Abstract
Mitochondrial Ca2+ uptake and release play a fundamental role in the control of different physiological processes, such as cytoplasmic Ca2+ signalling, ATP production and hormone metabolism, while dysregulation of mitochondrial Ca2+ handling triggers the cascade of events that lead to cell death. The basic mechanisms of mitochondrial Ca2+ homeostasis have been firmly established for decades, but the molecular identities of the channels and transporters responsible for Ca2+ uptake and release have remained mysterious until very recently. Here, we briefly review the main findings that have led to our present understanding of mitochondrial Ca2+ homeostasis and its integration in cell physiology. We will then discuss the recent work that has unravelled the biochemical identity of three key molecules: NCLX, the mitochondrial Na+/Ca2+ antiporter, MCU, the pore-forming subunit of the mitochondrial Ca2+ uptake channel, and MICU1, one of its regulatory subunits.
Keywords: calcium, MCU, MICU1, mitochondria, NCLX
Introduction
All membrane enclosed organelles (with the notable exception of nucleus and peroxisomes that appear to be in rapid, passive, equilibrium with the cytosol) are endowed with mechanisms that allow an energy-dependent Ca2+ accumulation and a release dependent on the cation concentration gradient between the organelle lumen and the cytosol (for review see Rizzuto and Pozzan, 2006). In most cases, the uptake mechanism is due to Ca2+ pumps (i.e. vectorial enzymes that utilize the energy liberated by ATP hydrolysis to drive Ca2+ accumulation into the organelle lumen); the release channels, on the contrary, are controlled by different second messengers and allow Ca2+ efflux into the cytoplasm. Sparse—and debated—evidence has been provided, indicating that lysosomes and other acidic organelles may use ATP indirectly, by generating a H+ gradient (acid inside) through ATP hydrolysis, followed by Ca2+ accumulation via a H+/Ca2+ exchanger (Rizzuto and Pozzan, 2006). Mitochondrial Ca2+ accumulation and release are based on an opposite strategy: not only they do not need ATP for uptake, but they utilize gated channels for Ca2+ uptake and exchangers (Na+ or H+/Ca2+ exchangers) for release. This apparent paradox depends on the fact that the driving force for Ca2+ accumulation in the mitochondrial matrix is the membrane potential (negative inside) (ΔΨ) across the inner membrane; the exchangers use the concentration gradient of Ca2+, H+ and Na+ across the inner membrane to cause the release of Ca2+ back into the cytosol (Nicholls, 2005). This unique mitochondrial toolkit ensures the maintenance of a low matrix Ca2+ concentration ([Ca2+]) in resting cells and a rapid Ca2+ accumulation by the organelle when cytosolic Ca2+ is elevated during activation. In turn, mitochondrial Ca2+ uptake and release is central not only for the regulation of cellular Ca2+ homeostasis, but is vital also for the regulation of intramitochondrial enzymes concerned with the utilization of oxidizable substrates. Finally, excess Ca2+ accumulation by mitochondria is a common event in the process of cell death, by both necrosis and apoptosis (Rizzuto and Pozzan, 2006; Giacomello et al, 2007).
Despite the importance and the general interest for this problem, and over 40 years of intense and frustrating research on the topic, the molecular nature of the components of the mitochondrial Ca2+ homeostatic machinery has remained mysterious. In the last year, however, three novel proteins have been identified that appear to fulfil all the characteristics expected by the mitochondrial Ca2+ uptake channels and by the Na+/Ca2+ exchanger (Palty et al, 2010; Baughman et al, 2011; De Stefani et al, 2011). We here briefly summarize the historical development of the research on this topic, the key findings that have unravelled the physiopathological role of mitochondrial Ca2+ homeostasis and finally we describe the latest findings concerned with the molecular identification of the key players of the mitochondrial Ca2+ accumulation and release machinery.
Historical background
Mitochondria were identified as the powerhouse for energy production in eukaryotic cells; thanks to decades of extensive biochemical work on carbohydrate metabolism and organelle morpho-functional characterization carried out, in the first half of the 20th century, by leading scientific figures such as Krebs, Corey, Claude, Palade and many others. It soon became clear that Ca2+ played a special role in mitochondrial physiology and, in particular, Ca2+ was demonstrated to behave as a unique uncoupler of oxidative phosphorylation, OXPHOS (reviewed in Carafoli, 2010). The reason for this uncoupling effect of Ca2+ remained, however, mysterious for some time, until in 1961 and 1962 two groups demonstrated that it was due to the ability of mitochondria to efficiently take up Ca2+ from the medium (and accumulate it in the matrix) at the expenses of energy consumption (Deluca and Engstrom, 1961; Vasington and Murphy, 1962). Two of the basic characteristics of Ca2+ accumulation by mitochondria were immediately highlighted: (i) Ca2+ uptake by the organelle requires an energy source in the form of either oxidizable substrates or ATP and (ii) Ca2+ can uncouple oxidation of substrates from ATP synthesis. A number of groups quickly jumped on the topic and many aspects of the Ca2+ uptake system of mitochondria were rapidly clarified; most of these findings still remain undisputed. In particular, it was shown that (i) the Ca2+ uptake system can take up also other divalent cations (Mn2+ and Sr2+ in particular), while Mg2+ acts as a competitive inhibitor; (ii) Ca2+ entry is blocked by uncouplers of OXPHOS such as dinitrophenol or FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone); (iii) this Ca2+ entry does not require ATP hydrolysis, although it can be supported by ATP if the respiratory chain (RC) is blocked; and (iv) the uptake is saturable (suggesting the existence of a ‘carrier’) and is accompanied by stoichiometric H+ extrusion from the matrix.
