TNFα-converting enzyme (TACE, also known as ADAM17) (Peschon et al, 1998), TGFα (Luetteke et al, 1993; Mann et al, 1993) and EGFR (Luetteke et al, 1994), as discussed below. Unlike humans with PA-PLA1α-null mutations, PA-PLA1α−/− mice did not seem to develop alopecia. Besides hair abnormalities, we could not find any difference between PA-PLA1α−/− mice and WT or heterozygous littermates such as fertility or growth rate under normal conditions (data not shown).
Reduced 2-acyl-LPA in PA-PLA1α−/− mice
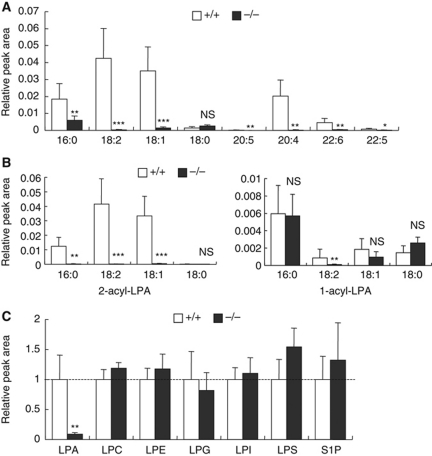
LPA detected in vivo consists of a mixture of LPA species with various fatty acids attached to either the sn-1 (1-acyl-LPA) or sn-2 (2-acyl-LPA) position of the glycerol backbone. To examine which LPA species, if any, are produced by PA-PLA1α in skin and hair follicles, which generate hair shafts, we analysed LPA species of tissue extracts using a newly developed liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. LPA ions were selectively detected by the selective reaction monitoring mode (e.g. m/z 409.3 for the negatively charged parent ion of 16:0-LPA and m/z 153.0 for the fragment ion of 16:0-LPA). Consistent with a previous reversed-phase LC condition (Creer and Gross, 1985), we observed two separate peaks corresponding to the 2-acyl isomer (first elution) and the 1-acyl isomer (second elution) (Supplementary Figure S2A). Among eight LPA species examined, five of six unsaturated LPA species (18:2, 18:1, 20:5, 20:4 and 22:6) were significantly reduced in both isolated vibrissa hair follicles and dorsal skin of PA-PLA1α−/− mice (Figure 2A and data not shown). Notably, in hair follicle of PA-PLA1α−/− mice, the six unsaturated LPA species were reduced by >90%, whereas saturated LPA species were unchanged (18:0) or slightly decreased (16:0) (Figure 2A). When 1-acyl isomer and 2-acyl isomer of LPA were separately quantified, 2-acyl-LPA in three predominant LPA species (16:0, 18:2 and 18:1) were significantly lowered in PA-PLA1α−/− hair follicles, whereas 1-acyl-LPA remained largely unchanged (Figure 2B). These data are consistent with the asymmetry of fatty acid composition of phospholipids in which saturated fatty acid and (poly)unsaturated fatty acid are generally attached at the sn-1 and sn-2 positions of the glycerol backbone, respectively. The levels of six other lysophospholipids including LPC (lysophosphatidylcholine), LPE (lysophosphatidylethanolamine), LPG (lysophosphatidylglycerol), LPI (lysophosphatidylinositol), LPS (lysophosphatidylserine) and S1P (sphingosine 1-phosphate) were unchanged in both isolated vibrissa hair follicles and dorsal skin of PA-PLA1α−/− mice (Figure 2C; Supplementary Figure S2B and C and data not shown), confirming that LPA was specifically decreased in PA-PLA1α−/− mice. These data demonstrate that PA-PLA1α produces LPA, especially 2-acyl-LPA with unsaturated fatty acids, in hair follicle.
Figure 2.
LPA is lowered in PA-PLA1α−/− hair follicles. (A) Relative abundance of eight LPA species in vibrissa hair follicles. Lipids in vibrissa hair follicles from P15 WT (+/+) and PA-PLA1α−/− (−/−) mice (during the hair growth phase) were extracted with methanol and analysed by an LC-MS/MS method. Peak areas of 1-acyl-LPA and 2-acyl-LPA were combined and normalized to the internal standard 17:0-LPA (n=8). Numbers in the x axis denote acyl chains that are attached to LPA. 16:0, palmitoyl; 18:2, linoleoyl; 18:1, oleoyl; 18:0, stearoyl; 20:5, eicosapentanoyl; 20:4, arachidonoyl; 22:6, docosahexanoyl; 22:5, docosapentaenoyl. (B) Same as (A), but 2-acyl-LPA and 1-acyl-LPA in (A) were separately quantified. (C) LC-MS/MS analysis of lysophospholipids in vibrissa hair follicles. Except for S1P, eight acyl moieties of each lysophospholipid were monitored and the sum of the peak areas was used for quantification (see Supplementary Figure S2 for details). The mean value in +/+ sample was set at 1 (n=8). Asterisks, *P<0.05, **P<0.01, ***P<0.001 versus +/+. NS, not significant difference between +/+ and −/−.
Impaired IRS formation in PA-PLA1α−/− hair follicles
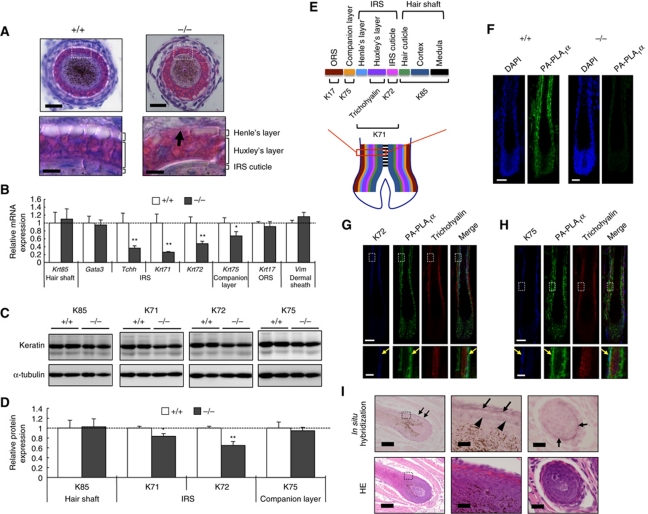
Among hair follicle layer-specific keratins, wavy hair phenotype appears only in mice with mutations for IRS-specific keratins (Krt25, Krt27 and Krt71) (Kikkawa et al, 2003; Peters et al, 2003; Tanaka et al, 2007). Thus, the irregular hair in PA-PLA1α−/− mice occurred most likely due to defects in the formation of the IRS. Indeed, histological analysis of cross sections of vibrissa hair follicles with haematoxylin and eosin (HE) showed that the IRS of PA-PLA1α−/− hair follicles, compared with that of WT hair follicles, was irregularly structured (Figure 3A). Other hair follicle structures such as the outer root sheath (ORS) and the dermal papilla appeared normal (Figure 3A and data not shown). The IRS consists of the three layers (Henle's layer, Huxley's layer and the IRS cuticle, from outermost to innermost; Figure 3E) and plays an important role in anchoring hair shaft and supporting of its development. In WT hair follicles, Henle's layer was recognized by fully keratinized and continuous cells that were unstained by HE, and Huxley's layer consisted of multiple-layered cells containing Eosin-positive trichohyalin granules (Figure 3A). In contrast, in PA-PLA1α−/− hair follicles, Henle's layer appeared to be discontinuous and Huxley's layer protruded into the ORS (Figure 3A). We noticed, however, that the IRS length was not significantly different between the two genotypes (Supplementary Figure S3A–D), suggesting that the IRS in PA-PLA1α−/− hair follicle, although morphologically deformed, could still undergo maturation/keratinization processes. The hair cycle and epidermal structure were also unaffected in PA-PLA1α−/− mice (data not shown). To confirm IRS malformation at the biochemical level, we next examined expressions of layer-specific marker genes. Quantitative RT–PCR revealed remarkable reductions in IRS markers (Krt71, Krt72 and Tchh encoding Keratin 71 (K71), K72, trichohyalin, respectively) (Langbein et al, 2003, 2006) as well as a slight reduction in a companion layer marker (Krt75) in PA-PLA1α−/− hair follicles (Figure 3B). Expression levels of other hair follicle markers including hair shaft (Krt85), ORS (Krt17) or dermal sheath (Vim encoding Vimentin) (Schweizer et al, 2007) were not different between the two genotypes (Figure 3B). Notably, the expression of an early IRS differentiation marker (Gata3) (Kaufman et al, 2003) was not affected in PA-PLA1α−/− mice (Figure 3B), in agreement with the existence of IRS structures (Figure 3A). At the protein level, IRS markers (K71 and K72) were also significantly decreased in PA-PLA1α−/− hair follicles whereas a hair shaft marker (K85) and a companion layer marker (K75) were not (Figure 3C and D). These data again suggest that, among the multiple layers in hair follicles, the IRS is specifically affected by disruption of PA-PLA1α, which may lead to impaired guidance and maturation of developing hair shafts, thus resulting in wavy hair formation.
