Abstract
Histone deacetylases (HDACs) deacetylate histones and non-histone proteins, thereby affecting protein activity and gene expression. The regulation and function of the cytoplasmic class IIb HDAC6 in endothelial cells (ECs) is largely unexplored. Here, we demonstrate that HDAC6 is upregulated by hypoxia and is essential for angiogenesis. Silencing of HDAC6 in ECs decreases sprouting and migration in vitro and formation of functional vascular networks in matrigel plugs in vivo. HDAC6 regulates zebrafish vessel formation, and HDAC6-deficient mice showed a reduced formation of perfused vessels in matrigel plugs. Consistently, overexpression of wild-type HDAC6 increases sprouting from spheroids. HDAC6 function requires the catalytic activity but is independent of ubiquitin binding and deacetylation of α-tubulin. Instead, we found that HDAC6 interacts with and deacetylates the actin-remodelling protein cortactin in ECs, which is essential for zebrafish vessel formation and which mediates the angiogenic effect of HDAC6. In summary, we show that HDAC6 is necessary for angiogenesis in vivo and in vitro, involving the interaction and deacetylation of cortactin that regulates EC migration and sprouting.
Keywords: angiogenesis, cortactin, deacetylation, endothelial cells, histone deacetylases
Introduction
Histone deacetylases (HDACs) are known repressors of gene transcription by deacetylating histone proteins, favouring condensation of chromatin structure (for review see Narlikar et al (2002)). Interestingly, HDACs remove acetyl groups from lysine residues of both histone and non-histone proteins, thereby affecting protein stability, activity and binding affinity as recently described (Bolden et al, 2006; Choudhary et al, 2009; Spange et al, 2009). The HDAC family consists of four classes: class I (HDAC1, 2, 3 and 8), class II (class IIa: HDAC4, 5, 7 and 9; class IIb: HDAC6 and 10), class III (sirtuin 1–7) and class IV (HDAC11).
Broad-spectrum inhibitors of class I and II HDACs inhibit angiogenesis in vitro and in animal models (Kim et al, 2001; Rossig et al, 2002; Qian et al, 2006b). In addition, class I and II HDAC inhibitors abrogate endothelial differentiation of progenitor cells (Rossig et al, 2005). Because of their repressive effects on tumour-driven angiogenesis, HDAC inhibitors meanwhile represent promising anti-cancer agents in early phase clinical trials (Carew et al, 2008; Mottet and Castronovo, 2010). On the basis of these findings, recent studies addressed the specific function of individual HDAC isoenzymes for endothelial cells (ECs) and angiogenesis (Chang et al, 2006; Mottet et al, 2007; Ha et al, 2008; Martin et al, 2008; Wang et al, 2008; Urbich et al, 2009). In particular, class IIa HDAC7 is an essential regulator of embryonic blood vessel development (Chang et al, 2006). Moreover, HDAC7 controls endothelial angiogenic functions, such as tube formation, migration and proliferation in vitro (Mottet et al, 2007; Martin et al, 2008; Margariti et al, 2010). Recent studies further indicate that protein kinase D-dependent phosphorylation and nuclear export (or cytoplasmic accumulation) of HDAC5 and HDAC7 plays an important role in VEGF-induced angiogenesis in vitro (Ha et al, 2008; Wang et al, 2008). In our previous study, we identified HDAC5 as a repressor of angiogenic gene expression in EC and angiogenesis (Urbich et al, 2009).
In contrast to class IIa HDACs, the role of class IIb HDACs for angiogenesis is largely unexplored. HDAC6 is localized predominantly in the cytoplasm and is the only member of the HDAC family that harbours a full duplication of its deacetylase homology region, followed by a specific ubiquitin-binding domain at the C-terminal end (Valenzuela-Fernandez et al, 2008). HDAC6 interacts with misfolded ubiquitinated proteins (Seigneurin-Berny et al, 2001; Hook et al, 2002), concentrates toxic protein aggregates and facilitates the aggresome-dependent clearance of these proteins (Kawaguchi et al, 2003; Boyault et al, 2007; Lee et al, 2010). Thus, the HDAC6-dependent clearance of misfolded proteins plays a role in the pathogenesis of neurodegenerative proteinopathies (Pandey et al, 2007). Consistent with its localization in the cytoplasm, the activities of HDAC6 are mainly independent of histones, but instead involve the deacetylation of cytoplasmic substrates such as α-tubulin, Hsp90 and cortactin (Hubbert et al, 2002; Matsuyama et al, 2002; Zhang et al, 2003, 2007; Kovacs et al, 2005). HDAC6 is a major determinant in the control of cell motility by regulation of the tubulin as well as the actin network (Gao et al, 2007; Zhang et al, 2007). HDAC6 regulates the binding of cortactin to actin in a deacetylation-dependent manner, and thereby controls the branching of the actin network at the leading edge of cells (Zhang et al, 2007). Beyond deacetylation, HDAC6 also modulates cell migration by deacetylase-independent mechanisms (Cabrero et al, 2006; Zilberman et al, 2009). Recently, it has been shown that HDAC6 and HDAC10 play an important role in Hsp-mediated regulation of VEGFR in cancer cells (Park et al, 2008). Moreover, HDAC6 associates with HIF-1α to increase its stability and transcriptional activity in cancer cells (Qian et al, 2006a). During revision of this manuscript, one study demonstrates that HDAC6 promotes angiogenesis by regulating the polarization and migration of vascular ECs in a microtubule end-binding protein 1-dependent manner (Li et al, 2011). Here, we assessed the role of HDAC6 for EC migration and sprout formation in vitro and angiogenesis in vivo, and explored the underlying mechanism involving the deacetylation of cortactin as a target of HDAC6 in ECs.
Results
HDAC6 is required for angiogenesis in vitro
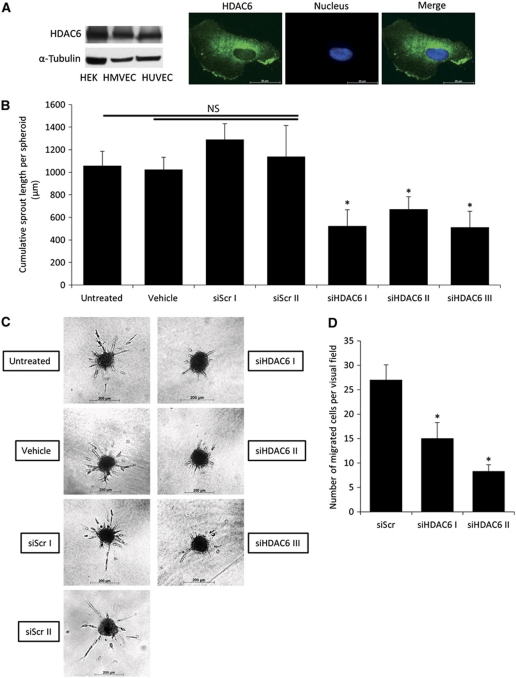
To study the role of HDAC6 for EC functions and angiogenesis, we first addressed the expression and localization of HDAC6 in EC. HDAC6 mRNA is expressed in ECs from different sources, such as human umbilical vein endothelial cells (HUVECs), microvascular endothelial cells and coronary artery endothelial cells, as well as in non-ECs such as coronary artery smooth muscle cells and cardiomyocytes (Supplementary Figure 1A). HDAC6 protein expression was detectable in ECs (Figure 1A, left panel and Supplementary Figure 1B), and HDAC6 protein is mainly localized in the cytoplasm of HUVECs (Figure 1A, right panel). To assess whether HDAC6 contributes to angiogenic functions in ECs, we performed different in vitro angiogenesis assays. Downregulation of HDAC6 in HUVECs with three independent siRNA oligonucleotides efficiently decreases HDAC6 expression, without affecting the expression of other HDACs, such as HDAC9 (Supplementary Figure 1C). Silencing of HDAC6 does not affect cell viability (Supplementary Figure 1D), but profoundly reduced sprouting in a three-dimensional spheroid assay as measured by the cumulative sprout length (Figure 1B and C), number of sprouts (Supplementary Figure 1E) and number of branch points (Supplementary Figure 1F) per spheroid. Moreover, downregulation of HDAC6 in HUVECs decreases tube formation in a matrigel assay (Supplementary Figure 1G), reduced migration of HUVECs in a scratched wound assay (Supplementary Figure 1H) and significantly inhibits cell migration in a transwell migration assay (Figure 1D).
Figure 1.