In the beginning, the driving force for such Ca2+ accumulation was unclear: the main idea was that it depended on the generation of a high energy phosphorylated chemical intermediate (denomitated X∼P; Chance, 1965), but with the general acceptance of the chemiosmotic hypothesis, it became clear that the driving force for Ca2+ accumulation is the ΔΨ across the mitochondrial inner membrane (Mitchell and Moyle, 1967). Thus, Ca2+ enters the mitochondrial matrix down its electrochemical gradient, that can be generated either by the electron flow in the RC or by reversal of the ATP synthase. Indeed, in those years, one of the most convincing pieces of evidence that supported the chemiosmotic hypothesis came from the study of mitochondrial Ca2+ accumulation: it was demonstrated that, in mitochondria with both the RC and the ATP synthase blocked, Ca2+ uptake could be activated by the K+ diffusion potential generated by adding valinomycin in the presence of low K+ in the medium (Scarpa and Azzone, 1970).
For some time, the net positive charges (2 or 1) transported across the membrane during Ca2+ accumulation was a matter of debate. Two models were proposed: in the first case (two positive charges), the Ca2+ carrier was defined mitochondrial Ca2+ ‘uniporter’, MCU (Brand et al, 1976) while in the second (one positive charge), it was define as a H+/Ca2+ antiporter (Moyle and Mitchell, 1977). The controversy was eventually settled (but has surfaced again recently; see below) and the general agreement was that Ca2+ accumulation results in the net transfer of two positive charges into the matrix; that is, it is mediated by the MCU. Ca2+ influx, in turn, results in the drop of ΔΨ that is regenerated by the extrusion (through the RC or ATP hydrolysis) of 2H+ per Ca2+ accumulated. Thus, to maintain gross electroneutrality, the H+/Ca2+ stoichiometry is 2.
An obvious consequence of Ca2+-dependent H+ extrusion is the alkalinization of the mitochondrial matrix that eventually blocks further Ca2+ uptake. Indeed, addition of permeant anions such as inorganic phosphate (Pi) or acetate collapses the ΔpH and allows massive Ca2+ accumulation into the matrix. When Pi is used, Ca2+ tends to precipitate in the matrix as hydroxiapatite, a process favoured by the alkaline pH inside the organelle, with damage of the mitochondrial integrity and irreversible uncoupling. Such Ca2+–Pi precipitates were observed in cells of damaged tissues, suggesting that this may occur also in vivo.
If Ca2+ accumulation depends solely on the ΔΨ across the inner membrane, it should reach electrochemical equilibrium. For a value of 180 mV (negative inside) this would imply, according to the Nernst equation, about 1 million fold accumulation of Ca2+ in the matrix, clearly incompatible not only with experimental observations in living cells (Somlyo et al, 1979, 1985), but also with general cell physiology concepts. The solution of this paradox came from the observation that, in isolated mitochondria, a slow and complete release of Ca2+ is observed if, after Ca2+ accumulation, the MCU is blocked by Ruthenium Red (RR). The conclusion was therefore that an ‘electroneutral Ca2+ efflux’ must exist, whose activity prevents attainment of electrochemical equilibrium. Steady-state Ca2+ accumulation is reached when the rate of Ca2+ influx through the MCU equals that of the efflux (antiport). In non-excitable tissues (liver, kidney), such an antiport appears to be predominantly an H+/Ca2+ antiport, while in excitable tissues (heart, brain) it appears to be primarily a Na+/Ca2+ exchanger (Puskin et al, 1976; Brierley et al, 1994). The kinetic equilibrium between MCU-dependent influx and antiport-dependent efflux thus results in a futile (energy consuming) cycle of Ca2+ across the mitochondrial inner membrane. As to the stoichiometry of such antiporters, the question remained unsettled for some time, but a general consensus has been reached, at least for the Na+/Ca2+ antiport, that catalyses the exchange of 3(4)Na+ per (1)Ca2+, that is, similarly to its counterpart in the plasma membrane (PM). In coupled mitochondria, the highly negative (inside) membrane potential favours the efflux of Ca2+ and the influx of Na+ and prevents its reverse functioning. No consensus has yet been reached on the stoichiometry for the H+/Ca2+ antiport, that is, whether it is electroneutral (2H+ per Ca2+) or electrogenic (⩾3 H+ per Ca2+).