Figure 3.
Impaired IRS formation in PA-PLA1α−/− hair follicles. (A) HE staining of vibrissa hair follicles. Magnified views of the boxed areas are shown in the bottom panels. The arrow indicates the discontinuous Henle's layer in a PA-PLA1α−/− vibrissa hair follicle. Scale bars, 20 μm (top) and 5 μm (bottom) (B) Quantitative RT–PCR (qRT–PCR) analysis of hair follicle layer-specific markers in P4 dorsal hair follicles. The number of transcripts was normalized to the housekeeping gene, Gapdh, in the same sample and the mean value in +/+ sample was set at 1 (n=4). (C, D) Immunoblot analysis of hair follicle layer-specific markers in P10 dorsal skin and densitometric quantification. The marker intensity was normalized to α-tubulin in the same sample and the mean value in +/+ sample was set at 1 (n=4). (E) A schematic illustration of hair follicle layers and marker expressions in follicular layers. (F–H) Immunofluorescent detection of PA-PLA1α in dorsal hair follicles. Nuclei were stained with DAPI. Specificity of anti-PA-PLA1α antibody was confirmed using PA-PLA1α−/− hair follicle (F). K72 (G) and K75 (H) (detected with Alexa Fluor 647) were pseudo-coloured ‘blue.’ Magnified views of the boxed areas are shown in the bottom panels. The arrows indicate co-expression of PA-PLA1α with K72 (G) and K75 (H) in WT hair follicles. Scale bars, 20 μm (top) and 5 μm (bottom). (I) In situ hybridization of PA-PLA1α mRNA in P15 WT vibrissa hair follicles (upper panels, purple hybridization signal). A serial section was stained with HE (lower panels). No significant signals were detected using a sense probe (data not shown). Note that brown dots inside the hair cuticle in the middle panel are melanin granules, not a hybridization signal. Left panels, longitudinal sections of hair follicles; middle panels, enlarged images of the boxed areas in the left panels; right panels, cross sections of hair follicles. Arrows, IRS; arrowheads, hair cuticle. Scale bars represent 100 μm in left panels and 20 μm in middle and right panels. *P<0.05, **P<0.01 versus +/+.
In situ localization of PA-PLA1α expression in the hair follicles was analysed by immunofluorescent staining and in situ mRNA hybridization techniques. Immunofluorescent staining detected the presence of PA-PLA1α protein in hair follicle epithelial cells (Figure 3F). The PA-PLA1α signals were not observed in PA-PLA1α−/− hair follicles (Figure 3F), confirming the specificity of the anti-PA-PLA1α rat monoclonal antibody established in this study. We also performed double immunostaining using anti-PA-PLA1α and hair follicle layer-specific markers. An intense PA-PLA1α signal within the hair follicles was co-localized in two layers with an IRS cuticle marker (K72) and a companion layer marker (K75) (Figure 3G and H). The localization of PA-PLA1α was also confirmed by an in situ mRNA hybridization analysis, which showed that an intense mRNA signal in the IRS and a less intense signal in the companion layer (Figure 3I). This expression pattern was consistent with the observation that PA-PLA1α−/− hair follicles exhibited aberrant IRS (Figure 3A–D), strongly suggesting that the primary defect in PA-PLA1α−/− hair follicle is in the IRS.
PA-PLA1α is co-expressed with TACE, TGFα and EGFR in hair follicles
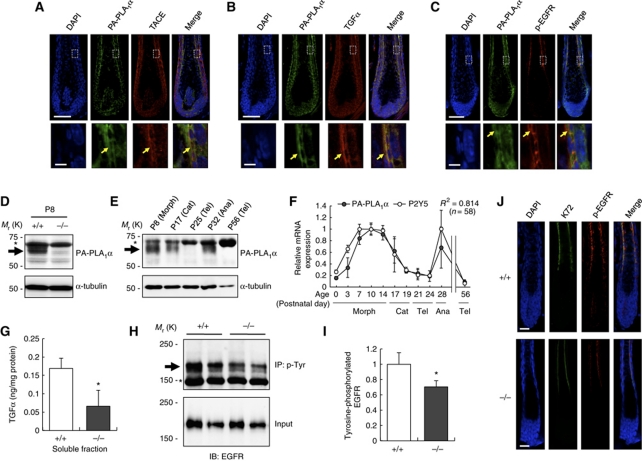
We noticed that the wavy hair phenotype of PA-PLA1α−/− mice was similar to the phenotypes of TACE-deficient (Peschon et al, 1998), TACE mutant (known as woe) (Hassemer et al, 2010), TGFα-deficient, TGFα mutant (known as wa-1) (Luetteke et al, 1993; Mann et al, 1993) and EGFR mutant (known as wa-2) mice (Luetteke et al, 1994). TACE, a member of a disintegrin and metalloprotease (ADAM) family, is capable of cleaving a transmembrane proform of TGFα (Sahin et al, 2004), a process referred to as ectodomain shedding, and the subsequent binding of soluble TGFα to EGFR results in activation of EGFR, known as transactivation. Thus, the TACE–TGFα–EGFR pathway has been recognized as an important regulator of hair follicle development. The phenotypic resemblance of these mutant mice to PA-PLA1α−/− mice led us to hypothesize that the TACE–TGFα–EGFR pathway is regulated by PA-PLA1α, most likely through production of LPA and the subsequent activation of P2Y5 in hair follicles. Immunofluorescent staining revealed that TACE, TGFα and the phosphorylated form of EGFR (p-EGFR; phosphorylated at Tyr1068, a critical site for autophosphorylation), were co-expressed with PA-PLA1α in the IRS, specifically in the IRS cuticle of the K72-positive layer (Figure 4A–C). We found that PA-PLA1α was upregulated during the growth (morphogenesis or anagen) phase of the hair cycle and downregulated during the regression (catagen) and resting (telogen) phases at both the protein and mRNA levels (Figure 4D–F). The expression pattern of P2Y5 was also hair cycle dependent and was nearly identical to that of PA-PLA1α in both the developmental and adult stages (Figure 4F; Supplementary Figure S4), suggesting that both P2Y5 and PA-PLA1α have cooperative roles during the anagen phase. Our results are consistent with previous observations that TGFα and P2Y5 were expressed in the IRS (Luetteke et al, 1993; Shimomura et al, 2008). These data strongly support our hypothesis that PA-PLA1α and P2Y5 regulates hair follicle formation through the TACE–TGFα–EGFR pathway.
