Abstract
The epigenetic changes of the chromatin represent an attractive molecular substrate for adaptation to the environment. We examined here the role of CREB-binding protein (CBP), a histone acetyltransferase involved in mental retardation, in the genesis and maintenance of long-lasting systemic and behavioural adaptations to environmental enrichment (EE). Morphological and behavioural analyses demonstrated that EE ameliorates deficits associated to CBP deficiency. However, CBP-deficient mice also showed a strong defect in environment-induced neurogenesis and impaired EE-mediated enhancement of spatial navigation and pattern separation ability. These defects correlated with an attenuation of the transcriptional programme induced in response to EE and with deficits in histone acetylation at the promoters of EE-regulated, neurogenesis-related genes. Additional experiments in CBP restricted and inducible knockout mice indicated that environment-induced adult neurogenesis is extrinsically regulated by CBP function in mature granule cells. Overall, our experiments demonstrate that the environment alters gene expression by impinging on activities involved in modifying the epigenome and identify CBP-dependent transcriptional neuroadaptation as an important mediator of EE-induced benefits, a finding with important implications for mental retardation therapeutics.
Keywords: CBP, epigenetics, learning and memory, mental retardation, neurogenesis
Introduction
Animals have developed a complex nervous system for adapting their conduct to the ever-changing environmental conditions. This flexibility at the behavioural level depends on the ability of neuronal circuits to evolve based on previous experiences through cellular mechanisms, such as functional and structural plasticity. These processes rely on the activation of specific and complex transcriptional programmes. A novel idea that has come under discussion in recent years is that these gene programmes are also subjected to activity-driven modulation through epigenetic modification of the chromatin of neural cells (Borrelli et al, 2008; Zocchi and Sassone-Corsi, 2010). Importantly, the malfunction of these processes can contribute to the molecular aetiology of cognitive disorders (Graff and Mansuy, 2009). A good example of epigenetic disorder is the Rubinstein–Taybi syndrome (RSTS), a complex autosomal-dominant disease characterized by cognitive impairments and skeletal abnormalities (Rubinstein and Taybi, 1963; Wiley et al, 2003) associated to mutations in the gene encoding the CREB-binding protein (CBP) (Petrij et al, 1995). CBP is a transcriptional co-activator with lysine acetyltransferase (KAT) activity and thereby it has the ability to leave epigenetic marks on the chromatin (Chan and La Thangue, 2001). The recent characterization of several mouse models for RSTS has demonstrated a direct role of the KAT activity of CBP in RSTS pathology and highlighted the importance of histone acetylation in neuronal plasticity and memory in the normal brain (Bourtchouladze et al, 2003; Alarcon et al, 2004; Korzus et al, 2004; Wood et al, 2005, 2006; Chen et al, 2010; Viosca et al, 2010; Barrett et al, 2011; Valor et al, 2011).
Environmental enrichment (EE) has been found to be beneficial in a number of cognitive disorders (Nithianantharajah and Hannan, 2006), including different forms of mental retardation. Exposing laboratory rodents to continuous or repeated sessions of EE increase dendritic branching and spine number in hippocampal neurons, promotes neurogenesis and the integration of newborn neurons in functional circuits and improves learning and memory (van Praag et al, 2000; Nithianantharajah and Hannan, 2006). These events likely require the activation of complex gene networks, but the nature and sequence of the genetic programme underlying these experience-driven changes in the structure and function of neuronal circuits remains elusive. Interestingly, EE has been shown to promote hippocampal histone acetylation (Fischer et al, 2007), a process that is impaired in RSTS mice (Alarcon et al, 2004).
We explore here the benefits of EE in a mouse model of RSTS mice. Our results reveal that EE promoted synaptic growth and alleviated some behavioural and cognitive deficits associated to RSTS. However, CBP-deficient mice showed a strong defect in environment-induced neurogenesis that correlated with attenuation of the transcriptional programme associated with this process and with impaired EE-mediated enhancement of spatial navigation and pattern separation. Our results support an specific role for CBP in environment-induced neurogenesis and identify CBP-dependent transcription and histone acetylation as important mediators of environment-induced benefits.
Results
EE ameliorates some behavioural deficits in cbp+/− mice, but does not cause an improvement of spatial memory and discrimination
EE is known to trigger major structural and functional changes in the hippocampus (Nithianantharajah and Hannan, 2006). To examine the efficacy of behavioural therapy in RSTS, we compared cohorts of cbp+/− and control littermate mice housed either in standard cages (SC) or in a large EE in a number of paradigms.
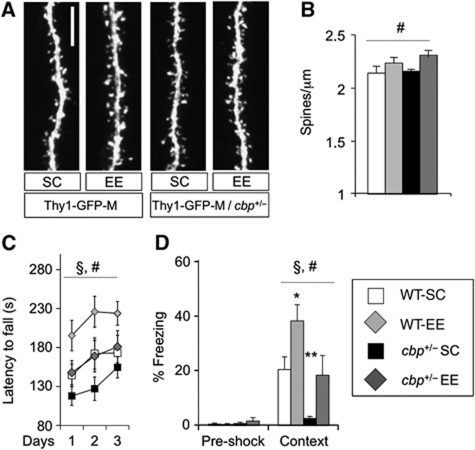
We first examined whether EE promoted synaptic growth in this mouse models of RSTS by assessing dendritic spine density and morphology in CA1 pyramidal neurons of Thy1-EGFP-M/cbp+/− double mutants in different housing conditions. These animals exhibit an in vivo Golgi-like staining in the CA1 area in which few neurons are intensively labelled with EGFP expression (Feng et al, 2000). The examination of the number of dendritic spines in the stratum radiatum demonstrated that EE triggered similar structural changes in hippocampal pyramidal neurons of cbp+/− mice and control littermates (Figure 1A and B; spine density two-way ANOVA: F(1,24)housing=5.32, P=0.03). Neither EE nor CBP deficiency altered the morphology of dendritic spines and hippocampal dendritic organization (Supplementary Figure S1).
Figure 1.
EE-mediated structural, behavioural and cognitive benefits. (A) Representative confocal images showing spines protruding from dendritic segments of hippocampal CA1 pyramidal neurons from cbp+/− mice and control littermates in SC or EE conditions. Scale bar: 5 μm. (B) Mice exposed to EE, independently of the genotype, show increased density of dendritic spines compared with animals kept in SC. Two-way ANOVA, #: significant housing effect; n=6–8 mice per group. These EE-dependent changes did not affect the morphology of dendritic spines (Supplementary Figure S1A–C) or the general cytoarchitecture of the hippocampus (Supplementary Figure S1D and E). (C) Cbp+/− mice show deficits in RotaRod performance and the exposure to EE causes a recovery in mutant mice and an improvement of the performance of WT mice. Two-way ANOVA, #: significant housing effect, §: significant genotype effect; n=10 mice per group. (D) Cbp+/− mice show an impairment in contextual fear conditioning. EE rescues this deficit and improves the contextual memory of WT mice. Two-way ANOVA, #: significant housing effect, §: significant genotype effect. t-Tests compared with WT-SC group: *P<0.05; **P<0.005; n=9–10 mice per group.