Knockdown of HDAC6 decreases endothelial cell sprouting and migration in vitro. (A) Western blot analysis of HDAC6 expression in cultured endothelial and non-endothelial cells (left panel). α-Tubulin serves as loading control. Immunofluorescence analysis of HDAC6 (shown in green) localization in HUVECs (right panel). Nuclei are stained with Hoechst 33342 in blue. (B) Capillary-like sprouting from spheroids was measured after HDAC6 silencing with three independent HDAC6 siRNAs compared to two different control siRNAs, untreated and vehicle (only transfection reagent)-treated cells. The spheroid assay was performed 24 h after siRNA transfection. Data are shown as mean cumulative sprout length per spheroid (*P<0.05 versus Scr I and Scr II, n=3–10). (C) Representative pictures are shown. (D) HUVECs were transfected with HDAC6 or control siRNA for 48 h, and cell migration was monitored in a transwell migration assay for 4 h under basal conditions. Migrated cells were counted by staining the nuclei with DAPI (n=4). HEK, human embryonic kidney cell.
Since spheroid sprouting was most efficiently suppressed by HDAC6 silencing and sprouting angiogenesis requires the specification of the ECs to tip or stalk cells (Gerhardt et al, 2003), we determined whether HDAC6 silencing alters the position of ECs within the sprouts. In competitive spheroid assays, in which control or HDAC6-silenced HUVECs were labelled with the fluorescent proteins CFP or YFP, the HDAC6-deficient ECs showed a similar positioning in the growing sprouts compared to the control cells (Supplementary Figure 2A and B), indicating that HDAC6 depletion does not influence the ability of ECs to become tip or stalk cells.
Hypoxia transcriptionally upregulates HDAC6
Because the physiological regulation of HDAC6 is largely unknown, we tested several stimuli that are known to control endothelial responses. Whereas prolonged shear stress for 72 h does not affect HDAC6 mRNA expression in HUVECs (Supplementary Figure 2C), HDAC6 mRNA expression at 24 h and HDAC6 protein expression at 36 h is increased by hypoxia (Supplementary Figure 2D–G).
HDAC6 contributes to vessel formation during zebrafish embryonic development
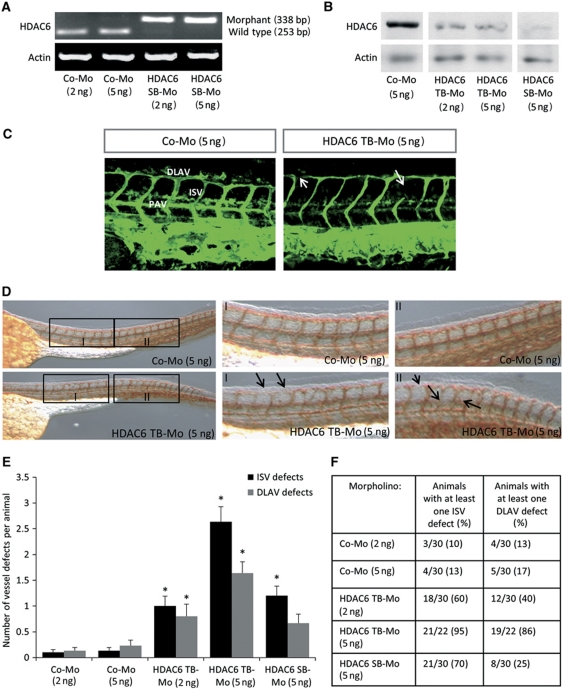
Having shown that knockdown of HDAC6 decreases in vitro angiogenesis, we determined whether HDAC6 contributes to endothelial sprouting and vessel formation in vivo. Therefore, we studied the effect of HDAC6 silencing during zebrafish embryonic development in a tg(fli1:EGFP) zebrafish line expressing EGFP in the developing vessels under the control of the fli-1 promoter. PCR of FACS-sorted EGFP+ ECs from the developing zebrafish revealed that HDAC6 mRNA is present in ECs yielding a band of 253 bp (Supplementary Figure 3A and B). Knockdown of HDAC6 was achieved by injection of morpholinos (Mos) targeting the ATG-start codon for translation-blocking (HDAC6 TB-Mo) or by injection of a splice-blocking Mo (HDAC6 SB-Mo), resulting in an aberrant splicing of HDAC6 mRNA (Figure 2A and Supplementary Figure 3C). PCR and western Blot analyses confirmed a robust silencing of HDAC6 with both Mos (Figure 2A and B). Confocal fluorescence microscopy shows defects in intersegmental vessels (ISVs) and dorsal longitudinal anastomotic vessels (DLAVs) for the HDAC6 morphants at 48 h after fertilization (Figure 2C), without other obvious morphological defects. For quantification of vessel defects, zebrafish embryos were stained against EGFP by whole-mount antibody staining (Figure 2D). Silencing of HDAC6 significantly increases the number of defects for ISVs and DLAVs for both tested Mos compared to a control Mo (Figure 2E and F). For the translation-blocking Mo, a similar increase of defects for the ISVs and the DLAVs was observed, which was dose dependent. In summary, these data indicate that HDAC6 contributes to vessel formation in zebrafish.
Figure 2.
Silencing of HDAC6 impairs embryonic vessel formation in zebrafish. (A) Aberrant splicing of Danio rerio HDAC6 mRNA after HDAC6 splice-blocking Mo injection by PCR. Injection of the HDAC6 SB-Mo generated at 24 h post fertilization a morphant signal of 338 bp, whereas the HDAC6 wt signal completely disappeared (253 bp), showing the functionality of the Mo. Whole-zebrafish embryo mRNA was isolated 24 h after Mo injection and subjected to RT–PCR. Actin mRNA expression serves as loading control. (B) HDAC6 protein expression was analysed in whole-zebrafish embryo lysate at 24 h after injection of HDAC6 translation-blocking or splice-blocking Mo. Protein lysates were subjected to western blotting with HDAC6-specific antibody. Actin was used as loading control. C–F phenotyping of HDAC6 morphants 48 h post fertilization. (C) Representative confocal fluorescence pictures of vessel in the anterior part of tg(fli1:EGFP) zebrafish embryos after injection of HDAC6 translation-blocking or control Mo. Arrows indicate vessel defects. (D–F) For quantification of vessel defects, HDAC6 Mo- or control Mo-treated zebrafish embryos were stained for GFP using anti-GFP antibody. (D) Representative overview pictures and higher magnification of two regions of the anterior part of control-Mo-injected and HDAC6 TB-Mo-injected embryos are shown. Arrows indicate vessel defects. (E) Quantification of defects in ISVs and DLAVs for HDAC6 and control morphants. Statistical significance was calculated for the respective Mo concentration (n=22–30). (F) Penetration of vessel defects for HDAC6 or control Mo. Numbers represent the number of animals and percentage of animals with at least one ISV or DLAV defect. DLAV, dorsal longitudinal anastomotic vessel; ISV, intersegmental vessels; PAV, parachordal vessel. Figure source data can be found with the Supplementary Information.
HDAC6 contributes to human vessel maturation and perfusion in vivo
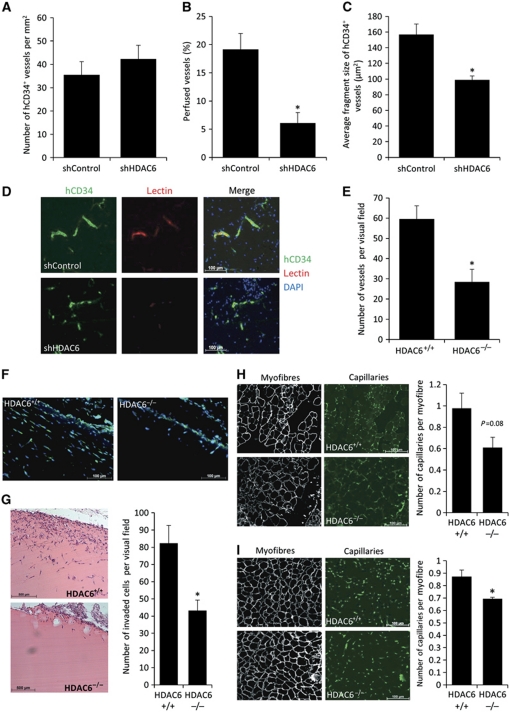
As a model for human vessel formation, we used an in vivo spheroid assay in mice as previously described (Alajati et al, 2008). Therefore, we transduced HUVEC with control or HDAC6 shRNA lentiviral vectors, allowing long-term silencing. Knockdown of HDAC6 was assessed by western blot (Supplementary Figure 3D), and the specificity of HDAC6 shRNA was further confirmed by demonstrating that overexpression of a non-targeting HDAC6 (HDAC6 wild-type (wt) lacking the binding site for shRNA within the 3′UTR) rescued the effect of HDAC6 silencing on sprouting in vitro (Supplementary Figure 3E). For the in vivo assay, spheroids of the transduced cells were mixed with matrigel and injected subcutaneously into mice for 3 weeks (Supplementary Figure 3F). Immunohistological analysis shows that silencing of HDAC6 in human ECs does not affect the overall microvascular density (Figure 3A and D), but significantly reduces the perfusion of the vasculature compared to control (Figure 3B and D). Furthermore, the vessels of the developing vasculature were significantly smaller in vessel size compared to control vessels (Figure 3C and D). To further assess the in vivo relevance of HDAC6, neovascularization was determined in implanted matrigel plugs in HDAC6 knockout mice. The number of perfused vessels that invaded into the implanted matrigel plugs in vivo is decreased in HDAC6 knockout mice (Figure 3E and F). Additionally, the number of invaded cells measured by H&E staining is significantly decreased (Figure 3G). Furthermore, 14 days after induction of hind limb ischaemia HDAC6 knockout mice tend to have a reduced capillary density in the thigh muscle (Figure 3H) and show a significant reduction of capillary density in the lower leg (Figure 3I) compared to wt mice. However, in contrast to zebrafish, we did not observe developmental vessel defects in HDAC6 knockout mice as assessed by the vascular outgrowth of the retina (Supplementary Figure 3G and H). Taken together, HDAC6 contributes to the formation and perfusion of mature human and mouse vessels in vivo in adult mice.