Figure 4.
PA-PLA1α is co-expressed with TACE, TGFα and p-EGFR in hair follicles and EGFR signalling is reduced in PA-PLA1α−/− mice. (A–C) Immunofluorescent staining of PA-PLA1α with TACE (A), TGFα (B) and phosphorylated form of EGFR (p-EGFR) at Tyr1068 (C) in WT dorsal hair follicles. The arrows indicate co-expression of PA-PLA1α with each protein. Nuclei were stained with DAPI. Magnified views of the boxed areas are shown in the bottom panels. (D) Immunoblot analysis of PA-PLA1α in dorsal skin lysate. Lysate of skin from P8 WT (+/+) and PA-PLA1α−/− (−/−) mice was subjected to immunoblot using anti-PA-PLA1α antibody. The membrane was stripped and reprobed with anti-α-tubulin antibody as a loading control. The arrow and asterisk denote PA-PLA1α and non-specific bands, respectively. (E) Hair cycle-dependent expression of PA-PLA1α in dorsal skin. Skin specimens at different hair cycle stages were analysed by immunoblot as described above. Note that first hair cycle starts with catagen (P17). Morph, morphogenesis; Cat, catagen (regression); Tel, telogen (resting); Ana, anagen (growth). (F) qRT–PCR analysis of hair cycle-dependent expression of PA-PLA1α and P2Y5 mRNA in WT dorsal skin. The number of transcripts was normalized to Gapdh and the maximal value of each gene was set at 1 (n=3–5). R2, squared correlation coefficient between PA-PLA1α level and P2Y5 level. (G) Soluble TGFα level in vibrissa hair follicles from WT and PA-PLA1α−/− mice. Lysate of vibrissa hair follicles during the growth phase were ultracentrifuged and the supernatants were subjected to TGFα sandwich ELISA. Amount of soluble TGFα was normalized to total protein in the same sample determined by the BCA method (mean±s.e.m.; n=4). (H) p-EGFR level in dorsal skin. Tyrosine-phosphorylated proteins were immunoprecipitated from skin lysate during the hair growth phase using anti-phosphotyrosine (p-Tyr) monoclonal antibody (PY20). The immunoprecipitates (IP) and aliquots of total dorsal skin lysate (Input) were subjected to immunoblot with anti-EGFR antibody. The arrow and asterisk indicate bands corresponding to p-EGFR and IgG, respectively. (I) Densitometric analysis of p-EGFR in (H). The IP intensity was normalized to the input intensity in the same sample and the mean value in +/+ sample was set at 1 (mean±s.e.m.; n=4). (J) Immunofluorescent staining of p-EGFR at Tyr1068 and K72 in WT and PA-PLA1α−/− hair follicles. Nuclei were stained with DAPI. Scale bars represent 50 μm (A–C, upper panels), 5 μm (A–C, bottom panels) and 20 μm (J). *P<0.05 versus +/+.
Impaired TGFα-EGFR signalling in PA-PLA1α−/− hair follicles
To test this hypothesis, we first examined the level of TGFα in PA-PLA1α−/− hair follicles. The soluble TGFα was significantly lower (by 61% reduction) in PA-PLA1α−/− hair follicles than in WT hair follicles (Figure 4G), whereas TGFα mRNA expression and membrane-bound TGFα protein were not significantly different between the two genotypes (Supplementary Figure S5A and B). Immunofluorescent staining also showed no apparent differences in the TGFα expression patterns (Supplementary Figure S6B). Immunoprecipitation and immunoblot analyses of dorsal skin lysate revealed that tyrosine-phosphorylated EGFR (i.e. activated EGFR) was lowered in PA-PLA1α−/− mice than in WT mice (Figure 4H and I). Immunofluorescent staining using an antibody against p-EGFR confirmed that p-EGFR signal in the IRS was reduced in PA-PLA1α−/− hair follicles (Figure 4J). These results indicate that PA-PLA1α induces TGFα release and subsequent EGFR phosphorylation in vivo, which is required for the proper development of hair follicles.
P2Y5 and TACE mediate LPA-induced TGFα release and EGFR transactivation in keratinocytes
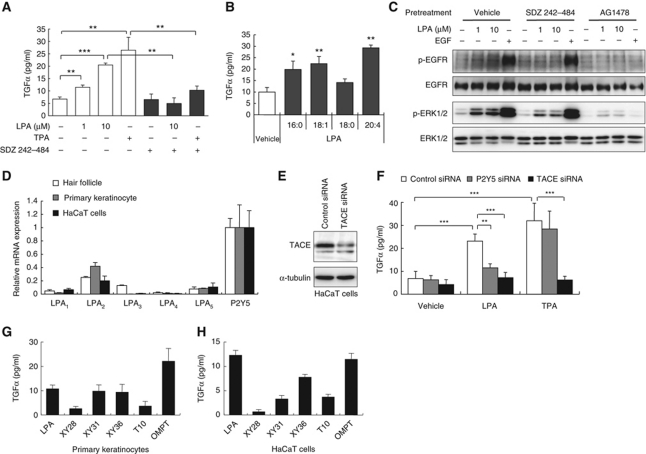
LPA and other GPCR agonists have been shown to induce transactivation of EGFR in various cell types in vitro (Daub et al, 1997). A critical step of EGFR transactivation is ADAM-mediated ectodomain shedding of EGFR ligands such as TGFα and heparin-binding EGF-like growth factor (HB-EGF) (Peschon et al, 1998; Prenzel et al, 1999; Sahin et al, 2004). We first examined whether LPA induces TGFα release and EGFR transactivation in keratinocytes. When primary epidermal keratinocytes were treated with LPA (18:1), the cells released significant amounts of TGFα (Figure 5A), which was equivalent to the amount observed in cells treated with 12-O-tetradecanolyphorbol-13-acetate (TPA) (Figure 5A), a well-established PKC-dependent ADAM activator. The LPA-induced TGFα release was completely blocked by pretreatment with a broad-spectrum metalloprotease inhibitor, SDZ 242–484 (Kottirsch et al, 2002) (Figure 5A). Among the LPA species tested, LPA (20:4) was the most potent in inducing TGFα release in keratinocytes (Figure 5B). Interestingly, we found that LPA species that were abundantly present in hair follicles (16:0, 18:1 and 20:4) (Figure 2A) had higher ability to induce TGFα release (Figure 5B). In the primary keratinocytes, LPA also induced an increase in the level of phosphorylated EGFR at Tyr1068 (p-EGFR) as well as phosphorylated ERK1/2 (p-ERK1/2), one of the downstream targets of EGFR (Figure 5C). Both p-EGFR and p-ERK were attenuated by pretreatment with SDZ 242–484 or an EGFR inhibitor, AG1478. These in vitro studies clearly demonstrate that LPA transactivates EGFR through ADAMs, which consequently leads to activation of ERK.
Figure 5.