EE is also known to have important consequences in the animals’ behaviour (Nithianantharajah and Hannan, 2006). We observed that cbp+/− and control littermates behaved similarly in the open field regardless of their housing condition (Supplementary Figure S2A, B, and that both genotype and housing had a mild impact in the elevated plus maze task (Supplementary Figure S2C, D). EE, however, caused a remarkable improvement of the animals’ performance in an accelerated RotaRod task in both genotypes (Figure 1C; latency to fall two-way ANOVA repeated measures: F(1,36)genotype=5.55, P=0.02; F(1,36)housing=5.21, P=0.03), reversing previously described deficit of cbp+/− mice (Alarcon et al, 2004). The cbp+/−-EE group stayed in the RotaRod as long as the wild type (WT)-SC group, but did not reach the performance level of the WT-EE group. Importantly, the reversal of behavioural deficits was not restricted to locomotor impairments. Cbp+/− mice, like other CBP-deficient strains (Barco, 2007), are impaired in diverse memory tasks, including contextual fear conditioning (Alarcon et al, 2004). We subjected cohorts of cbp+/− and control littermates housed either in SC or in EE to contextual fear conditioning and observed a similar effect of EE (Figure 1D; freezing time two-way ANOVA: F(1,35)genotype=13.52, P=0.001; F(1,35)housing=10.72, #: P=0.002). The cbp+/−-EE group showed similar memory as compared with the WT-SC group (t-tests; cbp+/−-SC versus WT-SC: t(18)= 3.76, P=0.001; cbp+/−-EE versus WT-SC: t(17)= 0.24, P=0.81), but lower than the WT-EE group (t-test; WT-EE versus cbp+/−-EE: t(17)= 2.14, P=0.05).
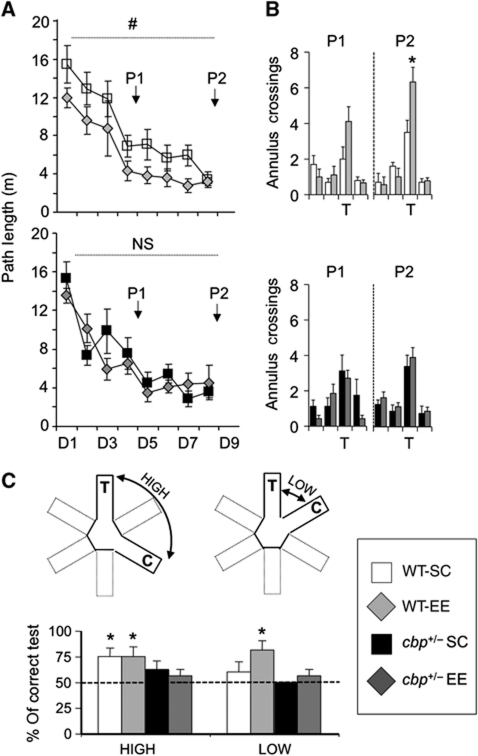
We also examined whether EE has a beneficial impact in the navigation skills of cbp+/− mice. In agreement with the reversal of motor deficits in the RotaRod task, the cbp+/−-EE group increased their swimming speed in the Morris water maze (MWM) task (Supplementary Figure S2E), reversing the previously reported locomotor deficit (Alarcon et al, 2004). As previously reported (Alarcon et al, 2004), cbp+/− and control littermates maintained in SC performed equally well the hidden platform task in the MWM (F(1,16)genotype=2.44, P=0.14). As expected, EE improved the performance of WT mice both during training (F(1,17)housing=6.97, P=0.02; Figure 2A, upper panel) and in the two probe trials (Figure 2B, upper panel). In contrast, cbp+/− mice showed the same performance regardless of the housing condition (F(1,14)housing=0.07, P=0.79; Figure 2A and B, lower panels). Interestingly, the large number of annulus crossings in the WT-EE group indicates that these animals show better spatial discrimination ability than the other three groups (Figure 2B).
Figure 2.
Cbp+/− mice show impaired EE-enhanced spatial navigation and pattern recognition ability. (A) The two-way ANOVA analysis of path lengths in the water maze task revealed a significant housing effect (F(1,31)housing=4.81, P=0.04), no genotype effect (F(1,31)genotype=0.37, P=0.55), and indicated a possible genotype × housing interaction (F(1,31)genotype × housing=3.53, P=0.07). More precisely, EE improved the performance in WT mice (upper panel, two-way ANOVA, #: significant housing effect), but not in cbp+/− mice (lower panel, NS: non-significant); n=8–10 mice per group. (B) EE housed wt mice showed more annulus crossings in the first and the second probe trials (upper panels: P1, P=0.06 no significant difference; P2, *P=0.03), whereas cbp+/− mice did not exhibit any housing effect (lower panels: P1, P=0.90; P2, P=0.70). (C) (Upper panels) Schematic representation of the WRM tests used to measure pattern separation. Mice were tested for their pattern separation ability by comparing their performance in two types of tests: low separation tests (LOW) in which the target arm (T, where the platform is located) and the choice arm (C) were contiguous, and high separation tests (HIGH) in which the target and the choice arms were separated by a closed arm. Graph: In the third day of training, the mice were subjected to two symmetrical low and high separation tasks and the average performance was calculated. WT mice housed in an enriched environment (WT-EE) performed well both kind of tests (percentage of correct HIGH: t(7)=2.65, P=0.03; percentage of correct LOW: t(7):3.42, P=0.01), whereas WT housed in standard cages (WT-SC) were only successful in the high separation tests (percentage of correct HIGH: WT-SC, t(9)=3.00, P=0.02). In contrast, cbp+/− mutants failed in both the HIGH and the LOW tests. *P<0.05 t-tests versus 50 (chance); n=8–10 mice per group.
To complete this comprehensive behavioural analysis, we assessed working memory and pattern separation ability in a water radial maze (WRM) task in which the mice were tested for the ability to select, from a choice of two arms, the arm harbouring the escaping platform (Figure 2C). We performed four probe trials (T1–T4) and tested whether mice could differentiate between locations that were presented closely in space (LOW: T2 and T4) versus those that were more highly separated (HIGH: T1 and T3). Cbp+/− mice were impaired in the spatial discrimination task (two-way repeated measures ANOVA: F(1,30)genotype=5.55, P=0.02). In addition, only WT-EE mice performed equally well at low and high separations, whereas cbp+/− mice (regardless of the housing condition) and WT-SC mice could not discriminate between the choice and the target arms when presented in close spatial proximity (Figure 2C). These results indicate that EE enhanced pattern separation ability in WT mice, an skill that is likely to be in higher demand when the animal lives in an enriched, changing environment.
Overall, our comprehensive behavioural analysis demonstrated that EE has a beneficial effect in various motor and cognitive abilities in cbp+/− mice and unveiled new deficits associated to impaired CBP function, namely reduced EE-enhanced spatial navigation and pattern separation ability.
CBP is specifically required for environment-induced neurogenesis
According to current theoretical models describing the role of the DG in the processing and storage of spatial information, newborn neurons in the subgranular zone (SGZ) are hypothesized to facilitate pattern separation and spatial memory resolution (Deng et al, 2010; Aimone et al, 2011; Sahay et al, 2011b). Therefore, our results in the MWM and WRM may suggest a specific defect in environment-induced neurogenesis. To assess this hypothesis, we examined adult neurogenesis in cbp+/− mice by BrdU uptake.
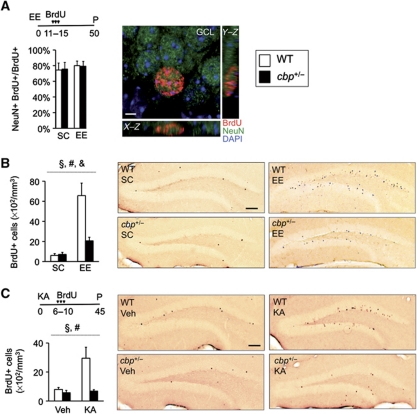
Adult neurogenesis is a complex and dynamic phenomenon that takes several weeks. Neural progenitors go through different stages before becoming mature granule cells integrated in functional circuits (Kronenberg et al, 2003; Zhao et al, 2008). Five weeks after BrdU administration, most BrdU+ cells (LRC, label-retaining cells) were double labelled with the marker for mature neurons NeuN (Figure 3A). In this condition, the quantification of LRC in the two neurogenic regions in the adult mouse brain, the SGZ and the SVZ (subventricular zone), demonstrated that basal adult neurogenesis is not affected in cbp+/− mice (Figure 3B and C; Supplementary Figure S3). We next examined EE-induced neurogenesis and found that EE increased >10-fold the number of newborn neurons in the SGZ of WT mice, whereas cbp+/− mice only showed a modest increase (Figure 3B; F(1,13)housing=40.05, P<0.001; F(1,13)genotype=14.57, P<0.01; F(1,13)genotype × housing=15.91, P<0.01). Our experiments therefore revealed a good correlation between EE-enhanced neurogenesis and spatial navigation and pattern separation abilities, supporting a specific role for newborn neurons in these skills.