Figure 3.
HDAC6 contributes to human vessel maturation and perfusion in an in vivo mouse model. (A–C) Immunohistological analysis of human neovessel formation after subcutaneous injection of HUVEC spheroids in a matrigel–fibrin matrix in mice. Human vessels were stained with hCD34 antibody. (A) Quantification of microvascular density. (B) Perfusion was analysed as percentage of TRIC-lectin-positive vessels in relation to hCD34-positive vessels. (C) Average fragment size of human vessel was determined measuring the total outline surface of hCD34-positive vessels in relation to the total number of human vessels using ImageJ. (A–C) shControl, n=5; shHDAC6, n=4. *P<0.05. (D) Representative pictures of the human vasculature (shown in green) and perfusion (shown in red). Nuclei are shown in blue. (E) HDAC6 knockout mice (HDAC6−/−, n=6) and wild-type (HDAC6+/+, n=5) mice were subjected to a matrigel plug assay. Lectin-positive structures were counted manually. *P<0.05. (F) Representative images of invaded lectin-positive vessels into the matrigel plug. (G) Quantification of invaded cells into the matrigel plug by H&E staining (right panel, n=5 each). Left panel shows representative images. *P<0.05. (H, I) HDAC6 knockout mice (HDAC6−/−) and wild-type (HDAC6+/+) mice were subjected to hind limb ischaemia. Capillary density was examined histologically in the thigh (H; n=4 for HDAC6+/+ versus n=6 for HDAC6−/−) and the lower leg (I; n=5 each). Left panels show representative images of the capillaries stained for lectin and myofibres stained for laminin. Right panels show the quantification of number of capillaries per myofibre. *P<0.05.
HDAC6 does not contribute to tumour vascularization in a Lewis lung carcinoma model
Broad-spectrum HDAC inhibitors abrogate tumour growth, in part, by inhibition of tumour vascularization (Kim et al, 2001). Having shown that HDAC6 is required for neovascularization in adult mice, we asked whether HDAC6 might contribute to tumour vascularization. Therefore, we injected wt Lewis lung carcinoma cells subcutaneously into male HDAC6 knockout mice and control mice. Unexpectedly, male HDAC6 knockout mice show an increased tumour growth (Supplementary Figure 4A–C) compared to control mice, whereas tumour vascularization was not affected (Supplementary Figure 4D). Since HDAC6 was implicated in oestrogen signalling and breast cancer (Azuma et al, 2009), we additionally reproduced this study in female mice with similar results (Supplementary Figure 4E–I). These data suggest that in mice lacking HDAC6, the growth of subcutaneously injected Lewis lung carcinoma cells is promoted, whereas angiogenesis is not affected.
HDAC6 promotes angiogenesis independently of α-tubulin deacetylation and ubiquitin binding
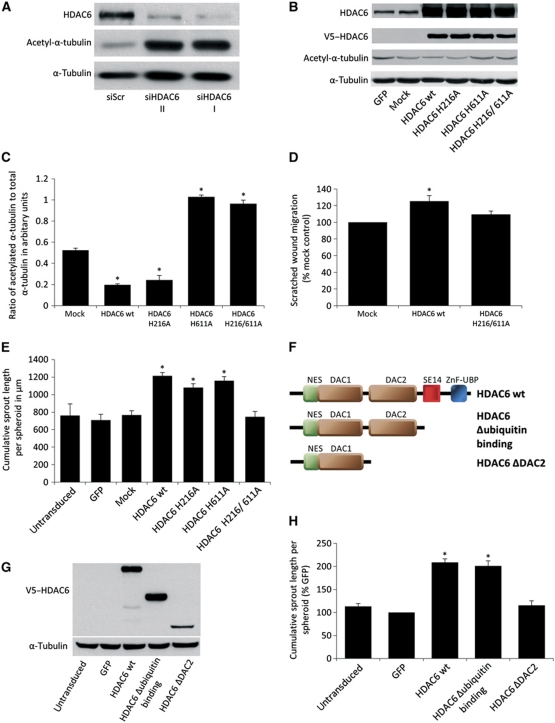
HDAC6-dependent regulation of cell migration was attributed, in part, to its ability to interact with the tubulin cytoskeleton (Hubbert et al, 2002). The stability of a dynamic pool of tubulin fibres depends on the acetylation state of α-tubulin. Thus, deacetylated α-tubulin is less stable and facilitates migration by an increased fibre turnover (Matsuyama et al, 2002). In ECs, silencing of HDAC6 by two independent siRNAs increases the acetylation of α-tubulin (Figure 4A). To determine whether the control of angiogenesis by HDAC6 depends on its α-tubulin deacetylation activity, we used the HDAC6-specific inhibitor tubacin, which mainly blocks the α-tubulin deacetylation activity, whereas the HDAC activity is mainly unaffected (Haggarty et al, 2003). Application of 10 μM tubacin increases α-tubulin acetylation about 25-fold in HUVECs (Supplementary Figure 5A and B), but does not affect EC sprouting, migration and network formation (Supplementary Figure 5C), indicating that acetylation of α-tubulin does not directly regulate HUVEC migration. To further confirm these results and to determine whether the deacetylation activity of HDAC6 is necessary for angiogenesis, we overexpressed different HDAC6 constructs exhibiting single mutations in either one (HDAC6 H216A and HDAC6 H611A) or both deacetylation domains (HDAC6 H216/611A). The contribution of the single HDAC domains for catalytic activity is currently controversially discussed (Grozinger et al, 1999; Haggarty et al, 2003; Zhang et al, 2003; Zou et al, 2006) (for details see Supplementary Figure 5D). However, all studies consistently indicate that mutations in both HDAC domains abolish catalytic activity. Under basal conditions, overexpression of these constructs only modestly affect α-tubulin acetylation (Figure 4B), probably because of the low levels of acetylated α-tubulin in HUVECs. To increase the sensitivity of our experimental readout, we acetylated α-tubulin in HUVECs by treatment with tubacin and monitored the activity of the constructs by ELISA after washout of the inhibitor. We observed an increased α-tubulin deacetylation in HDAC6 wt and HDAC6 H216A-overexpressing cells compared to mock-transduced cells (Figure 4C and Supplementary Figure 5E), demonstrating the α-tubulin deacetylation activity of these constructs. In contrast, HDAC6 H611A- and HDAC6 H216/611A-overexpressing cells show an even higher acetylation of α-tubulin compared to mock-transduced cells (Figure 4C and Supplementary Figure 5E), indicating that these constructs exhibit no tubulin deacetylation activity and might compete with endogenous HDAC6 for α-tubulin. Under basal conditions, overexpression of HDAC6 wt increases EC migration (Figure 4D and Supplementary Figure 5F) and sprout formation (Figure 4E), whereas the deacetylation-deficient construct HDAC6 H216/611A shows no effect. Additionally, overexpression of the single-mutation constructs HDAC6 H216A and HDAC6 H611A increases endothelial sprouting, although the HDAC6 H611A mutant shows no α-tubulin deacetylation activity. In summary, these data indicate that HDAC6 regulates cell migration and angiogenesis dependent on deacetylation activity, but independent of α-tubulin deacetylation.
Figure 4.