LPA induces P2Y5- and TACE-dependent TGFα release and consequent EGFR transactivation in keratinocytes. (A) LPA-induced TGFα release from primary keratinocytes in a metalloprotease-dependent manner. Keratinocytes were treated with LPA (1-oleoyl, unless otherwise noted) or a phorbol ester, TPA (100 nM), in the presence or absence of a broad-spectrum metalloprotease inhibitor, SDZ 242–484 (10 μM) (**P<0.01, ***P<0.001). (B) LPA-induced TGFα release from mouse primary keratinocytes. Keratinocytes were treated with LPA (10 μM) containing different fatty acids (1-palmitoyl (16:0), 1-oleoyl (18:1) and 1-stearoyl (18:0) and 1-arachidonoyl (20:4)). TGFα in the conditioned media was measured by sandwich ELISA (*P<0.05, **P<0.01 versus vehicle treatment). (C) Immunoblot analyses of EGFR and ERK phosphorylation in primary keratinocytes. Cells were treated with LPA or EGF (100 ng/ml) for 5 min (p-EGFR) or 15 min (p-ERK) in the presence or absence of SDZ 242–484 (10 μM) or an EGFR inhibitor, AG1478 (1 μM). (D) Expressions of LPA receptors in P4 dorsal hair follicles, primary keratinocyte and human HaCaT keratinocytes (n=4). The number of transcripts was normalized to Gapdh and the mean value of P2Y5 expression level was set at 1 (n=3–4). (E) Immunoblot analysis of siRNA-mediated knockdown of TACE in HaCaT cells. (F) TGFα release from siRNA-transfected HaCaT cells. Control, P2Y5 or TACE-knockdown cells were treated with LPA (10 μM) or TPA (100 nM) and TGFα release was measured (**P<0.01, ***P<0.001). Note that knockdown efficiency (mRNA reduction level quantified by qRT–PCR) of TACE siRNA and P2Y5 siRNA was 76±4.5% (mean±s.d., n=3) and 67±4.8%, respectively. (G, H) LPA analogues (10 μM) were tested for TGFα-releasing activities from primary keratinocytes (D) and HaCaT cells (E). The TGFα concentration in vehicle-treated cells was set as the baseline. Note that TGFα release was potently induced by P2Y5 agonists (XY31, XY36 and OMPT), but not by LPA analogues that lack P2Y5-agonistic activity (XY28 and T10). The structures and agonistic activities of these LPA analogues at LPA receptors are shown in Supplementary data. (A, B, F, G, H) Data are representative of three independent experiments and show the mean±s.d. of at least triplicate cultures.
We further examined whether P2Y5 and TACE were involved in the process of LPA-induced TGFα release in keratinocytes. Quantitative RT–PCR analysis showed that, among the six LPA receptors, P2Y5 was most abundantly expressed in both mouse primary epidermal keratinocytes and human HaCaT keratinocytes. In addition, the expression patterns of LPA receptors in the epidermal keratinocytes and HaCaT cells were nearly identical to those in mouse hair follicles (Figure 5D). To assess the involvement of P2Y5 and TACE in TGFα release, we used RNA interference to knockdown endogenous P2Y5 and TACE in HaCaT cells. Small interfering RNA (siRNA)-mediated knockdown of P2Y5 attenuated the LPA-induced, but not the TPA-induced, TGFα release, whereas knockdown of TACE, which was confirmed by immunoblot analysis (Figure 5E), almost completely inhibited both LPA-induced and TPA-induced TGFα release (Figure 5F). Furthermore, the P2Y5 agonists (XY31, XY36 and OMPT) identified in this study (Supplementary Figure S9A and B) induced TGFα release from both primary keratinocytes and HaCaT cells (Figure 5G and H). Notably, a strong correlation was found between the P2Y5-agonistic activity (Supplementary Figure S9B) and TGFα-releasing activity (Figure 5G and H) of these LPA analogues. Taken together, these data demonstrate that P2Y5 and TACE mediate LPA-induced TGFα release and the consequent EGFR transactivation in keratinocytes.
Reconstitution of LPA-induced TGFα release in HEK293 cells
As a further test of whether P2Y5 has a role in TGFα release, we reconstituted a cell-based system that mimics ectodomain shedding of TGFα by utilizing an alkaline phosphatase (AP)-tagged TGFα (AP-TGFα) (Tokumaru et al, 2000), which we refer to as an AP-TGFα shedding assay (Figure 6A). In this system, AP-TGFα release into the conditioned media can be quantitatively measured by a colorimetric reaction using p-nitrophenylphosphate (p-NPP). When HEK293 cells transiently expressing AP-TGFα and P2Y5 (either mouse P2Y5 or human P2Y5) were treated with LPA, AP activity was dose dependently increased in the conditioned media (Figure 6B), showing that P2Y5 mediated LPA-induced AP-TGFα release. P2Y5-expressing cells and mock-transfected (control) cells were equally sensitive to TPA stimulation (Figure 6C), indicating that expression of P2Y5 did not affect PKC-induced AP-TGFα release. Importantly, no significant AP-TGFα release was induced from P2Y5-expressing cells by the other six lysophospholipids (Figure 6C; Supplementary Figure S8A), confirming that P2Y5 is an LPA-specific receptor. We noticed that a small amount of AP-TGFα was also released from control cells, possibly via endogenously expressed LPA receptors in HEK293 cells (Supplementary Figure S7). Indeed, siRNA-mediated knockdown of P2Y5 endogenously expressed in HEK293 cells attenuated LPA-induced AP-TGFα release by 45%, but did not attenuate TPA-induced AP-TGFα release (Figure 6D). Similar to TGFα release from keratinocytes, both LPA-induced and TPA-induced AP-TGFα release from HEK293 cells was almost completely suppressed by knockdown of TACE and pretreatment with a broad metalloprotease inhibitor, SDZ 242–484 (Figure 6E–G). Using the reconstituted model, we further examined signal transduction of P2Y5 leading to AP-TGFα release in HEK293 cells. Among four inhibitors that specifically block four distinct subclasses of heterotrimeric Gα proteins (Gs, Gi, Gq and G12/13), only Y27632 (a Rho kinase (ROCK) inhibitor; G12/13 pathway) inhibited LPA/P2Y5-induced AP-TGFα release, whereas NF449 (a Gs inhibitor), Pertussis Toxin (PTX; a Gi inhibitor) and YM254890 (a Gq inhibitor) did not have the inhibitory effect (Figure 6H). In addition, Ro31-8425 (a pan-PKC inhibitor), but not U73122 (a PLCβ inhibitor), blocked LPA/P2Y5-induced AP-TGFα release. Co-transfection of dominant-negative (DN) forms of G proteins including G12, G13 and RhoA also inhibited LPA/P2Y5-induced AP-TGFα release (Figure 6I), confirming the involvement of G12/13 signalling. Thus, we concluded that LPA/P2Y5-induced G12/13 signalling underlies TACE-dependent TGFα release (Figure 6J).
Figure 6.