Figure 3.
Impaired induced neurogenesis in the SGZ of cbp+/− mice. (A) Animals received two daily injections of BrdU (100 mg/kg) for 5 consecutive days starting at day 11 of EE and were perfused (P) 35 days later. The right image shows cells in the SGZ immunolabelled with antibodies against BrdU (red) and NeuN (green) and nuclei counterstained with DAPI (blue). Five weeks after the last administration of BrdU, the vast majority of surviving cells were NeuN+. Scale bar: 2 μm. (B) CBP-deficient mice show a severe impairment in EE-induced neurogenesis in the SGZ. The right panels show representative images of BrdU (brown nuclei) immunostaining showing newborn neurons in the SGZ of WT and cbp+/− mice in SC and EE. Two-way ANOVA, #: significant housing effect, §: significant genotype effect, &: significant genotype × housing interaction; n=3–4 mice per group. Scale bars: 100 μm. (C) KA-induced neurogenesis is also impaired in cbp+/− mice. Six days after a single administration of KA (20 mg/kg) (KA) or vehicle (Veh), WT and cbp+/− mice received two daily BrdU injections for 5 consecutive days. Five weeks later, the mice were perfused (P) and adult newborn cells were stained for BrdU. The right panels show representative images of LRC immunolabelling in the SGZ of WT and cbp+/− mice treated with KA or vehicle. Two-way ANOVA, #: significant housing effect, §: significant genotype effect; n=4–5 per group. Scale bars: 100 μm.
To define more precisely the impairment associated to CBP deficiency, we examined the anatomy and cellular composition of the hippocampus of mutant mice and control littermates by magnetic resonance imaging (MRI; Supplementary Figure S4) and histological analyses (Supplementary Figure S5). Cbp+/− mice had normal hippocampal structure, cell density and hippocampus/brain ratio despite of their slightly smaller body size, facial dysmorphia and skull abnormalities (Viosca et al, 2010). We also examined neuronal differentiation in the SGZ using different markers. The number of quiescent type-1 progenitors (radial nestin+ cells) did not show significant difference between genotypes or housing conditions (Supplementary Figure S6A). In contrast, the examination of maturing neurons with doublecortin (dcx) immunolabelling revealed no difference between genotypes in the basal condition and a reduction in cbp+/− mice housed in an EE (Supplementary Figure S6B; F(1,22)housing=15.03, P<0.01; F(1,22)genotype=4.23, P=0.05; F(1,22)genotype × housing=4.39, P<0.05). This result indicates that EE-induced neurogenesis is already impaired at early differentiation stages. In line with this finding, the determination of immature neurons by LRC count at earlier times after BrdU administration revealed no difference between genotypes in the basal condition (Supplementary Figure S6C).
We examined next whether CBP deficiency also interfered with activity-dependent regulation of neurogenesis in a paradigm unrelated with EE: the cellular response to a single injection of the pro-epileptic drug kainic acid (KA), a manipulation which is known to trigger long-term structural and functional changes in the hippocampus (Parent et al, 1997). As expected, KA-injected WT mice displayed more BrdU/NeuN-positive cells than those injected with vehicle, whereas there was no significant increase in neurogenesis in the case of cbp+/− mice (Figure 3C; F(1,19)treatment=6.97, P<0.05; F(1,19)genotype=5.65, P<0.05; F(1,19)genotype × treatment=3.48, P=0.08).
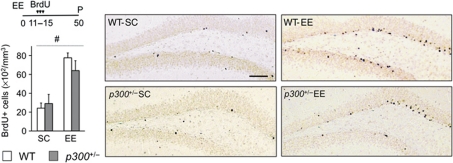
CBP's paralog p300 has been also associated to RSTS; however, the cognitive deficits associated to p300 deficiency in both humans and mice are more modest than for CBP (Zimmermann et al, 2007; Viosca et al, 2010). To evaluate the specificity of the role of CBP in environment-induced neurogenesis, we performed equivalent BrdU uptake experiments in p300+/− mice. This mutant strain showed normal adult neurogenesis in the SGZ both in standard conditions and after EE (Figure 4; F(1,18)housing=31.17, P<0.001; F(1,18)genotype=0.31, P=0.59).
Figure 4.
Environment-induced neurogenesis is not altered in p300+/− mice. p300-deficient mice show normal basal and EE-induced neurogenesis in the SGZ. The right panels present representative images of BrdU (brown nuclei) immunostainings. Two-way ANOVA, #: significant housing effect; n=3–6 per group. Scale bar: 100 μm.
Together, these results indicate that CBP plays a specific and important role in the adaptative response to tonic changes in the activity of hippocampal circuits triggered by experience.
Impaired transcriptional neuroadaptation and histone acetylation in the hippocampus of cbp+/− mice
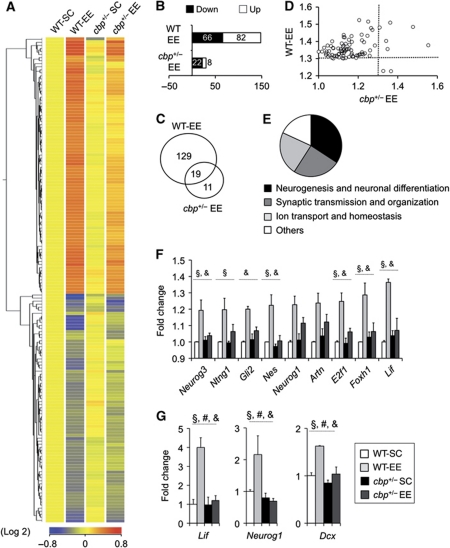
To identify the genes downstream of CBP involved in these deficits, we determined the gene expression profile of CBP-deficient mice and WT littermates housed in SC or after 2 weeks of EE using microarrays. This analysis revealed that CBP deficiency had a very modest effect on basal gene expression, whereas EE triggered changes in >150 genes (Figure 5A).
Figure 5.
Impaired neuroadaptative transcriptional response to EE in the hippocampus of cbp+/− mice. (A) The hierarchical cluster of the 159 TCs differentially regulated in response to EE (corrected P-value <0.05, FC>1.3) reveals an attenuated transcriptional response in cbp+/− mice. (B) Number of TCs upregulated (white) and downregulated (black) in response to EE in WT and cbp+/− mice (referred to the respective SC groups) with FC>1.3. (C) Venn diagram showing the number of EE-regulated TCs. (D) Scatter plot comparing, in WT and cbp+/− mice, the FC of the 84 TCs differentially upregulated in response to EE. The dotted line indicates the threshold for FC. Most dots are located in the upper left quadrant, indicating that the changes are larger in WT animals. r(82)=0.31, P<0.05. (E) Pie diagram showing the number of unique entities associated to a GO term in each of the major functional categories identified in the analysis of gene sets differentially expressed in EE mice. (F) Bar graph showing the expression level of specific neurogenesis-related genes whose induction by EE is impaired in cbp+/− mice (expression values extracted from microarray data). Two-way ANOVA, §: significant genotype effect, &: significant genotype × housing interaction (non-corrected P-values). All these genes show a significant housing effect. Some interesting genes showing borderline significance are also presented. (G) qRT–PCR validation of EE-mediated hippocampal induction for the neurogenesis-related genes lif, neurog1 and dcx. Two-way ANOVA, #: significant housing effect, §: significant genotype effect, &: significant genotype × housing interaction; n=3 per group.