HDAC6-mediated endothelial sprouting depends on catalytic activity, but is independent of α-tubulin deacetylation and ubiquitin binding. (A) Western blot of HUVEC protein lysate showing the level of acetylated α-tubulin at 72 h after transfection with two different HDAC6 siRNAs. α-Tubulin serves as loading control (n=3). (B–E) HUVECs were transduced with virus encoding HDAC6 wt and mutated HDAC6 constructs possessing single-point mutations in either one (HDAC6 H216A and HDAC6 H611A) or both deacetylation domains (HDAC6 H216/611A). As controls serve either untransduced, GFP or mock (empty pLenti4 virus)-transduced cells. (B) HUVECs were transduced with different HDAC6 constructs for 72 h. Overexpression of the constructs and level of the α-tubulin acetylation were measured by western blot. Total level of α-tubulin serves as loading control (n=3). (C) HUVEC overexpressing different HDAC6 constructs were treated for 4 h with 2.5 μM tubacin following washout of the inhibitor. Cell lysates were taken 90 min after washout, and levels of acetylated α-tubulin and total tubulin were measured by ELISA. Data are presented as the ratio of acetylated α-tubulin to total α-tubulin (n=3). (D) HUVECs were transduced with different HDAC6 constructs for 6 days, followed by scratched wound migration assay for 24 h. Data are presented as migrated distance in percentage (%) mock control (n=4). (E) HUVECs were transduced with different HDAC6 constructs for 24 h followed by spheroid assay (*P<0.05 versus untransduced control, GFP and mock control, n=3). (F–H) HUVECs were transduced with virus encoding HDAC6 wt and HDAC6 C-terminal deletion constructs. The HDAC6 Δubiquitin binding constructs lacks the ZnF-UBP domain, which is necessary to bind ubiquitinated proteins. The HDAC6 ΔDAC2 construct lacks the second catalytic domain and the C terminus. (F) Scheme of HDAC6 wt and HDAC6 deletion constructs. (G) HUVECs were stably transduced with HDAC6 wt and C-terminal deletion constructs. Overexpression of the constructs was monitored by western blot using V5 tag antibody. α-Tubulin serves as loading control. (H) HUVECs were stably transduced with HDAC6 wt and C-terminal deletion constructs and subjected to spheroid assay (n=5). Data are presented as percentage (%) GFP (*P<0.05 versus GFP). DAC, deacetylation domain; NES, nuclear export sequence; SE14, repeat region containing serine and glutamic acid; ZnF-UBP, zinc finger ubiquitin-binding domain. Figure source data can be found with the Supplementary Information.
HDAC6 possesses two separate catalytic domains and a ZnF-UBP domain, which interact with polyubiquitinated proteins, thereby facilitating clearance of misfolded proteins (Seigneurin-Berny et al, 2001; Hook et al, 2002; Kawaguchi et al, 2003). To define which of these domains are necessary for angiogenesis, we overexpressed different HDAC6 C-terminal deletion constructs (Figure 4F and G). Overexpression of a HDAC6 construct lacking the ubiquitin-binding domain (HDAC6 Δubiquitin binding) still increases sprouting (Figure 4H), indicating that the transport of misfolded proteins under these conditions does not contribute to the proangiogenic effect of HDAC6. In contrast, overexpression of a HDAC6 construct lacking the second deacetylation domain and the C terminus (HDAC6 ΔDAC2) does not induce endothelial sprouting (Figure 4H), suggesting that either the activity of the second domain or binding to the second domain is necessary.
HDAC6 deacetylates cortactin in ECs
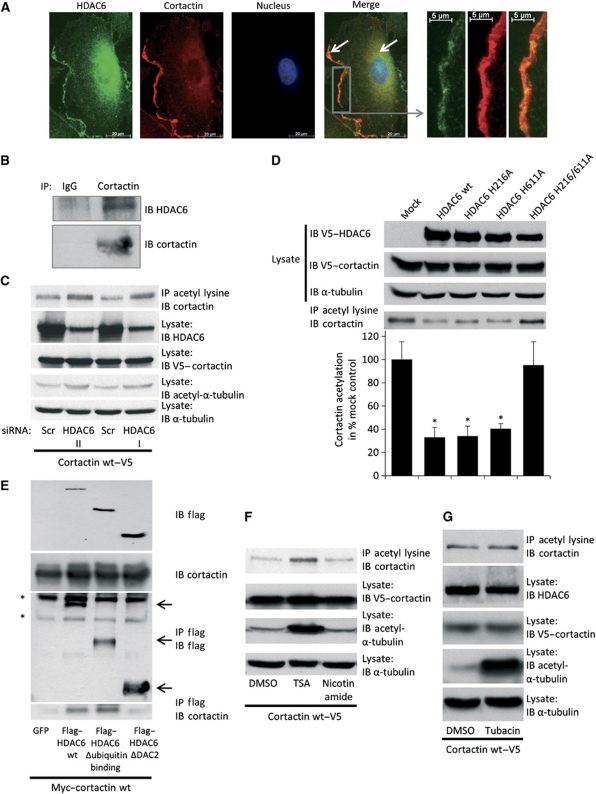
Having demonstrated that deacetylation of α-tubulin is not responsible for the regulation of angiogenesis by HDAC6, we ask whether HDAC6 regulates migration by interfering with the actin cytoskeleton. HDAC6 was shown to interact with the actin-remodelling and -stabilizing enzyme cortactin in HeLa cells (Zhang et al, 2007). In ECs, cortactin colocalizes with HDAC6 in the cytoplasm and at the membrane (Figure 5A). In addition, cortactin as well as HDAC6 colocalize with actin at the cell membrane at the leading edge of HUVECs (Supplementary Figure 6A and B). In cells overexpressing cortactin, the tagged protein co-immunoprecipitates with endogenous HDAC6 (Supplementary Figure 6C), and vice versa, overexpressed HDAC6 co-immunoprecipitates with endogenous cortactin (Supplementary Figure 6D). Moreover, endogenous HDAC6 interacts with endogenous cortactin (Figure 5B). Knockdown of HDAC6 by siRNA increases acetylation of cortactin (Figure 5C and Supplementary Figure 6E). Furthermore, overexpression of the sprouting-promoting constructs HDAC6 wt, HDAC6 H216A and HDAC6 H611A decreases cortactin acetylation under basal conditions, whereas HDAC6 H216/611A shows no effect (Figure 5D). HDAC6 wt and mutants with a maintained proangiogenic activity (Figure 4H) were shown to bind to cortactin (Figure 5E and (Zhang et al, 2007)), whereas another mutant (HDAC6 ΔDAC2), which does not affect sprouting, did not bind to cortactin (Figures 4H and 5E).
Figure 5.
HDAC6 deacetylates cortactin in endothelial cells. (A) Immunofluorescence analysis of HDAC6 and cortactin localization in HUVECs. HUVECs were stained with HDAC6- (shown in green) and cortactin- (shown in red) specific antibodies and Alexa 488- and Alexa 594-conjugated secondary antibodies. Nuclei were stained with Hoechst 33342 (shown in blue). White arrows indicate colocalization of HDAC6 and cortactin at the cell membrane and in the cytoplasm. Right panel shows a higher magnification of the cell membrane at the leading edge of the cell. (B) For endogenous immunoprecipitation, HUVEC lysate was incubated with cortactin-specific antibody or control IgG antibody. The ability of endogenous HDAC6 to bind endogenous cortactin was assessed by western blot of the precipitate using an HDAC6-specific antibody (n=2). (C) HUVECs were stably transduced with V5-tagged cortactin wt virus, followed by HDAC6 or control siRNA transfection for 72 h. Equal amounts of protein lysate were subjected to immunoprecipitation reaction with anti-acetyl-lysine antibody conjugated to agarose beads. Bound proteins were resolved and used for western blot analysis of cortactin acetylation levels utilizing anti-cortactin-specific antibody. HDAC6 and acetylated α-tubulin levels were measured in total cell lysate. As loading controls serve α-tubulin and cortactin expression in total cell lysate before precipitation (n=3). (D) HUVECs were stably transduced with V5-tagged cortactin wt virus, followed by transduction with different HDAC6 constructs. Immunoprecipitation was done as described in C. Lower panel shows quantification (n=4). (E) HUVECs were transiently transfected with Myc-tagged cortactin plasmid together with either GFP control or different HDAC6 constructs for 24 h. Equal amounts of protein lysates were incubated with flag-antibody bound to agarose. The ability of the different HDAC6 constructs to bind to cortactin was determined with anti-cortactin-specific antibody (lower panel). Overexpression of cortactin and HDAC6 constructs was tested in whole-cell lysates (upper panel, n=3). Asterisks represent unspecific bands in western blot. (F, G) HUVECs were stably transduced with V5-tagged cortactin wt virus and incubated with broad-spectrum HDAC inhibitors (F) or 10 μM tubacin (G) for 16 h. DMSO serves as control. A total amount of 1 μg/ml trichostatin A (TSA) or 20 mM nicotinamide in DMSO was applied. Immunoprecipitation (upper panel) was done as described in C. Levels of cortactin, acetylated α-tubulin and α-tubulin were determined in whole-cell lysates (n=3). Figure source data can be found with the Supplementary Information.