LPA induces P2Y5- and TACE-dependent TGFα release via a G12/13–RhoA–ROCK pathway in HEK293 cells. (A) Scheme of AP-tagged (AP-TGFα) shedding assay. HEK293 cells transiently expressing AP-TGFα and P2Y5 were treated with a compound for 1 h. Amount of AP-TGFα release was quantified by measuring AP activities in both conditioned media and cell surface using a colorimetric reaction of AP substrate, p-NPP. Note that protease that cleaves AP-TGFα is endogenously expressed in HEK293 cells. AP activity detected in vehicle treatment was set as the baseline. (B) LPA induces AP-TGFα release from P2Y5-expressing cells. HEK293 cells that were transfected with mouse P2Y5 (mP2Y5), human P2Y5 (hP2Y5) or control vector were stimulated with 3–1000 nM of LPA (n=4; *P<0.05, **P<0.01 versus control cells). (C) Same as (B), but treated with various lysophospholipids (1 μM) and TPA (100 nM). Note that five lysophospholipids other than LPA did not induce P2Y5-dependent AP-TGFα release. Also note that transient expression of P2Y5 did not affect TPA (100 nM)-induced AP-TGFα release. (D) siRNA-mediated knockdown of P2Y5 endogenously expressing in HEK293 cells attenuated LPA-induced, but not TPA-induced, AP-TGFα release. Note that knockdown efficiency (mRNA reduction level quantified by qRT–PCR) with P2Y5 siRNA was 85±1.8% (mean±s.d., n=3). (E–G) SDZ 242–484 (a metalloprotease inhibitor; 1 μM) (E) or siRNA-mediated knockdown of TACE (F, G) inhibited both LPA (1 μM)-induced and TPA (100 nM)-induced AP-TGFα release. Note that knockdown efficiency with TACE siRNA was 90±0.7% (mean±s.d., n=3). (H) LPA/P2Y5-induced AP-TGFα release was blocked by Y27632 (a ROCK inhibitor; 10 μM; 30 min pretreatment, unless otherwise noted) and Ro31-8425 (Ro; a pan-PKC inhibitor; 10 μM), but not by NF449 (a Gs inhibitor, 100 μM), PTX (a Gi inhibitor; 100 ng/ml; 16 h), YM254890 (YM; a Gq inhibitor; 10 μM) and U73122 (a PLCβ inhibitor; 3 μM). (I) LPA/P2Y5-induced AP-TGFα release was blocked by co-transfection of DN forms of G proteins including G12, G13 (heterotrimeric Gα proteins) and RhoA (small G protein). Concentration of LPA was 1 μM (H, I). Bars and error bars in (C–E, G–I) represent mean values and s.d., respectively (n=3–4; *P<0.05, **P<0.01, ***P<0.001; NS, not significant difference). (J) Scheme of signal transduction leading to TGFα release in HEK293 cells. Upon binding of LPA, P2Y5 activates G12/13–RhoA–ROCK signalling, which leads to TACE-dependent ectodomain shedding of pro-TGFα, partially through a PKC pathway, and results in TGFα release.
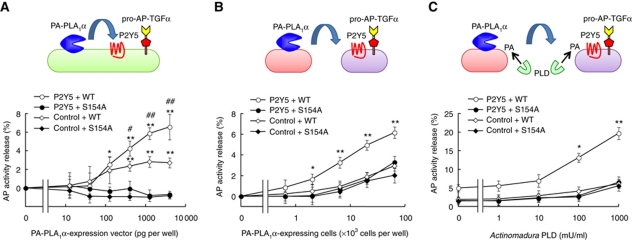
PA-PLA1α initiates P2Y5-mediated TGFα release in HEK293 cells
We finally asked whether PA-PLA1α itself is capable of inducing P2Y5-mediated AP-TGFα release. Co-expression of P2Y5 and PA-PLA1α, but not a catalytically inactive PA-PLA1α mutant (S154A) (Sonoda et al, 2002), dramatically enhanced AP-TGFα release (Figure 7A). A small but significant AP-TGFα release was observed when PA-PLA1α vector was introduced alone (Figure 7A, control+WT), probably via endogenously expressed LPA receptors in HEK293 cells (Supplementary Figure S7), as was observed in LPA-stimulated mock-transfected cells (Figure 6B and D). Next, we tested whether PA-PLA1α was capable of activating P2Y5 in a juxtacrine manner. When PA-PLA1α-expressing cells and P2Y5-expressing cells were prepared separately and mixed together, significant AP-TGFα release from P2Y5-expressing cells was observed (Figure 7B). The AP-TGFα release was also dependent on the catalytic activity of PA-PLA1α and the transfection of the P2Y5 vector. The PA-PLA1α-initiated AP-TGFα release from P2Y5-expressing cells was further elevated upon treatment with bacterial phospholipase D (PLD) (Figure 7C), which was previously shown to generate PA on the outer leaflet of plasma membrane (Sonoda et al, 2002; Aoki, 2004). These data clearly show that PA-PLA1α is capable of activating P2Y5 in an autocrine and/or juxtacrine manner via hydrolysis of PA on the plasma membrane and production of LPA.
Figure 7.
PA-PLA1α induces P2Y5-dependent TGFα release. PA-PLA1α-mediated activation of P2Y5 in autocrine (A) and paracrine (B, C) manners. (A) Spontaneous AP-TGFα release from HEK293 cells expressing catalytically active PA-PLA1α (WT), P2Y5 and AP-TGFα. Note that AP-TGFα release was not observed in cells expressing catalytically inactive PA-PLA1α mutant harbouring a serine-to-alanine substitution at residue 154 (S154A). Also note that AP-TGFα release was significantly reduced when P2Y5 vector was replaced with an empty vector (control) (n=4; *P<0.05, **P<0.01 versus control vector-transfected cells; #P<0.05, ##P<0.01 versus S154A-expressing cells). (B) PA-PLA1α-induced paracrine activation of P2Y5. P2Y5- and AP-TGFα-expressing cells were mixed with PA-PLA1α-expressing cells that were separately prepared (n=4; *P<0.05, **P<0.01 versus control vector-transfected cells and S154A-expressing cells). (C) Exogenous phospholipase D (PLD) potently enhanced the PA-PLA1α-mediated AP-TGFα release. Same as B (2 × 103 PA-PLA1α-expressing cells per well), but in the presence of bacterial PLD. AP activity in the absence of PA-PLA1α-expressing cells and PLD treatment was used as the baseline (n=4; *P<0.05, **P<0.01 versus control vector-transfected cells and S154A-expressing cells).
Discussion
Genetic studies of human hair disorders have suggested that LPA signalling has a crucial role in hair follicle development. However, the underlying molecular mechanism, even the involvement of LPA itself, has until now been unclear. The data presented here show that LPA generated by an LPA-producing enzyme, PA-PLA1α, regulates hair follicle formation through a mechanism that involves TGFα release and EGFR transactivation via the most recently identified LPA receptor, P2Y5 (Figure 8). This model is supported by the following observations: (i) PA-PLA1α−/− mice exhibited a wavy hair phenotype that resembles the hair phenotype of mutant mice defective in TGFα-related genes (TACE, TGFα and EGFR) (Luetteke et al, 1993, 1994; Mann et al, 1993; Peschon et al, 1998; Hassemer et al, 2010); (ii) PA-PLA1α, TACE, TGFα and p-EGFR were co-expressed in the IRS and, PA-PLA1α and P2Y5 showed similar hair cycle-dependent expression; (iii) the activation states of TGFα and EGFR were decreased in PA-PLA1α−/− mice; (iv) LPA species with unsaturated fatty acids, which were found to be potent agonists for P2Y5, were selectively lowered in PA-PLA1α−/− mice; (v) LPA induced TGFα release and EGFR transactivation via P2Y5 and TACE in keratinocytes; and (vi) LPA and PA-PLA1α induced P2Y5-mediated TGFα release in a TACE-dependent manner in reconstituted HEK293 cells. In vitro studies have demonstrated that LPA is an initiator of EGFR transactivation in a variety of cells such as corneal epithelial cells and lung epithelial cells (Prenzel et al, 1999; Zhao et al, 2006; Xu et al, 2007). This study is the first to demonstrate the in vivo significance of LPA-induced EGFR transactivation. Moreover, GPCR-induced EGFR transactivation has been implicated in several pathophysiological conditions such as renal lesions (Lautrette et al, 2005), wound healing (mediated by AT1) (Yahata et al, 2006), cardiac hypertrophy (AT1 and β1) (Zhai et al, 2006; Noma et al, 2007) and regulation of blood pressure (endothelin-1 receptors) (Chansel et al, 2006). However, these previous studies examined disease models that were, in most cases, induced by exogenously administered GPCR agonists. Thus, our data provide the first evidence of the physiological role of GPCR-induced EGFR transactivation in vivo.