None of the very few transcript clusters (TCs) significantly altered in the hippocampus of cbp+/− mice at the basal stage showed a fold change (FC) larger than 1.2 (Supplementary Table SI). Interestingly, although the function of most of these low confidence candidate genes is poorly understood, some of them have been previously associated with mental retardation (e.g. asl, spred2, srgap3) and the development of the nervous system (e.g. rtn4rl1, fjx1, crim1) and may, therefore, contribute to RSTS neurological traits. As a validation of the microarray experiment, analysis of the individual probe sets comprised into CBP's TC confirmed a 50% reduction in the signal corresponding to exon 2, as it would be expected considering the gene targeting strategy used to generate these mice (Supplementary Figure S7A). Quantitative RT–PCR (qRT–PCR) assays using independent samples reproduced this result and confirmed that the reduction of CBP levels does not cause a compensatory upregulation of the paralog gene Ep300 (Supplementary Figure S7B).
Regarding the transcriptional response to EE, our microarray analysis provided a comprehensive list of EE-induced genes in the mouse hippocampus (Supplementary Table SII). Interestingly, the in silico prediction tool PSCAN (Zambelli et al, 2009), which searches for TF consensus binding sequences in promoter regions, revealed a significant enrichment for CREB-binding sites, among other CBP-interacting transcription factors (Kasper et al, 2006), within the promoter region of these genes (Supplementary Table SIII). Gene Ontology (GO) analysis identified Neurogenesis and neuron differentiation, Ion transport and homeostasis and Synaptic transmission as the main biological functions affected by this condition (Figure 5E; Supplementary Figure S7C; Supplementary Table SIV).
The transcriptional programme induced by EE was clearly attenuated in cbp+/− mice. Both gene upregulations and downregulations were affected (Figure 5A–D). Although all the GO functional groups listed above showed a significant interaction with genotype (Supplementary Figure S7D), Neurogenesis and neuron differentiation showed the most pronounced effect, manifested both in the number of entities affected and the level of statistical significance. The list of EE-regulated related to Neurogenesis and neuron differentiation (Supplementary Table SV) included 13 genes showing a significant genotype effect and 16 genes showing significant genotype × housing interaction. Figure 5F presents several examples of genes directly related to neurogenesis whose expression was enhanced in WT mice housed in an EE, but not in cbp+/− mice. Interestingly, PSCAN analysis revealed that the promoters of these genes were enriched in transcription factor binding site motifs recognized by some relevant CBP partners, such as NF-κβ, p53 and NRSF (Supplementary Table SVI). Independent qRT–PCR assays for some of these genes, such as the pro-differentiative cytokine lif, the pro-neurogenic transcription factor neurog1 and the microtubule-associate protein dcx, confirmed the microarray data (Figure 5G).
Given the cellular heterogeneity of the hippocampus, our gene profiling experiment cannot discriminate between transcriptional changes in specific cellular types and changes in the cellular composition of the tissue. Interestingly, the list of neurogenesis-related genes showing a significant condition per genotype interaction (Supplementary Table SV) included both genes that likely play a cell-autonomous effect (i.e. nes, gli2) and genes encoding for proteins that may have a paracrine function in neurogenesis (i.e. lif), suggesting that transcriptional changes in both mature and newborn neurons can contribute to the altered gene profile. Importantly, in agreement with our observations regarding structural changes in CA1 neurons, the microarray data and independent qRT–PCR assays confirmed that CBP deficiency does not cause a general impairment in EE-regulated gene expression since we observed similar upregulation of a number of genes related to synaptogenesis, such as bdnf, nptx2 and vgf, in both genotypes (Supplementary Figure 7E and F).
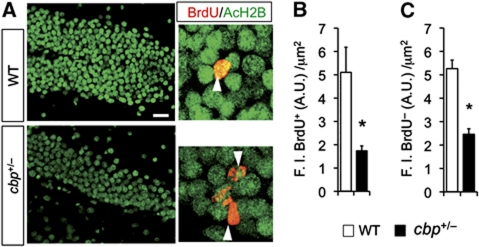
We next examined the acetylation state of mature neurons and neuroprogenitor cells in the DG of cbp+/− mice. Previous analyses in these mice had demonstrated a reduction in hippocampal histone H2B acetylation. This modification has been associated to active transcription (Karlic et al, 2010) and appears to be one of the main reaction catalysed by the KAT activity of CBP in vivo (Alarcon et al, 2004; Valor et al, 2011). In agreement with this observation, we found that both, the proliferating cells in the SGZ (Figure 6A and B) and the surrounding mature granule cells (Figure 6C; Supplementary Figure S8A), have lower level of acetylated histone H2B (AcH2B) than the corresponding cells in WT mice. We, however, did not observe any effect of long-term exposure to EE in the bulk acetylation level of different histones and specific lysine residues (Supplementary Figure S8B and C).
Figure 6.
Reduced histone acetylation in the dentate gyrus of cbp+/− mice. (A) Representative confocal images of the DG labelled with BrdU (red) and acetylated histone H2B (green). Scale bar: 25 μm. (B) Quantification of fluorescence intensity in individual BrdU+ cells demonstrates that the acetylation of histone H2B is reduced in the proliferating neuroprogenitors of cbp+/− mice. *P<0.05 (unpaired two-tailed t-test), n=12–16 cells per group. (C) Quantification of fluorescence intensity in individual granule cells (BrdU− cells adjacent to the BrdU+ cells shown in B) demonstrates a general reduction of histone H2B acetylation in the DG of cbp+/− mice. *P<0.05 (unpaired two-tailed t-test), n=46–50 cells per group.
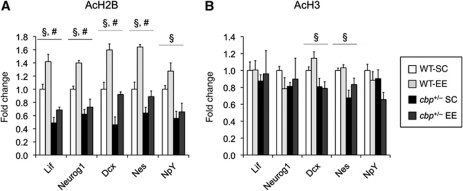
Next, we explored through chromatin immunoprecipitation (ChIP) assays whether the reduced bulk histone H2B acetylation affected the acetylation state of the promoters of some of the neurogenesis-related genes differentially induced by EE in cbp+/− mice and control littermates. Two-way ANOVA of ChIP data revealed significant effects of both genotype and housing condition. Whereas CBP deficiency caused a significant reduction in the acetylation state of histone H2B, EE caused a significant increase. We also found that CBP deficiency prevented the increase of AcH2B induced by EE (Figure 7A). Interestingly, ChIP assays using antibodies against AcH3 showed that CBP deficiency also caused deficits in the acetylation of histone H3 at the promoters of the genes dcx and nes (Figure 7B) despite of the absence of significant deficits at bulk acetylation level (Supplementary Figure S8B and C). These local histone acetylation deficits were not restricted to the neurogenesis-related genes differentially induced in cbp+/− mice, NPY which is not affected at the transcription level by genotype also showed reduced levels of histone H2B acetylation at its promoters (Figure 7A and B).
Figure 7.
Reduced histone acetylation at the promoters of neurogenesis-related genes. (A) ChIP assays using an antibody against AcH2B. (B) ChIP assays using an antibody against AcH3. Two-way ANOVA, #: significant housing effect, §: significant genotype effect; n=3 mice per sample, three samples per condition.