Besides HDAC6, it was shown that class III HDAC Sirt1 and Sirt2 can interact with and deacetylate cortactin (Zhang et al, 2007, 2009). However, in EC inhibition of class III HDACs by nicotinamide does not affect cortactin acetylation, whereas inhibition of class I and II HDACs by trichostatin A increases acetylation of cortactin (Figure 5F). In accordance with the data demonstrating that tubacin does not affect endothelial sprouting, we did not detect an increase of cortactin acetylation after treatment with tubacin (Figure 5G).
Cortactin is essential for EC migration and vessel formation in zebrafish
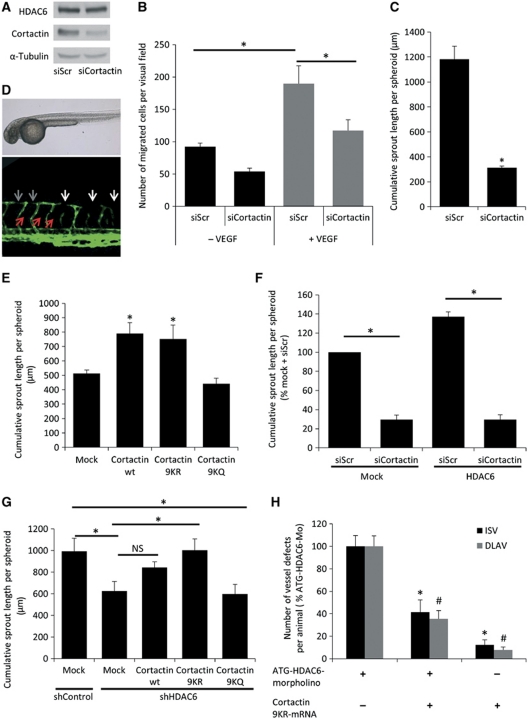
The interaction between HDAC6 and cortactin suggests that HDAC6 regulates angiogenesis through modulation of cortactin acetylation. Therefore, we studied the effect of cortactin silencing in vitro and in vivo. siRNA-mediated downregulation of cortactin decreases basal as well as VEGF-induced HUVEC migration (Figure 6A and B). Furthermore, knockdown of cortactin inhibits endothelial sprouting in vitro (Figure 6C), indicating that cortactin is crucial for angiogenesis. Moreover, cortactin is expressed in the developing vessels in zebrafish (Supplementary Figure 7A). Silencing of cortactin by application of a splice-blocking Mo (Supplementary Figure 7B) leads to a complete loss of the parachordal vessel (Figure 6D, red arrows, and Supplementary Figure 7C and D) in more than 90% of the embryos. Furthermore, the numbers of ISV and DLAV defects are increased (Supplementary Figure 7E and F), and the DLAV shows only faint connections (Figure 6D, grey arrows). Moreover, the diameters of ISVs and DLAVs are significantly reduced compared to control embryos (Supplementary Figure 7G), demonstrating that cortactin regulates vessel development in zebrafish.
Figure 6.
Cortactin is crucial for angiogenesis and mediates HDAC6-dependent sprouting. (A) HUVECs were transfected with cortactin or control siRNA for 48 h. Cortactin expression was measured by western blot. Expression of HDAC6 and α-tubulin were used as loading control (n=3). (B) HUVECs were transfected with cortactin or control siRNA for 48 h, followed by a transwell migration assay for 4 h under basal (black bars) or stimulatory conditions with 50 ng/ml VEGF (grey bars). Migrated cells were counted manually (n=4). (C) HUVECs were transfected with cortactin or control siRNA for 24 h, followed by a spheroid assay (n=3). (D) Representative images of tg(fli1:EGFP) zebrafish embryos injected with cortactin splice-blocking Mo (cortactin-SB-Mo) at 48 h post fertilization. Upper panel shows transmitted light images of the whole embryo. Lower panel shows fluorescent confocal images of the anterior part. White arrows indicate vessel defects, grey arrows indicate faint connections of the DLAV and red arrows indicate the absence of the parachordal vessel. (E) HUVECs were transduced with cortactin wt and different mutated cortactin constructs for 24 h, followed by a spheroid assay. For deacetylation mimic cortactin (cortactin 9KR), nine lysine residues of the repeat region were mutated to arginines. For acetylation mimic cortactin (cortactin 9KQ), nine lysine residues of the repeat region were mutated to glutamines. Mock-transduced cells serve as control (n=5). (F) HUVECs were stably transduced with HDAC6 wt virus or control virus, and subsequently transfected with either cortactin or control siRNA for 24 h. Spheroid assay was performed, and the cumulative sprout length was measured (n=4). (G) HUVECs were stably transduced with HDAC6 or control shRNA virus, and subsequently transduced with mock control or different cortactin constructs (see figure legend E). (H) Quantification of cumulative sprout length in a spheroid assay (n=5). (H) Quantification of ISV and DLAV vessel defects in ATG-HDAC6 morphants with or without 100 pg of cortactin 9KR-mRNA 48 h post fertilization. As control group serves 100 pg of cortactin 9KR-mRNA. Data are presented as percentage (%) of ATG-HDAC6-Mo. *P<0.05 for ISV; #P<0.05 for DLAV (n=56 for ATG-HDAC6-Mo; n=49 for ATG-HDAC6-Mo+cortactin 9KR-mRNA; and n=48 for cortactin 9KR-mRNA). Figure source data can be found with the Supplementary Information.
HDAC6 regulates EC migration and sprouting by deacetylation of cortactin
Because HDAC6 deacetylates cortactin in the repeat region (Zhang et al, 2007), we determined whether deacetylation of lysine residues in the repeat region of cortactin is important for endothelial sprouting. Therefore, we used different cortactin constructs in which nine lysine residues were mutated to either arginines (cortactin 9KR), which mimics deacetylation, or glutamines (cortactin 9KQ), which mimics acetylation and inhibits actin binding to cortactin (Zhang et al, 2007). Overexpression of cortactin wt and cortactin 9KR significantly increases sprouting, whereas overexpression of acetylation mimic cortactin 9KQ does not affect sprouting (Figure 6E), demonstrating that deacetylation of cortactin is important for its function in EC.
Downregulation of cortactin by siRNA completely abolishes the pro-sprouting effect of HDAC6 overexpression (Figure 6F), indicating that cortactin might mediate the sprouting-promoting effect of HDAC6. Furthermore, overexpression of deacetylation mimic cortactin completely rescues the sprouting defect of HDAC6 depletion, whereas overexpression of cortactin wt or acetylation mimic cortactin fails to do so (Figure 6G and Supplementary Figure 8A). Moreover, deacetylation mimic cortactin enhances HUVEC migration after HDAC6 knockdown in a transwell assay (Supplementary Figure 8B). To study whether deacetylation mimic cortactin rescues the vessel defects of HDAC6 morphant zebrafishes, we injected mRNA of the murine cortactin 9KR construct (Supplementary Figure 8C) together with the HDAC6-ATG-Mo into the developing zebrafish. Injection of cortactin 9KR partially rescues the vessel defects after HDAC6 depletion (Figure 6H and Supplementary Figure 8D and E). Therefore, HDAC6 regulates EC migration and angiogenesis by deacetylation of cortactin.
Discussion
Increasing evidence indicates the contribution of class IIa HDACs, such as HDAC5 and HDAC7, to angiogenic functions of ECs (Chang et al, 2006; Ha et al, 2008; Wang et al, 2008; Urbich et al, 2009). However, the roles of class IIb HDACs (HDAC6 and HDAC10) for angiogenesis are largely unknown. Here, we demonstrate that HDAC6 is necessary for in vitro angiogenesis, vessel formation in zebrafish, vascularization of implanted matrigel plugs and ischaemia-induced neovascularization in vivo. Consistently with previous studies (Zhang et al, 2008), we did not observe developmental defects in HDAC6 knockout mice, and the retina vasculature is formed normally, indicating that developmental angiogenesis is not affected. One explanation for the lack of a developmental phenotype in HDAC6−/− mice might be that another class IIb HDAC, namely, HDAC10, compensates the defect of HDAC6. Although silencing of HDAC10 alone in HUVEC did not affect basal sprouting of HUVEC or acetylation of cortactin (Supplementary Figure 9A–C), overexpression of HDAC10 mRNA rescues, in part, vessel defects after depletion of HDAC6 in zebrafish embryos (Supplementary Figure 9D–G). Thus, further studies are required to test whether other HDACs might compensate for loss of HDAC6 with regard to the regulation of cortactin.