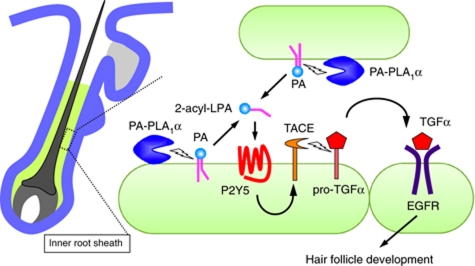
Figure 8.
Proposed model for PA-PLA1α- and P2Y5-mediated hair follicle development. PA-PLA1α is expressed and secreted in the developing IRS of hair follicles and produces 2-acyl-LPA from PA on the outer leaflet of the plasma membrane by hydrolyzing the acyl chain at the sn-1 position. The resulting 2-acyl-LPA activates P2Y5 in a paracrine and/or autocrine manner, eliciting TACE-dependent shedding of membrane-bound TGFα (pro-TGFα). Soluble TGFα binds to EGFR in the IRS of hair follicles. Activated/phosphorylated EGFR induces development of the IRS, which is required for proper formation of the hair shaft. Genetic deletions of these molecules (PA-PLA1α in this study and Kazantseva et al (2006), P2Y5 (Pasternack et al, 2008; Shimomura et al, 2008), TACE (Peschon et al, 1998; Hassemer et al, 2010), TGFα (Luetteke et al, 1993; Mann et al, 1993), EGFR (Luetteke et al, 1994)) result in aberrant hair formation in mice and/or humans.
Recent experiments with mouse embryonic fibroblasts suggest that LPA signalling shares a common pathway with EGFR signalling (Stortelers et al, 2008). Because LPA-induced EGFR transactivation can be mediated by not only P2Y5 but also by other LPA receptors such as LPA1 and LPA3 (Supplementary Figure S10B and C), it is reasonable to assume that LPA-producing enzymes and LPA receptors may cooperatively function in EGFR transactivation, thereby affecting other EGFR-involved physiological and pathophysiological conditions such as wound healing, development of heart and bone and progression of cancer (Sibilia and Wagner, 1995; Threadgill et al, 1995).
Activity of ADAMs is regulated by several kinases mainly through phosphorylation of a cytoplasmic region of these proteases in some systems (Mezyk et al, 2003; Edwards et al, 2008). These kinases are activated downstream of both receptor tyrosine kinases and GPCRs. Although some G proteins such as Gi and Gq are implicated in activation of ADAMs and consequent EGFR ligand release (Ohtsu et al, 2006; Uchiyama et al, 2009), little is known about G proteins involved in TACE activation. Our data establish a G12/13–RhoA–ROCK pathway, induced by LPA/P2Y5, as a novel regulator of TACE activation (Figure 6H–J). It should be noted that, consistent with our observation, Yanagida et al (2009) also showed that P2Y5 coupled with G13 and induced Rho signalling pathway. In general, G12/13 signalling, compared with Gs, Gi and Gq signalling, is considered an experimentally difficult pathway to detect in conventional GPCR assays such as cAMP, Ca2+ mobilization and promoter-driven luciferase (Siehler, 2009). Our results suggest that AP-TGFα release may be a useful readout, which may uncover previously unidentified or unappreciated GPCRs coupled with G12/13.
So far physiological importance of 2-acyl-LPA has not been elucidated. The present study demonstrated for the first time that 2-acyl-LPA is present in hair follicles and has a critical and specific role in hair follicle formation. Using the LC-MS/MS method established in this study, we demonstrated that the levels of 2-acyl-LPA species with unsaturated fatty acids were reduced in PA-PLA1α−/− hair follicles (Figure 2A–C), which demonstrates that PA-PLA1α produces 2-acyl-LPA in vivo. Thus, it seems likely that 2-acyl-LPA species with unsaturated fatty acids act as ligands of P2Y5 in hair follicles. In agreement with this, we found that P2Y5 was more strongly activated by LPA species with unsaturated fatty acids than by LPA species with saturated fatty acids (Supplementary Figure S8C). We previously showed that 2-acyl-LPA with unsaturated fatty acids preferentially activated LPA3 (Bandoh et al, 2000), which has a critical role in embryo implantation in the uterus (Ye et al, 2005). Together with these previous studies on LPA3, the present study on PA-PLA1α and P2Y5 underscores the importance of 2-acyl-LPA in regulating physiological events.
It is likely that 2-acyl-LPA generated on a membrane by PA-PLA1α immediately acts on P2Y5 in an autocrine and/or juxtacrine manner. This model is supported by the following observations: (i) PA-PLA1α is a secreted membrane-bound enzyme (Sonoda et al, 2002) and is concentrated on the plasma membrane (Hiramatsu et al, 2003); (ii) PA-PLA1α-expressing cells (Figure 7A and B), but not the conditioned media from the cells (data not shown), efficiently induced P2Y5-mediated AP-TGFα release in an autocrine or juxtacrine manner; and (iii) both PA-PLA1α (Figure 3G) and P2Y5 (Shimomura et al, 2008) are expressed in the IRS of the hair follicles. This model is also supported by the fact that 2-acyl-LPA is generally unstable due to a reaction known as spontaneous acyl migration. Furthermore, the fact that PA-PLA1α directly activated P2Y5 indicates that PA is located in the outer leaflet of the plasma membrane and, more importantly, PA transporter/scramblase exists, similar to a recently identified PS scamblase (Suzuki et al, 2010), because PA is intracellularly synthesized.
PA-PLA1α belongs to the lipase family, which includes pancreatic lipase and lipoprotein lipase. PA-PLA1α, together with PA-PLA1β and PS-specific PLA1 (PS-PLA1), form a subfamily within the lipase family. Unlike other lipases, these PLA1s exhibit strict substrate specificity to either PA (PA-PLA1α and β) (Sonoda et al, 2002; Hiramatsu et al, 2003) or PS (PS-PLA1) (Sato et al, 1997; Aoki et al, 2007). Our recent studies (paper in preparation) indicate that PS-PLA1 produces in vivo LPS, another lysophospholipid mediator with a variety of biological functions (Aoki et al, 2007; Makide et al, 2009). It is noteworthy that a unique feature of the products of these enzymes is that they have a fatty acid attached at the sn-2 position of the glycerol backbone. Our results show that 2-acyl-LPA is a potent ligand of P2Y5. Thus, from biochemical and structural points of view, we speculate that an ancestral member of the lipase family that had a specific role in production of lysophospholipid mediators, such as LPA and LPS, emerged and evolved through gene duplication, which eventually formed the PLA1 subfamily.
Materials and methods
Reagents, antibodies and plasmids
See Supplementary data.
Animals
Mice were maintained according to the Guidelines for Animal Experimentation of Tohoku University and the protocol was approved by the Institutional Animal Care and Use Committee at Tohoku University (No. 21-Pharm-Animal-21). Generation of PA-PLA1α−/− mice is described in Supplementary data. In all experiments using homozygous mice, littermates were used for a control.
Histology and in situ hybridization analyses
See Supplementary data.
Immunofluorescent staining
Paraformaldehyde-fixed tissues were embedded in OTC compound (Sakura Finetek) or in paraffin wax. For paraffin sections, antigen retrieval was performed using 10 mM citrate buffer (pH 7.0) at 120°C for 10 min. PA-PLA1α was detected using biotinylated anti-PA-PLA1α monoclonal antibody (clone mask1), followed by ABC Elite kit (Vector Laboratories) and TSA Alexa Fluor 488 kit (Invitrogen). Except for anti-PA-PLA1α monoclonal antibody, primary antibodies were visualized with secondary antibodies conjugated to Alexa Fluor 488, 568, 594 and 647. Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI). Fluorescent images were captured with a Zeiss LSM700 confocal microscope (Carl Zeiss).