Environment-induced neurogenesis is extrinsically regulated by CBP function in granule cells
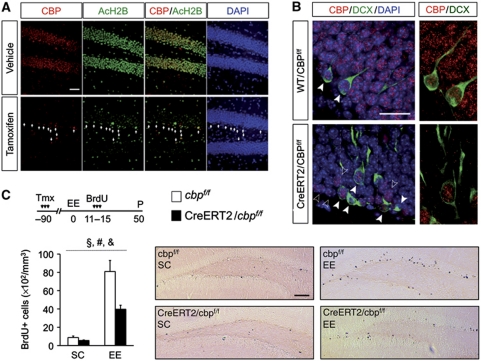
To further clarify the role of CBP-dependent mechanisms in gene expression in environment-induced neurogenesis, we examined the consequences in adult neurogenesis of restricted ablation of CBP in mature neuron using CaMKIIa-creERT2/CBPf/f mice. This regulatable and restricted CBP knockout strain shows a complete ablation of CBP activity in mature granule neurons of the dentate gyrus few days after tamoxifen administration together with a dramatic reduction in the acetylation level of neuronal histones (Figure 8A; Supplementary Figure S9), whereas neuroprogenitor cells and immature neurons show normal levels of CBP and histone acetylation (Figure 8A and B). CaMKIIa-creERT2/CBPf/f and control littermates in SC and upon EE exposure received BrdU injections 12 weeks after tamoxifen administration. The quantification of the number of LRC in these four experimental groups showed that the specific loss of CBP in mature granule cells is sufficient to cause impaired environment-induced neurogenesis in the SGZ (Figure 8C; F(1,17)housing=62.77, P<0.001; F(1,17)genotype=10.91, P<0.01; F(1,17)genotype × housing=8.03, P<0.05). This result discards that the reported adult neurogenesis deficit were a consequence of developmental defects, and indicates that activity-dependent extrinsic regulation of hippocampal neurogenesis requires proper levels of CBP in mature granule neurons of the dentate gyrus.
Figure 8.
The environment-induced neurogenesis defect is not developmental and depends on intact levels of CBP in mature granule cells. (A) In forebrain-restricted inducible CBP knockout mice, tamoxifen injection causes the elimination of CBP immunoreactivity (red) in granule cells and severe hypoacetylation (green). The few remaining CBP+ cells in the inner blade of the DG show normal levels of AcH2B (arrow heads) and are NeuN− (see Supplementary Figure S9), suggesting that only progenitors and newborn neurons still express CBP. Nuclei were stained with DAPI (blue). Scale bar: 50 μm. (B) Immunohistochemistry for CBP (red) and DCX (green) in coronal sections of CaMKIIa-creERT2/CBPf/f and WT/CBPf/f mice treated with tamoxifen demonstrate that most of the remaining CBP+ nuclei in the SGZ of CaMKIIa-creERT2/CBPf/f mice belong to Dcx+ cells (solid arrowheads). The empty arrowheads denote cells in the SGZ of the DG that were CBP+ and Dcx−. Nuclei were stained with DAPI (blue). Right, magnification of the dotted squares in the left images show CBP+ and DCX+ cells in the SGZ of the DG. Scale bar: 15 μm. (C) Tamoxifen was administered to 2-month-old mice and 12 weeks later, mice were housed in EE or maintained in SC. Animals received two daily injections of BrdU (100 mg/kg) for 5 consecutive days starting at day 11 of EE. Five weeks after the last administration of BrdU, the animals were perfused (P) for immunohistochemistry. CaMKIIa-creERT2/CBPf/f mice show a severe impairment in EE-induced neurogenesis in the SGZ. The right panels show representative images of BrdU (brown nuclei) immunostaining showing newborn neurons in the SGZ of CaMKIIa-creERT2/CBPf/f mice and control littermates housed in either SC or EE. Two-way ANOVA, #: significant housing effect, §: significant genotype effect, &: significant genotype × housing interaction; n=4–5 per group. Scale bar: 100 μm.
Discussion
Our experiments in a mouse model for RSTS mental retardation demonstrate that EE ameliorates some of the deficits associated to RSTS and unveil new impairments caused by CBP deficiency. Cbp+/− mice have a strong defect in environment-induced neurogenesis that correlates with the attenuated induction of EE-regulated genes, with impaired EE-dependent histone acetylation at specific promoters and with impaired modulation of pattern separation and spatial navigation abilities. Additional experiments with conditional knockout mice indicate that environment-induced neurogenesis is extrinsically regulated by CBP function in granule cells. Moreover, our rigorous microarray analysis contributes to elucidate the still poorly defined gene programme induced in the hippocampus in response to EE. This information will represent a useful resource for future studies since we identified dozens of new genes involved in the neuronal response to this condition. Although CBP deficiency had a minor impact in basal hippocampal gene expression, our screen also identified possible CBP downstream genes that may be relevant for RSTS pathology and should be further explored.
Reversal of behavioural deficits in cbp+/− mice: implications in RSTS therapeutics
Our interdisciplinary analyses demonstrate that long-term exposure to EE had a beneficial effect in various motor and cognitive abilities of cbp+/− mice and promoted synaptic growth and induced a number of plasticity-related genes in the hippocampus regardless of the genotype. Our data, therefore, suggest that behavioural therapy may be, as for other cognitive disorders (Nithianantharajah and Hannan, 2006), beneficial for RSTS children. However, EE also unveiled new deficits associated to CBP hemideficiency. The result of two independent water maze experiments, the classical hidden platform task and a radial maze task adapted to examine pattern separation ability, showed that cbp+/− mice, contrary to their control littermates, do not become more precise in the localization of the escape platform as result of the EE. There is a great deal of discussion about the role of hippocampal newborn neurons in learning and memory (Deng et al, 2010; Aimone et al, 2011; Ming and Song, 2011; Sahay et al, 2011b) and the extent to which increased neurogenesis is relevant for cognitive improvement in hippocampal-dependent tasks (Meshi et al, 2006; Jaholkowski et al, 2009). Our behavioural experiments provide additional correlative evidence supporting the recently presented hypothesis that newborn neurons in the DG play a specific role in spatial memory resolution and pattern separation (Clelland et al, 2009; Creer et al, 2010; Sahay et al, 2011a). In addition, these results indicate that there are two components in the neurological deficits observed in RSTS mice, one of them susceptible of recovery through EE (e.g. locomotor and fear conditioning impairments) and another resistant to this condition and related to the production of newborn neurons in the SGZ. Overall, these findings suggest a role of defective neurogenesis in RSTS cognitive impairments and predict that the efficacy of environmental therapies might be more limited than in other mental impairment disorders (Jirtle and Skinner, 2007). Our study might also have important implications for other neurological diseases since a similar specific defect in enrichment-mediated hippocampal neurogenesis has been reported for mice expressing Presenilin 1 (PS1) variants linked to early-onset familial Alzheimer's disease (FAD) (Choi et al, 2008) and for PS1-deficient mice (Feng et al, 2001). Since PS1 deficiency causes a reduction of the expression of CBP (Saura et al, 2004), our finding provides an attractive molecular explanation for FAD-linked defects in neurogenesis.
Role of CBP in environment-induced neurogenesis
Although CBP has been recently shown to regulate embryonic neural differentiation (Wang et al, 2010), our experiments indicate that a normal level of CBP is neglectable for steady-state neurogenesis in the adult hippocampus, but becomes again necessary for modulating the rate of neurogenesis in response to environmental challenges, such as EE and induced seizures, suggesting a general role for CBP in the adaptation of hippocampal circuits to external stimuli. CBP does so likely in coordination with other epigenetic factors like Gadd45b, a protein previously involved in DNA repair, whose loss also causes a deficit in environment-induced neurogenesis (Ma et al, 2009a). CBP plays a dual role in transcriptional regulation: it acts as transcriptional co-activator for a large number of transcription factors and as epigenetic enzyme with intrinsic KAT activity (Chan and La Thangue, 2001). It is likely that both functions will contribute to the neurogenesis and transcriptional defects observed in CBP-deficient mice.
The most studied partner of CBP is CREB, an activity-regulated transcription factor that plays important roles in cognition (Benito and Barco, 2010) and adult neurogenesis (Merz et al, 2011). CREB regulates different phenomena during neurogenesis, from proliferation and survival of neuroprogenitors to the maturation and integration of newborn neurons in neuronal circuits (Zhu et al, 2004; Jagasia et al, 2009; Merz et al, 2011). Other transcription factors involved in adult neurogenesis also use CBP as co-activator (Medrano and Scrable, 2005; Denis-Donini et al, 2008). These include NF-κβ and p53 that are, in addition, direct substrates of CBP's KAT activity (Ito et al, 2001; Nadiminty et al, 2006). Interestingly, we observed a strong and significant enrichment for the binding motifs of some of these transcription factors in the promoters of neurogenesis-related genes regulated by EE and showing a significant genotype effect or genotype × housing interaction.