We also assessed the role of HDAC6 in tumour vascularization in a Lewis lung carcinoma model in global HDAC6 knockout mice. Unexpectedly, tumour growth was increased in HDAC6 knockout mice. However, angiogenesis as measured by quantifying endomucin-positive vessels was not affected in HDAC6−/− versus wt mice. Lee et al (2008) demonstrated that HDAC6 is required for efficient oncogenic tumourigenesis and that HDAC6 knockout mice show a reduced number of skin tumours in a chemical carcinogen induced tumour model. However, to our knowledge, there is no study regarding the effect of HDAC6 deficiency in the host regulating tumour growth and tumour vascularization of implanted wt tumours. Our experiments might be explained by a function of HDAC6 in the tumour environment, for example, in inflammatory cells, which are the key regulators of tumour growth. Indeed, in comparison with ECs, HDAC6 mRNA is 2.4- and 2.7-fold higher expressed in CD3+ lymphocytes and CD14+ myeloid cells (Supplementary Figure 4J). Whereas previous studies clearly document a function of HDAC6 in lymphocytes (Serrador et al, 2004; Cabrero et al, 2006; de Zoeten et al, 2011), the role in myeloid cells is unclear and deserves further studies. Therefore, one may speculate that the deficiency of HDAC6 may modulate lymphocyte/myeloid cell functions to change the tumour environment.
Having shown that HDAC6 is a positive regulator of angiogenesis, we addressed the underlying mechanism. HDAC6 possesses two separate catalytic domains, and we demonstrate that both domains are required for the regulation of angiogenesis. The requirement of the single HDAC domains for catalytic activity is currently controversially discussed. However, all studies indicate that point mutations in both domains fully block catalytic activity. The group of Stuart Schreiber showed that a single mutation in either of the domains does not affect global deacetylation activity assessed by histone deacetylation (Grozinger et al, 1999; Haggarty et al, 2003), whereas a mutation in the second deacetylation domain inhibits α-tubulin deacetylation (Haggarty et al, 2003). In contrast, the group of Patrick Matthias showed that single mutations in either of the two deacetylation domains inhibits α-tubulin and histone deacetylation (Zhang et al, 2003). A third independent study demonstrated that only the second deacetylase domain exhibits catalytic activity towards α-tubulin as well as histones (Zou et al, 2006). The reason for the diverse results is currently unknown. All of these studies were done in cell-free assays. In contrast, we assessed the activity of different HDAC6 constructs directly in ECs in a cellular environment. Our results are in accordance with the studies by Stuart Schreiber, indicating that both the HDAC domains have global deacetylation activity (assessed by the deacetylation of cortactin), and that only the second deacetylation domain exhibits α-tubulin deacetylation activity (as shown in Figure 4C). From these results, we concluded that the overall catalytic activity of HDAC6 is necessary to induce endothelial migration and sprouting because the catalytic inactive construct HDAC6 H216/611A fails to increase migration and sprouting. Furthermore, we conclude that α-tubulin deacetylation is not responsible for the regulation of sprouting by HDAC6, because the HDAC6 H611A construct, which does not affect α-tubulin acetylation, increases sprouting comparable to the HDAC6 wt or the HDAC6 H216A construct. These results were confirmed by using the pharmacological inhibitor tubacin, which does not affect in vitro sprouting, migration and network formation. Tubacin is a HDAC6-specific inhibitor, which blocks α-tubulin deacetylation through interaction with the second deacetylation domain, whereas the overall activity of wt HDAC6, as measured by means of histone deacetylation, is only slightly affected (Haggarty et al, 2003). During revision of this manuscript, Li et al (2011) showed that HDAC6 regulates angiogenesis in vivo and in vitro, confirming the role of HDAC6 for angiogenesis. They demonstrate that pharmacological inhibition of HDAC6 with tubacin as well as silencing by siRNA inhibits HUVEC cell migration, tube formation and sprouting angiogenesis. However, as described above, we did not observe an effect by tubacin treatment. We used the well-described concentrations of 2.5–10 μM in our experiments. Since Li et al (2011) did not provide the concentration used for their experiments, differences in the experimental conditions or the concentration of tubacin may explain the differential experimental outcome. HDAC6 can interact with proteins that control microtubule turnover (Zilberman et al, 2009; Li et al, 2011), and thereby regulates the tubulin network independently of α-tubulin deacetylation. Thus, from our data, we cannot fully exclude that HDAC6-dependent regulation of angiogenesis is mediated, in part, by the tubulin network.
HDAC6 plays a pronounced role in the cellular stress response to misfolded protein by binding and aggregation of ubiquitiniated proteins (Seigneurin-Berny et al, 2001; Hook et al, 2002; Kawaguchi et al, 2003). In the present study, we show that overexpression of an ubiquitin-binding-deficient mutant of HDAC6 increases sprouting comparable to HDAC6 wt, indicating that the binding of HDAC6 to ubiquitinated misfolded proteins is not necessary for HDAC6-dependent regulation of sprouting under basal condition. However, Gao et al (2007) showed that the ubiquitin-binding-deficient mutant can interact with endogenous HDAC6 and is thereby recruited to target proteins. Under conditions when the catalytic activity of HDAC6 might not be sufficient to process the amount of misfolded proteins, overexpression of the ubiquitin-binding-deficient mutant that interacts with endogenous HDAC6 might help to facilitate the processing of misfolded proteins. However, one would expect that under basal conditions, ECs are not exposed to an excess of misfolded proteins and the ubiquitin-binding-deficient mutant might facilitate sprouting independently of interaction with ubiquitinated proteins. This does not imply that under ischaemic or stress conditions endothelial function or revascularization depends, in part, on HDAC6-mediated clearance of misfolded proteins. Furthermore, we cannot exclude that the Znf-UBP domain mediates effects independently of misfolded proteins under conditions when endogenous HDAC6 is present.
Because the deacetylation of α-tubulin and binding of misfolded proteins are not responsible for regulation of angiogenesis by HDAC6, we ask whether HDAC6 regulates migration by interfering with the actin cytoskeleton. HDAC6 was shown to interact with the actin-remodelling and -stabilizing enzyme cortactin in HeLa cells and MDA-MB-231 cells (Zhang et al, 2007; Rey et al, 2011). Cortactin regulates branching and stability of actin filaments at the leading edge of migrating cells and thereby facilitates migration (for review, see Cosen-Binker and Kapus (2006)). Deacetylation of cortactin in the repeat region by HDAC6 abolishes binding of actin, and therefore inhibits cell migration (Zhang et al, 2007) and the invasion capacity of breast cancer cells (Rey et al, 2011). Our results demonstrate that HDAC6 binds to and deacetylates cortactin in ECs. In contrast, class III HDACs, which can also interact with and deacetylate cortactin (Zhang et al, 2007, 2009), do not affect cortactin acetylation in ECs. On the basis of our finding that HDAC6 interacts with cortactin, we hypothesized that HDAC6 regulates angiogenesis through modulation of cortactin activity. Indeed, silencing of cortactin decreases EC migration and sprouting in vitro and vessel development in the zebrafish. Moreover, overexpression of cortactin wt and a deacetylation mimic cortactin enhances sprouting, whereas overexpression of acetylation mimic cortactin does not affect sprouting, demonstrating that deacetylation of cortactin is important for its function in ECs. In addition, we addressed the causal contribution of cortactin to HDAC6 function in two experiments. First, silencing of cortactin completely prevents the pro-sprouting effect of HDAC6 overexpression. Second, overexpression of deacetylation mimic cortactin completely rescues the sprouting defect of HDAC6 depletion, whereas overexpression of cortactin wt or acetylation mimic cortactin fails to do so. Consistently, overexpression of the deacetylation mimic cortactin rescues, in part, vessel defects after HDAC6 depletion in zebrafish embryos. Taken together, these data indicate that HDAC6 regulates EC migration and angiogenesis by deacetylation of cortactin.
The physiological regulation of HDAC6 is largely unknown. A recent study demonstrates that shear stress slightly increased the expression of HDAC6 protein after 12 h (Wang et al, 2010). In the current study, we did not observe a shear-stress-dependent regulation of HDAC6 mRNA after prolonged shear stress for 72 h. The difference might be explained by a stabilization of HDAC6 protein after 12 h as shown by Wang et al (2010) rather than a transcriptional up-regulation of HDAC6. Interestingly, hypoxia upregulates HDAC6 mRNA and protein expression in ECs, which is in line with a recent study showing that HDAC6 mRNA levels are elevated in hypoxia-induced hypertrophic hearts (Lemon et al, 2011). Because HDAC6 stabilizes HIF-1α and VEGFR1 and VEGFR2 in cancer cells, one may speculate that hypoxia upregulates HDAC6 to stabilize HIF-1α and VEGFRs. These data provide a therapeutic option for targeting HIF-1α and VEGFRs with HDAC inhibitors against class IIb isozymes to interfere with angiogenesis signalling in ECs.
Taken together, HDAC6 is upregulated by hypoxia and is necessary for angiogenesis in vitro and in vivo involving the interaction and deacetylation of cortactin that regulates EC migration and sprouting.
Materials and methods
Cell culture
Pooled HUVECs were purchased from Lonza and cultured in endothelial basal medium (EBM; Lonza) supplemented with hydrocortisone, bovine brain extract, epidermal growth factor, gentamycin sulphate, amphotericin-B and 10% fetal calf serum (FCS; Invitrogen) until the forth passage. After detachment with trypsin, cells were grown in 6 cm culture dishes for at least 24 h.