Quantitative RT–PCR analysis
See Supplementary data. The primers used in the qRT–PCR analysis are listed in Supplementary data.
Isolation of epidermal keratinocytes and dorsal hair follicles
See Supplementary data.
Cell culture and transfection
See Supplementary data.
Quantification of endogenous TGFα release from keratinocytes
The media of primary mouse epidermal keratinocytes and HaCaT cells were replaced with 250 μl of supplement-free K-SFM and serum-free DMEM, respectively, on the day of cell stimulation. One hour later, primary keratinocytes were treated with various compounds for 2 h. For metalloprotease inhibitor treatment, cells were pretreated with 10 μM SDZ 242–484 for 30 min. TGFα levels in conditioned media were measured with a TGFα sandwich ELISA (human TGFα DuoSet Kit, R&D Systems) according to the manufacturer's instructions. The TGFα sandwich ELISA kit can recognize murine TGFα.
Immunoblot and immunoprecipitation
See Supplementary data.
Determination of soluble TGFα in hair follicles
Vibrissa hair follicles were surgically isolated from P15 C57BL6/J mice by removing the collagen capsules in ice-cold PBS containing 1 mM EDTA and 10 μM SDZ 242–484 (see also Supplementary Figure S4A). Ten vibrissa hair follicles per a single mouse were extracted with a MS-100R beads cell disrupter in lysis buffer (25 mM HEPES (pH 7.3), 250 mM sucrose, 10 mM MgCl2, 1 mM EDTA, 10 μg/ml PMSF, 20 μg/ml leupeptin and 10 μM SDZ 242–484). Lysate was centrifuged at 1000 g for 10 min. The supernatant was centrifuged at 200 000 g for 30 min. Protein concentration in the resulting supernatant (soluble fraction) was measured with a Micro BCA Protein Assay Kit (Pierce) and an equal amount of protein was used for TGFα sandwich ELISA.
Quantification of lysophospholipids by LC-MS/MS
See Supplementary data.
AP-TGFα shedding assay
AP-TGFα release was assayed by the previously described method (Tokumaru et al, 2000) with several modifications. HEK293 cells transiently expressing AP-TGFα with or without P2Y5 were detached with 0.05% Trypsin/0.52 mM EDTA, resuspended in Hank's balanced salt solution (HBSS) containing 5 mM HEPES (pH 7.4), and seeded in 90 μl at 2 × 104 cells per well in a 96-well plate. After 30 min, cells were treated with 10 μl of 10 × concentration of compounds for 1 h. After transferring 80 μl of conditioned media in each well to a new 96-well plate, 80 μl of p-NPP solution (10 mM p-NPP, 20 mM Tris–HCl (pH 9.5), 20 mM NaCl, 5 mM MgCl2) was added to both conditioned media and cells. OD405 was measured before and after 1 h incubation at 37°C with a VersaMax microplate reader (Molecular Devices). AP activity release was calculated from the increase of OD405 during 1 h incubation in the conditioned media (ΔODmedia) and the cell (ΔODcell) as follows (also see Figure 7A): AP activity in the conditioned media (APmedia) (%) is defined as the ratio of ΔODmedia to total ΔOD values (ΔODmedia plus ΔODcell); AP activity release (%) is determined by subtracting basal APmedia from APmedia in the presence of a compound where basal APmedia is defined as APmedia in the absence of a compound. Note that basal APmedia varied from 5 to 12%, depending on assay conditions.
For autocrine PA-PLA1α-initiated AP-TGFα release, HEK293 cells were transfected with combinations of PA-PLA1α, P2Y5 and AP-TGFα vectors in 100 μl of Opti-MEM (GIBCO) at 2 × 104 cells per each 96 well. Twenty-four hours after transfection, AP activity release was determined as described above. For paracrine PA-PLA1α-initiated AP-TGFα release, P2Y5 and AP-TGFα-expressing cells were prepared and seeded in a 96-well plate as described above. PA-PLA1α-expressing HEK293 cells were resuspended in HBSS and added to P2Y5 and AP-TGFα-expressing cells. After 1 h of incubation at 37°C, AP activity release was determined. For exogenous PLD treatment, 2 × 104 of P2Y5 and AP-TGFα-expressing cells and 2 × 104 of PA-PLA1α-expressing cells were mixed and incubated for 1 h in the presence or absence of bacterial PLD. AP activity release was determined. Catalytically inactive PA-PLA1α mutant (S154A) and empty vector were used as negative controls for PA-PLA1α and P2Y5, respectively.
Statistics
Columns and error bars represent means and standard deviations of independent experiments, respectively, unless otherwise indicated. Student's t-test was used to determine the significance of differences between groups. One-way ANOVA with Bonferroni's posttest analyses of multiple groups was employed for analyses of multiple groups. For all statistical tests, the 0.05 level of confidence was accepted for statistical significance.
Supplementary Material
Acknowledgments
We thank Dr Hitoshi Okochi and Dr Aki Osada (National Center for Global Health and Medicine, Japan) for technical advice on hair follicle analyses. JA was supported by grants from the National Institute of Biomedical Innovation, the National Project on Protein Structural and Functional Analyses from the Ministry of Education, Science, Sports and Culture of Japan (MEXT) and PRESTO from Japan Science and Technology Corporation. IA was a research fellow of the Japan Society for the Promotion of Science (DC1 1811607). IA was supported by a Grant-in-Aid for Young Scientists (B) (KAKENHI 21790058), Takeda Science Foundation and The Naito Science & Engineering Foundation. GDP thanks the Human Frontier of Science Program and the NIH (NS29632) for financial support.
Author contributions: AI, NA and JI performed the experiments and analysed the data. GDP developed LPA analogues. AI and JA prepared the paper. HA and JA supervised the work.
Footnotes
The authors declare that they have no conflict of interest.