Histone acetylation also plays an important role in neurogenesis (Lee and Lee, 2010). In vitro experiments using HDAC inhibitors (HDACi), with few exceptions, have consistently shown that the treatment with HDACi reduces neural cell proliferation and promotes neuronal differentiation (Hao et al, 2004; Hsieh et al, 2004; Yu et al, 2009; Umka et al, 2010). However, the results of in vivo experiments are more difficult to interpret (Jessberger et al, 2007; Kim et al, 2009), probably because the reduction of proliferation by HDACi may result in a net reduction of neurogenesis in spite of the potential activity of these compounds to promote neuronal differentiation. Interestingly, the deficiency in HDAC2 results in specific and cell-autonomous defects in neural differentiation during adult neurogenesis (Jawerka et al, 2010). Therefore, the balance between histone acetylation/deacetylation seems to be critical for the correct activation and/or inactivation of neurogenic programmes.
CBP and neuroadaptation to environmental changes
It has been proposed that epigenetic mechanisms, such as DNA methylation or histone modification, serve as key conduits for the extrinsic regulation of adult neurogenesis by a wide variety of stimuli, including the environment and internal physiological states (Ma et al, 2009b, 2010). Since the recruitment of CBP to specific promoters is regulated by neuronal activity and depends on the sort of stimuli (Hardingham et al, 1999), this protein is in a privileged position to link neuronal circuit activity to epigenetic modification of the chromatin, leading to persistent or permanent changes in neuronal circuits through changes in gene expression.
We and others have proposed that the reduction of CBP and the subsequent hypoacetylation of histones may interfere with the transcriptional response driven by activity, thus contributing to the cognitive deficits observed in mouse models for RSTS (Barco, 2007). Acetylation marks in the chromatin are considered a feature of active transcription (Kouzarides, 2007). In particular, the presence of acetylated H2B has been associated to highly transcribed genes and with the maintenance of transcriptional competence at specific loci (Myers et al, 2003; Karlic et al, 2010). Recent results indicate that this may be a consequence rather than a cause of the high transcriptional activity (Kasper et al, 2010; Valor et al, 2011). Histone acetylation, or at least some specific histone acetylation marks, may be associated to the ability to respond to certain stimuli rather than be an exact readout of the transcriptional activity of the loci. This would explain why the histone acetylation defects shown in Figures 6 and 7 do not have a direct correlate in basal hippocampal gene expression and basal adult neurogenesis. Given the role of CBP in the setting of epigenetic marks, it is maybe not surprising that the transcriptional consequences of its deficiency became more evident in the context of the establishment of a new long-term transcriptional stage, such as the response to EE. In agreement with this view, our microarray experiment indicates that most of the transcriptional programme induced by EE was affected by CBP deficiency.
The study of gene–environment interactions has experimented important progress in the last years. The epigenetic modification of the genome provides mechanisms that allow the stable propagation of gene activity states from one generation of cells to the next (Jaenisch and Bird, 2003). Referring to neurons, the same epigenetic events can underlie the long-term maintenance, maybe for the whole life of the individual, of new gene activity states, providing a plausible link between experience and long-lasting alterations in gene expression in the brain (Fischer et al, 2007). In fact, recent studies have shown that some of the benefits of EE can be transmitted to the offspring, which necessarily involves the participation of epigenetic mechanisms (Arai et al, 2009). Our study demonstrates that the environment can alter gene expression and its functional outputs by impinging on activities involved in modifying the epigenome, highlighting the importance of neural histone acetylation in gene–environment interaction and adult neurogenesis (Hsieh and Eisch, 2010). It also identifies CBP-dependent transcription and histone acetylation as important mediators of environment-induced benefits.
Materials and methods
Maintenance, treatment and housing of mice
The generation of cbp+/− (Tanaka et al, 1997), p300+/− (Yao et al, 1998), CBPf/f (Zhang et al, 2004), CaMKIIa-creERT2 (Erdmann et al, 2007) and Thy1-EGFP (line M) (Feng et al, 2000) mice have been previously described. The experiments with cbp+/− mice were performed on a DBA and C57BL/6J mixed background, since these mutants are not viable in a pure C57BL/6J background (Alarcon et al, 2004). The genetic background of all other mice was C57BL/6J. Experiments were performed in 2- to 7-month-old animals and in all cases the mice used as control were littermates of the mutant mice. For CBP ablation experiments, tamoxifen (T5648; Sigma-Aldrich) was administered to ∼2-month-old CaMKIIa-creERT2 /CBPf/f mice using a gastric probe for 5 consecutive days (total consumption=20 mg per animal); control animals were CaMKIIa-creERT2/CBPf/f treated with the same volume of the vehicle (corn oil C8267; Sigma-Aldrich) and non-cre recombinase expressing CBPf/f treated with tamoxifen. To observe nuclear translocation of cre recombinase in a control experiment, tamoxifen was administered once more the day before sacrifice. Mice were maintained according to animal care standards established by the European Union and all the protocols were approved by the Institutional Animal Care and Use Committee. The mice were kept on a 12-h light/dark cycle and food and water were provided ad libitum. Standard housing consisted of 30 × 15 × 11 cm3 clear cages occupied by up to five mice. The EE boxes were large white plexiglass boxes (>1 m2) and were occupied by a maximum of 20 mice. We used natural materials, plastic tubing, running wheels and toys to create an EE whose configuration was modified every 48 h shortly before the start of the dark cycle. The samples from EE animals used in the western blot, immunohistochemistry, qRT–PCR and microarray experiments were obtained at least 16 h after the last change to prevent any interference between the response to novelty and to EE. Intraperitoneal (i.p.) KA injection (20 mg/kg in saline solution) caused overt seizures 5–10 min later, which lasted for ∼2–3 h before they spontaneously stopped. All mice displayed status epilepticus that started with jerking of the forelimbs, often followed by seesaws and involuntary falling. One death occurred among 12 animals both in the WT and in the cbp+/− group. For BrdU uptake experiments, the animals received i.p. injections of BrdU (100 mg/kg in saline solution) at the times indicated in the legends of Figures 3, 4 and 8; Supplementary Figures S3 and S9.
Antibodies
In this study, the following primary antibodies were used: α-AcH2A, α-AcH2B, α-AcH3 and α-AcH4 (Sanchis-Segura et al, 2009); α-H2B (07-371), α-AcH2B-K5 (07-382), α-AcH2B-K12 (07-336), α-AcH2B-K15 (07-343), α-AcH2B-K20 (07-347), α-H3 (05-499), α-pH3-S10 (06-570), α-AcH3-K14 (06-911), α-AcH4-K8 (07-328) and α-NeuN (MAB377) from Millipore (Billerica, MA, USA); α-AcH2A-K5 (5276) from Cell Signaling (Beverly, MA, USA); α-H3 (ab1791), α-BrdU (ab6326), α-dcx (ab18723), α-nestin (ab11306) and α-AcH3-K14 (ab52946) from Abcam (Cambridge, UK); α-β-actin (F5441), α-GFAP (G9269), and α-MAP2 (M4403) from Sigma-Aldrich Química SA (Madrid, Spain); α-GFP (A-11122) from Invitrogen (Carlsbad, CA, USA); α-CBP C1 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); α-Cre recombinase (Kellendonk et al, 1999). See Supplementary data for secondary antibodies details.
Quantitative western blotting
Western blot analyses were carried out as previously described (Sanchis-Segura et al, 2009). Equal amount of protein extracted from isolated hippocampi was loaded in each lane, and the intensity of the protein bands was measured using FUJIFILM LAS-100 equipment (Fujiphoto Film Co.) and quantified using Quantity One 4.6 software (Bio-Rad, Inc.).