Transfection
HUVECs were transfected at 60% confluence using GeneTrans II (Mobitec) according to the manufacturer's protocol with 67 nM siRNA. siRNAs were synthesized by Eurogentec (Cologne, Germany) or Sigma-Aldrich. The following sequences were used: control siRNA Scr I against firefly luciferase (5′-CGUACGCGGAAUACUUCGA-3′; Elbashir et al, 2001; used as standard control), Scr II (5′-GUGGGCACCGAUAUCUUGA-3′), siHDAC6 I (5′-UUAAUCGUCGCAGUUCUCU-3′), siHDAC6 II (5′-CAUCCAAGUCCAUCGCAGA-3′), siHDAC6 III (5′-GUGGCCGCAUUAUCCUUAUCCUAGA-3′) and siCortactin (5′-AAUGCCUGGAAAUUCCUCAUU-3′). For plasmid overexpression of HDAC6 or cortactin constructs, HUVECs were transfected 24 h prior analysis using GenJet (Gentaur) according to the manufacturer's protocol.
Reagents and plasmids
Tubacin and niltubacin were kindly provided by Stuart Schreiber and Ralph Mazitschek. Several plasmids used in this study have previously been described. Mouse myc-tagged cortactin wt, myc-tagged cortactin 9KR, myc-tagged cortactin 9KQ, flag-tagged HDAC6 wt, flag-tagged HDAC6 840aa (Δubiquitin binding) and flag-tagged HDAC6 503aa (ΔDAC 2) were kindly provided by Edward Seto (Zhang et al, 2007). For lentivirus production, cortactin wt, 9KR, 9KQ, HDAC6 840aa and 503aa were subcloned into pENTR-D-Topo vector (Invitrogen) by PCR without stop-codon and shuttled in pLenti4-V5-DEST (Invitrogen) by gateway cloning. Full-length HDAC6 in pDONR was obtained from Genecopoeia (clone X0536 corresponding NM_006044). For lentivirus production, full-length HDAC6 was shuttled in pLenti4-V5-DEST by gateway cloning. HDAC6 H216A, HDAC6 H611A and HDAC6 H216/611A were generated by site-directed mutagenesis (QuikChange Site-Directed Mutagenesis Kit, Stratagene) using full-length HDAC6 in pDONR and subsequently shuttled into pLenti4-V5-DEST. Lentivirus containing EGFP was previously described (Ziebart et al, 2008). For in vitro transcription of the cortactin 9KR mutant, cDNA was subcloned into pCS2plus using BamHI and EcoRI. pEGFP plasmid was purchased from Clontech.
Angiogenesis assay in vitro
EC spheroids of defined cell number were generated as described previously (Diehl et al, 2007). In vitro angiogenesis was quantified by measuring the cumulative length of the sprouts, the number of sprouts and the number of branch points that had grown out of each spheroid using a digital imaging software (Axiovision 4.6, Carl Zeiss Imaging Solutions GmbH, Munich, Germany) analysing 10 spheroids per group and experiment. To measure tube formation, 75 000 HUVECs were seeded in 1 ml EBM (10% FCS) on the matrigel basement membrane matrix as described previously (Carmona et al, 2009).
Zebrafish lines, antibodies and reagents
Embryos of AB wt and the tg(fli1:EGFP) line (Lawson et al, 2002) were raised and staged as described (Kimmel et al, 1995). Embryos were kept in E3 solution at 28.5°C with or without 0.003% 1-phenyl-2-thiourea (Sigma) to suppress pigmentation and staged according to somite number or hours post fertilization (Epting et al, 2010). We used following antibodies for this study: pan-actin (Dianova, clone Ab-5), rabbit anti-GFP (A-11122; Invitrogen), rabbit anti-HDAC6 (Millipore, clone CT) and HRP-conjugated antibodies (DAKO).
RNA and Mo injection
For synthesis of sense RNA, we cloned murine cortactin 9KR mutant into pCS2plus. Sense RNA was prepared from NotI-linearized cortactin 9KR-pCS2plus using SP6 mMessage mMachine Kit (Ambion). RNA was diluted in 0.1 M KCL to a concentration of 0.1 μg/μl. Mos were diluted in 0.1 M KCl to concentrations of 2 and 5 μg/μl. One nanolitre of RNA or Mo dilution was injected through the chorion of one-cell or two-cell stage embryos. To attenuate possible off-target effects, a p53 Mo was co-injected 1.5-fold to the other Mos used. The following TB/SB antisense Mos (Gene Tools) were used:
HDAC6-TB-Mo: 5′-CTTTGGTATCTGGAACCGCATCCAT-3′ (ATG codon)
HDAC6-SB-Mo: 5′-GATGTGTATTTCTACCTCCACTTGT-3′ (exon 5–intron 5 junction)
Cortactin-SB-Mo: 5′-GACATTCATCTCAGACTGACTTGAT-3′ (exon 2–intron 2 junction)
p53-TB-Mo: 5′-GCGCCATTGCTTTGCAAGAATTG-3′
Standard control Mo: 5′-CCTCTTACCTCAGTTACAATTTATA-3′
In vivo spheroid assay in mice
In vivo matrigel plug assay was performed as previously described (Alajati et al, 2008; Laib et al, 2009) with the following modifications. A total of 3 × 105 HUVECs were seeded in T25 flask, transduced with shRNA virus and 48 h after transduction HUVECs were selected with 0.4 μg/ml puromycin for 24 h until control cells completely died. Selected cells were grown for 4 days and spheroids were generated in EBM medium (Lonza). Spheroids were mixed with matrigel containing VEGF and FGF2, and subcutaneously injected (1000 spheroids/plug) into severe combined immunodeficiency mice for 3 weeks. Mice received 150 μg TRIC-lectin (Sigma-Aldrich) in physiological NaCl solution intravenously 20 min before sacrifice to stain perfused vessels. Immunohistological analysis was performed as previously described (Alajati et al, 2008; Laib et al, 2009). Microvascular density and size of the vessels were analysed using ImageJ (National Institutes of Health). Perfusion was analysed as TRIC-lectin-positive vessels in relation to hCD34-positive vessels.
Matrigel plug assay
HDAC6 knockout mice were kindly provided by Tso-Pang Yao (Gao et al, 2007). Eight- to twelve-week-old female mice from parallel breedings were injected subcutaneously with two matrigel basement matrix (BD) plugs (200 μl each). Matrigel plugs were harvested at day 7. In order to analyse perfused capillaries, 200 μl FITC-conjugated lectin (1 mg/ml) was injected intravenously 30 min before harvest. Lectin-positive structures were counted manually in five microscopic fields in three different sections of each plug (× 5/0.25 objective) using a computer-assisted fluorescence microscope (Axiovert 100M equipped with AxioCam camera, Carl Zeiss, Jena, Germany). The average vessel number per plug was calculated, and the mean value for both matrigel plugs for each mouse was taken for the statistical analysis using an unpaired Student's t-test. Images were taken with a laser-scanning microscope (LSM510 META with Software Release 4.0 SP2, Carl Zeiss, Jena, Germany) using the Plan-Neofluar × 40/1.3 oil objective. H&E staining was performed as described previously (Bonauer et al, 2009).
Hind limb ischaemia model
The hind limb ischaemia model was performed as previously described (Bonauer et al, 2009) using 8- to 12-week-old female HDAC6+/+ and HDAC6−/− mice.
Statistical analysis
Data are expressed as mean±s.e.m. Two treatment groups were compared by Student's t-test. Multiple group comparisons were done by ANOVA (post hoc analysis, SPSS Inc.). Results were considered statistically significant when P<0.05.
Supplementary Material
Acknowledgments
We are grateful to Stuart L. Schreiber and Ralph Mazitschek of the Broad Institute of Harvard and MIT for a gift of the small-molecule reagent tubacin. We are thankful to Nicole Konecny, Ariane Fischer, Tino Röxe, M Muhly-Reinholz and Eva Besemfelder for expert technical assistance and Katrin Bennewitz for excellent zebrafish work. This study was supported by the Deutsche Forschungsgemeinschaft, TR-SFB 23 (projects Z5 to JK, A3 to HGA, A2 to SD and B5 to CU).