References
- Anliker B, Chun J (2004) Lysophospholipid G protein-coupled receptors. J Biol Chem 279: 20555–20558 [DOI] [PubMed] [Google Scholar]
- Aoki J (2004) Mechanisms of lysophosphatidic acid production. Sem Cell Dev Biol 15: 477–489 [DOI] [PubMed] [Google Scholar]
- Aoki J, Inoue A, Makide K, Saiki N, Arai H (2007) Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 89: 197–204 [DOI] [PubMed] [Google Scholar]
- Aoki J, Inoue A, Okudaira S (2008) Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781: 513–518 [DOI] [PubMed] [Google Scholar]
- Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H (2002) Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277: 48737–48744 [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K (2000) Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett 478: 159–165 [DOI] [PubMed] [Google Scholar]
- Chansel D, Ciroldi M, Vandermeersch S, Jackson LF, Gomez AM, Henrion D, Lee DC, Coffman TM, Richard S, Dussaule JC, Tharaux PL (2006) Heparin binding EGF is necessary for vasospastic response to endothelin. FASEB J 20: 1936–1938 [DOI] [PubMed] [Google Scholar]
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J (2010) LPA receptors: subtypes and biological actions. Ann Rev Pharmacol Toxicol 50: 157–186 [DOI] [PubMed] [Google Scholar]
- Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH (2010) International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev 62: 579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer MH, Gross RW (1985) Separation of isomeric lysophospholipids by reverse phase HPLC. Lipids 20: 922–928 [DOI] [PubMed] [Google Scholar]
- Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A (1997) Signal characteristics of G protein-transactivated EGF receptor. EMBO J 16: 7032–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DR, Handsley MM, Pennington CJ (2008) The ADAM metalloproteinases. Mol Aspects Med 29: 258–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassemer EL, Le Gall SM, Liegel R, McNally M, Chang B, Zeiss CJ, Dubielzig RD, Horiuchi K, Kimura T, Okada Y, Blobel CP, Sidjanin DJ (2010) The waved with open eyelids (woe) locus is a hypomorphic mouse mutation in Adam17. Genetics 185: 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu T, Sonoda H, Takanezawa Y, Morikawa R, Ishida M, Kasahara K, Sanai Y, Taguchi R, Aoki J, Arai H (2003) Biochemical and molecular characterization of two phosphatidic acid-selective phospholipase A1s, mPA-PLA1alpha and mPA-PLA1beta. J Biol Chem 278: 49438–49447 [DOI] [PubMed] [Google Scholar]
- Ishii S, Noguchi K, Yanagida K (2009) Non-EDG family lysophosphatidic acid (LPA) receptors. Prostaglandins Other Lipid Mediat 89: 57–65 [DOI] [PubMed] [Google Scholar]
- Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD (2008) Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol 9: 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, Dai X, Alegre ML, Fuchs E (2003) GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev 17: 2108–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantseva A, Goltsov A, Zinchenko R, Grigorenko AP, Abrukova AV, Moliaka YK, Kirillov AG, Guo Z, Lyle S, Ginter EK, Rogaev EI (2006) Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science 314: 982–985 [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Oyama A, Ishii R, Miura I, Amano T, Ishii Y, Yoshikawa Y, Masuya H, Wakana S, Shiroishi T, Taya C, Yonekawa H (2003) A small deletion hotspot in the type II keratin gene mK6irs1/Krt2–6 g on mouse chromosome 15, a candidate for causing the wavy hair of the caracul (Ca) mutation. Genetics 165: 721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Keino-Masu K, Ohto T, Sugiyama F, Takahashi S, Masu M (2009) Autotaxin/lysophospholipase D-mediated LPA signaling is required to form distinctive large lysosomes in the visceral endoderm cells of the mouse yolk Sac. J Biol Chem 284: 33561–33570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottirsch G, Koch G, Feifel R, Neumann U (2002) Beta-aryl-succinic acid hydroxamates as dual inhibitors of matrix metalloproteinases and tumor necrosis factor alpha converting enzyme. J Med Chem 45: 2289–2293 [DOI] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Praetzel S, Winter H, Schweizer J (2003) K6irs1, K6irs2, K6irs3, and K6irs4 represent the inner-root-sheath-specific type II epithelial keratins of the human hair follicle. J Invest Dermatol 120: 512–522 [DOI] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Praetzel-Wunder S, Helmke B, Schirmacher P, Schweizer J (2006) K25 (k25irs1), k26 (k25irs2), k27 (k25irs3), and k28 (k25irs4) represent the type I inner root sheath keratins of the human hair follicle. J Invest Dermatol 126: 2377–2386 [DOI] [PubMed] [Google Scholar]
- Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F (2005) Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874 [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC (1994) The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 8: 399–413 [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC (1993) TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73: 263–278 [DOI] [PubMed] [Google Scholar]
- Makide K, Kitamura H, Sato Y, Okutani M, Aoki J (2009) Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat 89: 135–139 [DOI] [PubMed] [Google Scholar]
- Mann GB, Fowler KJ, Gabriel A, Nice EC, Williams RL, Dunn AR (1993) Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell 73: 249–261 [DOI] [PubMed] [Google Scholar]
- Mezyk R, Bzowska M, Bereta J (2003) Structure and functions of tumor necrosis factor-alpha converting enzyme. Acta Biochim Polonica 50: 625–645 [PubMed] [Google Scholar]
- Mills GB, Moolenaar WH (2003) The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3: 582–591 [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, van Meeteren LA, Giepmans BN (2004) The ins and outs of lysophosphatidic acid signaling. Bioessays 26: 870–881 [DOI] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA (2007) Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 117: 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu H, Dempsey PJ, Eguchi S (2006) ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol 291: C1–10 [DOI] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC (2008) G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet 40: 329–334 [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA (1998) An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284 [DOI] [PubMed] [Google Scholar]
- Peters T, Sedlmeier R, Bussow H, Runkel F, Luers GH, Korthaus D, Fuchs H, Hrabe de Angelis M, Stumm G, Russ AP, Porter RM, Augustin M, Franz T (2003) Alopecia in a novel mouse model RCO3 is caused by mK6irs1 deficiency. J Invest Dermatol 121: 674–680 [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888 [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Aoki J, Nagai Y, Dohmae N, Takio K, Doi T, Arai H, Inoue K (1997) Serine phospholipid-specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J Biol Chem 272: 2192–2198 [DOI] [PubMed] [Google Scholar]
- Schweizer J, Langbein L, Rogers MA, Winter H (2007) Hair follicle-specific keratins and their diseases. Exp Cell Res 313: 2010–2020 [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Wajid M, Ishii Y, Shapiro L, Petukhova L, Gordon D, Christiano AM (2008) Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat Genet 40: 335–339 [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Wajid M, Petukhova L, Shapiro L, Christiano AM (2009) Mutations in the lipase H gene underlie autosomal recessive woolly hair/hypotrichosis. J Invest Dermatol 129: 622–628 [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269: 234–238 [DOI] [PubMed] [Google Scholar]
- Siehler S (2009) Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol 158: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Hla T (2009) Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res 50 (Suppl): S293–S298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, Taguchi R, Inoue K, Arai H (2002) A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J Biol Chem 277: 34254–34263 [DOI] [PubMed] [Google Scholar]
- Stortelers C, Kerkhoven R, Moolenaar WH (2008) Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics 9: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Umeda M, Sims PJ, Nagata S (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468: 834–838 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H (2006) Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 281: 25822–25830 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Miura I, Yoshiki A, Kato Y, Yokoyama H, Shinogi A, Masuya H, Wakana S, Tamura M, Shiroishi T (2007) Mutations in the helix termination motif of mouse type I IRS keratin genes impair the assembly of keratin intermediate filament. Genomics 90: 703–711 [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, Barnard JA, Yuspa SH, Coffey RJ, Magnuson T (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269: 230–234 [DOI] [PubMed] [Google Scholar]
- Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, Hanakawa Y, Ohmoto H, Yoshino K, Shirakata Y, Matsuzawa Y, Hashimoto K, Taniguchi N (2000) Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol 151: 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama K, Saito M, Sasaki M, Obara Y, Higashiyama S, Nakahata N (2009) Thromboxane A2 receptor-mediated epidermal growth factor receptor transactivation: involvement of PKC-delta and PKC-epsilon in the shedding of epidermal growth factor receptor ligands. Eur J Pharm Sci 38: 504–511 [DOI] [PubMed] [Google Scholar]
- van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J (2006) Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 26: 5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Yin J, Yu FS (2007) Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci 48: 636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata Y, Shirakata Y, Tokumaru S, Yang L, Dai X, Tohyama M, Tsuda T, Sayama K, Iwai M, Horiuchi M, Hashimoto K (2006) A novel function of angiotensin II in skin wound healing. Induction of fibroblast and keratinocyte migration by angiotensin II via heparin-binding epidermal growth factor (EGF)-like growth factor-mediated EGF receptor transactivation. J Biol Chem 281: 13209–13216 [DOI] [PubMed] [Google Scholar]
- Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, Taguchi R, Shimizu T, Ishii S (2009) Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem 284: 17731–17741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J (2005) LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J (2006) An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II-mediated cardiac hypertrophy. Circ Res 99: 528–536 [DOI] [PubMed] [Google Scholar]
- Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, Natarajan V (2006) Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem 281: 19501–19511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.