Immunohistochemistry and structural analyses
Mice were anaesthetized with a ketamine/xylazine mixture immediately and perfused with paraformaldehyde (4% in 0.1 M phosphate buffer); brains were postfixed overnight. Immunostaining was performed on 50 μm free-floating sections. For BrdU staining, sections were incubated in HCl 2 N for 30 min at 37°C, rinsed in 0.1 M borate buffer, pH 8.5, and thoroughly washed in Tris-buffered saline, pH 7.4. For diaminobenzidine (Sigma) immunostaining, sections were pretreated with 0.6% H2O2 to block endogenous peroxidase reaction. For immunofluorescence, sections were counterstained with DAPI (Molecular Probes). For the structural analysis of dendritic spines, the transgenic line Thy1-EGFP (Feng et al, 2000) was crossed with cbp+/− strain to generate Thy1-EGFP/cbp+/− double mutants. Two-way ANOVA and two-tailed unpaired t-test were used to analyse histological data. The experimenters were blind to the genotypes and housing of the mice for all quantifications. See Supplementary data for additional details on the analysis of dendritic spines and description of MRI methods.
Microarray analysis
Total RNA extracted from the hippocampi of three to four age, sex- and genotype-matched mice were use to produce one pooled sample. We analysed 16 samples (8 per genotype): 5 control (SC) and 3 EE. RNA samples were hybridized to GeneChip® Mouse Gene 1.0 ST Arrays according to the manufacturer's protocol (Affymetrix, Santa Clara, CA). The microarray data were then analysed using GeneSpring GX 11 (Agilent Technologies, Inc., Santa Clara, CA). RMA (Robust Multichip Average) algorithm was used for data normalization. Principal component analysis revealed clustering of samples according to the batch of replicates; therefore, normalization was conducted using the median of the corresponding control samples values as reference. TCs were then filtered on signal intensity by establishing a lower cutoff at the 20th percentile (20th–100th percentile). Genotype and EE differentially regulated TC sets were obtained using two-way ANOVA. TCs differentially regulated by ‘genotype’ were also identified using unpaired t-test since no TC passed the thresholds defined in the two-way ANOVA analysis. In significance analysis, P-values were obtained by asymptotic computation and corrected for multiple testing with Benjamini–Hochberg FDR method. Hierarchical clustering was also performed using GeneSpring software. GO enrichment analysis for differentially regulated genes upon EE was performed using the web-based gene set analysis toolkit (WebGestalt) (Zhang et al, 2005). PSCAN Versión 1.1.1 was used for transcription factor binding site discovery (Zambelli et al, 2009). See Supplementary data for additional detail. Microarray data are accessible through the Gene Expression Omnibus (GEO) database (GEO Series accession number GSE30880).
Quantitative RT–PCR and ChIP assays
Whole hippocampi were dissected and treated with RNAlater solution (Qiagen). DG subregions were microdissected from two 500-μm slices obtained from dorsal hippocampus with a tissue chopper (Stoelting). Total RNA was extracted using RNeasy kit (Qiagen). Reverse transcription was performed using RevertAid First-Strand cDNA synthesis kit (Fermentas). qRT–PCR was carried out using SYBR GreenER mix (Invitrogen, Carlsbad, CA) and primers specific for the genes of interest. ChIP experiments were performed according to the protocol in the Millipore ChIP kit (Millipore) with minor modification based on Wells and Farnham (2002). See Supplementary data for addition details. Primer sequences used in ChIP and qRT–PCR assays are listed in Supplementary Table SVII.
Behaviour
For all behavioural tasks, we used adult female mutant and control littermates to prevent the fights observed between males housed in an EE. The experimenters were blind to genotypes. The result of the PCR-based genotyping was provided as a factor for statistical analysis of the behavioural data once the battery of tasks was concluded. The open field, elevated plus maze RotaRod, MWM and fear conditioning tasks were performed as previously described (Viosca et al, 2010). The training protocol in the MWM consisted of three trials per day with a 45-min inter-trial interval. If the mice did not find the platform after 120 s, they were gently guided to it. Memory retention trials of 60 s were performed at the beginning of day 5 (P1) and 24 h after concluding the training on day 8 (P2). The number of annulus crossings was calculated considering an area double than the platform. For the pattern separation experiment, we adapted the protocol described into a WRM (Clelland et al, 2009). A six-arm WRM was positioned in the centre of a pool (1.7 m of diameter) filled with opaque water. A platform of 10 cm diameter was placed 1 cm below the water level at the end of one selected arm. We positioned external cues in the walls to facilitate spatial navigation. The animals were trained per 3 consecutive days changing the platform position each day to ensure that mice use a hippocampus dependent, allocentric strategy to solve the task; the platform position and the target arm were changed and balanced between trials. The animals were exposed each day to 14 training trials and four probe trials. In each trial, mice were gently positioned in the centre of the maze facing to a closed arm and allowed to search for the platform for a maximum of 120 s. In the probe trials, the animals had to choose between two arms open (the target and the choice arms) at variable distance (HIGH: high spatial separation with one closed arm in between; or LOW: low spatial separation, in which the arms were contiguous). A trial was considered successful when the mouse entered into the target arm without making any error. See Supplementary data for addition details.
Statistical methods
Precise description of the statistical methods used in each experiment is presented in the text. In the graphs, error bars represent s.e.m.
Supplementary Material
Acknowledgments
We thank Eva Benito, Isabel Fariñas, Eloisa Herrera and Juan Lerma for critical reading of the manuscript; Begoña Fernández-Nuñez, Fabrizio Grasso, Román Olivares and Matías M Pulópulos for technical assistance; we also thank Eva Benito for help with probe set level data extraction and Christoph Kellendonk for the α-Cre antibody. Research at Barco's laboratory is supported by the grants from the Spanish Ministry of Science and Innovation BFU2008-00611, CSD2007-00023 and SAF2008-03194-E (part of the coordinated ERA-Net NEURON project Epitherapy), and grants from Fundación Ramón Areces and Fundació La Marató de TV3 (063510). SC is supported by grants from the Spanish Ministry of Science and Innovation BFU2009-09938 and CSD2007-00023, and the Fundación Mutua Madrileña (3140/2008). MG is supported by Telethon-Italy (Grant no. GGP09196). JLA has a Juan de la Cierva contract and LMV has a Ramón y Cajal contract both given by the Spanish Ministry of Science and Innovation.