Author contributions: DK, JK, SG, RAB, EH and LR performed research and analysed data; T-PY provided the HDAC6 knockout mice; ES provided the HDAC6 constructs; T-PY, MT, ES, HGA and AMZ gave conceptional and technological advice; LR, SD and CU designed the study; and DK, SD and CU wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alajati A, Laib AM, Weber H, Boos AM, Bartol A, Ikenberg K, Korff T, Zentgraf H, Obodozie C, Graeser R, Christian S, Finkenzeller G, Stark GB, Heroult M, Augustin HG (2008) Spheroid-based engineering of a human vasculature in mice. Nat Methods 5: 439–445 [DOI] [PubMed] [Google Scholar]
- Azuma K, Urano T, Horie-Inoue K, Hayashi S, Sakai R, Ouchi Y, Inoue S (2009) Association of estrogen receptor alpha and histone deacetylase 6 causes rapid deacetylation of tubulin in breast cancer cells. Cancer Res 69: 2935–2940 [DOI] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784 [DOI] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S (2009) MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324: 1710–1713 [DOI] [PubMed] [Google Scholar]
- Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, Garrido C, Yao TP, Vourc'h C, Matthias P, Khochbin S (2007) HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev 21: 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrero JR, Serrador JM, Barreiro O, Mittelbrunn M, Naranjo-Suarez S, Martin-Cofreces N, Vicente-Manzanares M, Mazitschek R, Bradner JE, Avila J, Valenzuela-Fernandez A, Sanchez-Madrid F (2006) Lymphocyte chemotaxis is regulated by histone deacetylase 6, independently of its deacetylase activity. Mol Biol Cell 17: 3435–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JS, Giles FJ, Nawrocki ST (2008) Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Lett 269: 7–17 [DOI] [PubMed] [Google Scholar]
- Carmona G, Gottig S, Orlandi A, Scheele J, Bauerle T, Jugold M, Kiessling F, Henschler R, Zeiher AM, Dimmeler S, Chavakis E (2009) Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood 113: 488–497 [DOI] [PubMed] [Google Scholar]
- Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN (2006) Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 126: 321–334 [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325: 834–840 [DOI] [PubMed] [Google Scholar]
- Cosen-Binker LI, Kapus A (2006) Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 21: 352–361 [DOI] [PubMed] [Google Scholar]
- de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, Hancock WW (2011) Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol 31: 2066–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl F, Rossig L, Zeiher AM, Dimmeler S, Urbich C (2007) The histone methyltransferase MLL is an upstream regulator of endothelial-cell sprout formation. Blood 109: 1472–1478 [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498 [DOI] [PubMed] [Google Scholar]
- Epting D, Wendik B, Bennewitz K, Dietz CT, Driever W, Kroll J (2010) The Rac1 regulator ELMO1 controls vascular morphogenesis in zebrafish. Circ Res 107: 45–55 [DOI] [PubMed] [Google Scholar]
- Gao YS, Hubbert CC, Lu J, Lee YS, Lee JY, Yao TP (2007) Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol 27: 8637–8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger CM, Hassig CA, Schreiber SL (1999) Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci USA 96: 4868–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG (2008) Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem 283: 14590–14599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA 100: 4389–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook SS, Orian A, Cowley SM, Eisenman RN (2002) Histone deacetylase 6 binds polyubiquitin through its zinc finger (PAZ domain) and copurifies with deubiquitinating enzymes. Proc Natl Acad Sci USA 99: 13425–13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115: 727–738 [DOI] [PubMed] [Google Scholar]
- Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW (2001) Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med 7: 437–443 [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310 [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18: 601–607 [DOI] [PubMed] [Google Scholar]
- Laib AM, Bartol A, Alajati A, Korff T, Weber H, Augustin HG (2009) Spheroid-based human endothelial cell microvessel formation in vivo. Nat Protoc 4: 1202–1215 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM (2002) sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3: 127–136 [DOI] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP (2010) Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol 189: 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, Ordentlich P, Wang XF, Counter CM, Yao TP (2008) The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 68: 7561–7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon DD, Horn TR, Cavasin MA, Jeong MY, Haubold KW, Long CS, Irwin DC, McCune SA, Chung E, Leinwand LA, McKinsey TA (2011) Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. J Mol Cell Cardiol 51: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Xie S, Ren Y, Huo L, Gao J, Cui D, Liu M, Zhou J (2011) Microtubule-associated deacetylase HDAC6 promotes angiogenesis by regulating cell migration in an EB1-dependent manner. Protein Cell 2: 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margariti A, Zampetaki A, Xiao Q, Zhou B, Karamariti E, Martin D, Yin X, Mayr M, Li H, Zhang Z, De Falco E, Hu Y, Cockerill G, Xu Q, Zeng L (2010) Histone deacetylase 7 controls endothelial cell growth through modulation of beta-catenin. Circ Res 106: 1202–1211 [DOI] [PubMed] [Google Scholar]
- Martin M, Potente M, Janssens V, Vertommen D, Twizere JC, Rider MH, Goris J, Dimmeler S, Kettmann R, Dequiedt F (2008) Protein phosphatase 2A controls the activity of histone deacetylase 7 during T cell apoptosis and angiogenesis. Proc Natl Acad Sci USA 105: 4727–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21: 6820–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet D, Bellahcene A, Pirotte S, Waltregny D, Deroanne C, Lamour V, Lidereau R, Castronovo V (2007) Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ Res 101: 1237–1246 [DOI] [PubMed] [Google Scholar]
- Mottet D, Castronovo V (2010) Histone deacetylases: anti-angiogenic targets in cancer therapy. Curr Cancer Drug Targets 10: 898–913 [DOI] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell 108: 475–487 [DOI] [PubMed] [Google Scholar]
- Pandey UB, Batlevi Y, Baehrecke EH, Taylor JP (2007) HDAC6 at the intersection of autophagy, the ubiquitin-proteasome system and neurodegeneration. Autophagy 3: 643–645 [DOI] [PubMed] [Google Scholar]
- Park JH, Kim SH, Choi MC, Lee J, Oh DY, Im SA, Bang YJ, Kim TY (2008) Class II histone deacetylases play pivotal roles in heat shock protein 90-mediated proteasomal degradation of vascular endothelial growth factor receptors. Biochem Biophys Res Commun 368: 318–322 [DOI] [PubMed] [Google Scholar]
- Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, Pili R (2006a) Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res 66: 8814–8821 [DOI] [PubMed] [Google Scholar]
- Qian DZ, Kato Y, Shabbeer S, Wei Y, Verheul HM, Salumbides B, Sanni T, Atadja P, Pili R (2006b) Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res 12: 634–642 [DOI] [PubMed] [Google Scholar]
- Rey M, Irondelle M, Waharte F, Lizarraga F, Chavrier P (2011) HDAC6 is required for invadopodia activity and invasion by breast tumor cells. Eur J Cell Biol 90: 128–135 [DOI] [PubMed] [Google Scholar]
- Rossig L, Li H, Fisslthaler B, Urbich C, Fleming I, Forstermann U, Zeiher AM, Dimmeler S (2002) Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ Res 91: 837–844 [DOI] [PubMed] [Google Scholar]
- Rossig L, Urbich C, Bruhl T, Dernbach E, Heeschen C, Chavakis E, Sasaki K, Aicher D, Diehl F, Seeger F, Potente M, Aicher A, Zanetta L, Dejana E, Zeiher AM, Dimmeler S (2005) Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J Exp Med 201: 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, Khochbin S (2001) Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol 21: 8035–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F (2004) HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity 20: 417–428 [DOI] [PubMed] [Google Scholar]
- Spange S, Wagner T, Heinzel T, Kramer OH (2009) Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol 41: 185–198 [DOI] [PubMed] [Google Scholar]
- Urbich C, Rossig L, Kaluza D, Potente M, Boeckel JN, Knau A, Diehl F, Geng JG, Hofmann WK, Zeiher AM, Dimmeler S (2009) HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood 113: 5669–5679 [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F (2008) HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol 18: 291–297 [DOI] [PubMed] [Google Scholar]
- Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN (2008) Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci USA 105: 7738–7743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Yan ZQ, Qi YX, Cheng BB, Wang XD, Zhao D, Shen BR, Jiang ZL (2010) Normal shear stress and vascular smooth muscle cells modulate migration of endothelial cells through histone deacetylase 6 activation and tubulin acetylation. Ann Biomed Eng 38: 729–737 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, Yao TP, Lane WS, Seto E (2007) HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell 27: 197–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, Matthias P (2008) Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 28: 1688–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22: 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, Nicosia SV, Zhang X (2009) Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene 28: 445–460 [DOI] [PubMed] [Google Scholar]
- Ziebart T, Yoon CH, Trepels T, Wietelmann A, Braun T, Kiessling F, Stein S, Grez M, Ihling C, Muhly-Reinholz M, Carmona G, Urbich C, Zeiher AM, Dimmeler S (2008) Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res 103: 1327–1334 [DOI] [PubMed] [Google Scholar]
- Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A (2009) Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci 122: 3531–3541 [DOI] [PubMed] [Google Scholar]
- Zou H, Wu Y, Navre M, Sang BC (2006) Characterization of the two catalytic domains in histone deacetylase 6. Biochem Biophys Res Commun 341: 45–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.