Author contributions: JPL-A and AB conceived and designed the study, and wrote the manuscript. JPL-A performed the gene expression analysis and most of the cellular and molecular biology work. AC and JV performed the behavioural experiments. AC and MG performed the dendritic spine analysis and some immunostainings with neuronal markers. LMV contributed to the analysis of CBP floxed mice. MJ-M assisted in the performance of immunostainings. SC performed the MRI analysis.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aimone JB, Deng W, Gage FH (2011) Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70: 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A (2004) Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42: 947–959 [DOI] [PubMed] [Google Scholar]
- Arai JA, Li S, Hartley DM, Feig LA (2009) Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J Neurosci 29: 1496–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A (2007) The Rubinstein-Taybi syndrome: modeling mental impairment in the mouse. Genes Brain Behav 6: 32–39 [DOI] [PubMed] [Google Scholar]
- Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, Lynch G, Greene RW, Wood MA (2011) Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology 36: 1545–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito E, Barco A (2010) CREB′s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33: 230–240 [DOI] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P (2008) Decoding the epigenetic language of neuronal plasticity. Neuron 60: 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T (2003) A mouse model of rubinstein-taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA 100: 10518–10522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB (2001) p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114: 2363–2373 [DOI] [PubMed] [Google Scholar]
- Chen G, Zou X, Watanabe H, van Deursen JM, Shen J (2010) CREB binding protein is required for both short-term and long-term memory formation. J Neurosci 30: 13066–13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS (2008) Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron 59: 568–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325: 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ (2010) Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA 107: 2367–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis-Donini S, Dellarole A, Crociara P, Francese MT, Bortolotto V, Quadrato G, Canonico PL, Orsetti M, Ghi P, Memo M, Bonini SA, Ferrari-Toninelli G, Grilli M (2008) Impaired adult neurogenesis associated with short-term memory defects in NF-kappaB p50-deficient mice. J Neurosci 28: 3911–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann G, Schutz G, Berger S (2007) Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51 [DOI] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ (2001) Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32: 911–926 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning and memory is associated with chromatin remodelling. Nature 447: 178–182 [DOI] [PubMed] [Google Scholar]
- Graff J, Mansuy IM (2009) Epigenetic dysregulation in cognitive disorders. Eur J Neurosci 30: 1–8 [DOI] [PubMed] [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G (2004) Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci 24: 6590–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Cruzalegui FH, Bading H (1999) Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron 22: 789–798 [DOI] [PubMed] [Google Scholar]
- Hsieh J, Eisch AJ (2010) Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiol Dis 39: 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH (2004) Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA 101: 16659–16664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Lai CH, Zhao X, Saito S, Hamilton MH, Appella E, Yao TP (2001) p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J 20: 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33: 245–254 [DOI] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC (2009) GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci 29: 7966–7977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK (2009) New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem 16: 439–451 [DOI] [PubMed] [Google Scholar]
- Jawerka M, Colak D, Dimou L, Spiller C, Lagger S, Montgomery RL, Olson EN, Wurst W, Gottlicher M, Gotz M (2010) The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol 6: 93–107 [DOI] [PubMed] [Google Scholar]
- Jessberger S, Nakashima K, Clemenson GD Jr, Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J (2007) Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 27: 5967–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlic R, Chung HR, Lasserre J, Vlahovicek K, Vingron M (2010) Histone modification levels are predictive for gene expression. Proc Natl Acad Sci USA 107: 2926–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Fukuyama T, Biesen MA, Boussouar F, Tong C, de Pauw A, Murray PJ, van Deursen JM, Brindle PK (2006) Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol Cell Biol 26: 789–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Lerach S, Wang J, Wu S, Jeevan T, Brindle PK (2010) CBP/p300 double null cells reveal effect of coactivator level and diversity on CREB transactivation. EMBO J 29: 3660–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Tronche F, Casanova E, Anlag K, Opherk C, Schutz G (1999) Inducible site-specific recombination in the brain. J Mol Biol 285: 175–182 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Leeds P, Chuang DM (2009) The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem 110: 1226–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M (2004) CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42: 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T (2007) Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467: 455–463 [DOI] [PubMed] [Google Scholar]
- Lee S, Lee SK (2010) Crucial roles of histone-modifying enzymes in mediating neural cell-type specification. Curr Opin Neurobiol 20: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H (2009a) Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323: 1074–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Kim WR, Ming GL, Song H (2009b) Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann NY Acad Sci 1170: 664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H (2010) Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci 13: 1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano S, Scrable H (2005) Maintaining appearances--the role of p53 in adult neurogenesis. Biochem Biophys Res Commun 331: 828–833 [DOI] [PubMed] [Google Scholar]
- Merz K, Herold S, Lie DC (2011) CREB in adult neurogenesis—master and partner in the development of adult-born neurons? Eur J Neurosci 33: 1078–1086 [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R (2006) Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci 9: 729–731 [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H (2011) Adult neurogenesis in the Mammalian brain: significant answers and significant questions. Neuron 70: 687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers FA, Chong W, Evans DR, Thorne AW, Crane-Robinson C (2003) Acetylation of histone H2B mirrors that of H4 and H3 at the chicken beta-globin locus but not at housekeeping genes. J Biol Chem 278: 36315–36322 [DOI] [PubMed] [Google Scholar]
- Nadiminty N, Lou W, Lee SO, Lin X, Trump DL, Gao AC (2006) Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci USA 103: 7264–7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7: 697–709 [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17: 3727–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ, Breuning MH (1995) Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376: 348–351 [DOI] [PubMed] [Google Scholar]
- Rubinstein JH, Taybi H (1963) Broad thumbs and toes and facial abnormalities. A possible mental retardation syndrome. Am J Dis Child 105: 588–608 [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011a) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472: 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R (2011b) Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70: 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Lopez-Atalaya JP, Barco A (2009) Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology 34: 2642–2654 [DOI] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ 3rd, Kandel ER, Duff K, Kirkwood A, Shen J (2004) Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42: 23–36 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Naruse I, Maekawa T, Masuya H, Shiroishi T, Ishii S (1997) Abnormal skeletal patterning in embryos lacking a single Cbp allele: a partial similarity with Rubinstein-Taybi syndrome. Proc Natl Acad Sci USA 94: 10215–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umka J, Mustafa S, ElBeltagy M, Thorpe A, Latif L, Bennett G, Wigmore PM (2010) Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 166: 15–22 [DOI] [PubMed] [Google Scholar]
- Valor LM, Pulopulos MM, Jimenez-Minchan M, Olivares R, Lutz B, Barco A (2011) Ablation of CBP in forebrain principal neurons causes modest memory and transcriptional defects and a dramatic reduction of histone acetylation, but does not affect cell viability. J Neurosci 31: 1652–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (2000) Neural consequences of environmental enrichment. Nat Rev Neurosci 1: 191–198 [DOI] [PubMed] [Google Scholar]
- Viosca J, Lopez-Atalaya JP, Olivares R, Eckner R, Barco A (2010) Syndromic features and mild cognitive impairment in mice with genetic reduction on p300 activity: differential contribution of p300 and CBP to Rubinstein-Taybi syndrome etiology. Neurobiol Dis 37: 186–194 [DOI] [PubMed] [Google Scholar]
- Wang J, Weaver IC, Gauthier-Fisher A, Wang H, He L, Yeomans J, Wondisford F, Kaplan DR, Miller FD (2010) CBP histone acetyltransferase activity regulates embryonic neural differentiation in the normal and Rubinstein-Taybi syndrome brain. Dev Cell 18: 114–125 [DOI] [PubMed] [Google Scholar]
- Wells J, Farnham PJ (2002) Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods 26: 48–56 [DOI] [PubMed] [Google Scholar]
- Wiley S, Swayne S, Rubinstein JH, Lanphear NE, Stevens CA (2003) Rubinstein-Taybi syndrome medical guidelines. Am J Med Genet A 119: 101–110 [DOI] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T (2006) A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem 13: 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T (2005) Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao TP, Oh SP, Fuchs M, Zhou ND, Ch′ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R (1998) Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93: 361–372 [DOI] [PubMed] [Google Scholar]
- Yu IT, Park JY, Kim SH, Lee JS, Kim YS, Son H (2009) Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology 56: 473–480 [DOI] [PubMed] [Google Scholar]
- Zambelli F, Pesole G, Pavesi G (2009) Pscan: finding over-represented transcription factor binding site motifs in sequences from co-regulated or co-expressed genes. Nucleic Acids Res 37: W247–W252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Kirov S, Snoddy J (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33: W741–W748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hofmann C, Casanova E, Schutz G, Lutz B (2004) Generation of a conditional allele of the CBP gene in mouse. Genesis 40: 82–89 [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132: 645–660 [DOI] [PubMed] [Google Scholar]
- Zhu DY, Lau L, Liu SH, Wei JS, Lu YM (2004) Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 101: 9453–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N, Acosta AM, Kohlhase J, Bartsch O (2007) Confirmation of EP300 gene mutations as a rare cause of Rubinstein-Taybi syndrome. Eur J Hum Genet 15: 837–842 [DOI] [PubMed] [Google Scholar]
- Zocchi L, Sassone-Corsi P (2010) Joining the dots: from chromatin remodeling to neuronal plasticity. Curr Opin Neurobiol 20: 432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.