Mitochondrial Ca2+ uptake and cell physiology
A second major question was apparently settled at the end of the 1970s, that is, the participation of mitochondria in the physiological control of Ca2+ in living cells. Not only it was demonstrated that in healthy cells the mitochondrial Ca2+ content is low (Somlyo et al, 1985), but it was also shown that the apparent affinity of the MCU for Ca2+, under physiological conditions (i.e. 1 mM Mg2+), is very low (apparent Kd of 20–30 μM) and the influx rate only becomes substantial when the extramitochondrial [Ca2+] reaches values above 5–10 μM, that is, concentrations rarely (or never) observed in the cytosol of healthy cells (Rizzuto and Pozzan, 2006). This conclusion drastically reduced the interest for the mitochondrial Ca2+ handling process and, for a long time, many investigators considered it simply an interesting laboratory artefact. Most remaining interest was further abated by the discovery of the second messenger IP3 (inositol trisphosphate) and the demonstration that the Ca2+ store that is mobilized during cell activation is the endoplasmic reticulum (ER) and not mitochondria (Streb et al, 1983). The last question concerning the role of Ca2+ uptake in cell physiology was primarily focused on the role of matrix [Ca2+] in the regulation of NADH dehydrogenases (Denton and McCormack, 1980; Denton, 2009). However, the affinity of these enzymes for Ca2+ is quite high and, accordingly, it was argued that even a slow and inefficient Ca2+ uptake by mitochondria was sufficient to fulfil this function.
Mitochondrial Ca2+ returned to the limelight in 1992 when our group generated a novel, genetically encoded chemiluminescent indicator, aequorin. This probe, specifically targeted to the mitochondrial matrix, allowed dynamic, accurate and specific monitoring of the [Ca2+] within the matrix of mitochondria in living cells (Rizzuto et al, 1992). With this new tool, we could show that mitochondria in situ are capable not only of taking up Ca2+ (even for normal physiological cytoplasmic Ca2+ rises), but also that mitochondria in living cells undergo very fast and large increases in their matrix Ca2+ levels, reaching peaks one or two orders of magnitude higher than those in the cytoplasm (Rizzuto et al, 1993; Rizzuto and Pozzan, 2006). The discrepancy between the low Ca2+ affinity of the MCU observed in vitro and the high efficiency discovered in vivo was explained on the basis of the microheterogeneity of cytoplasmic Ca2+ rises during stimulation. In particular, we suggested that microdomains of high [Ca2+] (10–20 μM) can be transiently formed in regions of close apposition between mitochondria and Ca2+ channels of the ER/SR (sarcoplasmic reticulum) or of the PM (Rizzuto et al, 1998). These high Ca2+ microdomains rapidly dissipate (due to diffusion) insuring that mitochondria do not overload with Ca2+. This hypothesis received a number of indirect confirmations in the last 20 years by different groups; more recently, such microdomains in selected regions of contact between ER and mitochondria were finally measured directly (Giacomello et al, 2010; Csordas et al, 2010). The interest of the scientific community in mitochondrial Ca2+ handling was further stimulated when it was demonstrated that excess Ca2+ uptake by mitochondria could initiate the apoptotic process through the opening of the so-called ‘permeability transition pore’, PTP (Bernardi, 1999; Zamzami and Kroemer, 2001). A large number of data have thus been published in the recent years concerned with the physiopathological role of mitochondrial Ca2+ in processes as different as ischaemic death, excitotoxicity and cancer (Contreras et al, 2010). The field of mitochondrial Ca2+ homeostasis has undergone a significant revival and nowadays hundreds of papers on this topic are published every year.
The molecular nature of the mitochondrial Ca2+ handling machinery: a 40-year long story
In spite of all the renewed interest, a key element in the mitochondrial Ca2+ saga continued to elude the scientific community. Indeed, until very recently, the molecular identification of the players in this choreography (schematized in Figure 1) thwarted all the groups that tried to elucidate this issue. Importantly, without this information it was impossible to try any genetic manipulation of this key aspect of cell physiopathology. Many attempts were made to identify the molecular nature of the MCU and antiporters, starting in the early 1970s, that is, soon after the discovery of this mitochondrial function. As far as the MCU is concerned, the early 1970s saw the purification of the so-called mitochondrial glycoprotein that, when added to lipid bilayers, induced the appearance of a Ca2+ current. The finding raised significant interest since the best-known inhibitor of the MCU was RR, a generic stain for glycoproteins, thus consistent with the proposal that this might indeed be the MCU (Sottocasa et al, 1972). Even more striking was the observation that an antibody raised against this glycoprotein was capable of inhibiting energy-dependent mitochondrial Ca2+ uptake in mitoplasts (mitochondria without the outer membrane) and even in intact organelles (Panfili et al, 1976). Soon after, however, it became clear that the glycoprotein was far from pure and that the glycosylated peptide was a contaminant. The specificity of the antibodies was also dubious and the story quietly and rapidly faded away. A new attempt at the purification of the MCU was made in the mid-1990s by the group of Saris. A 40-kDa protein was purified that catalysed RR-sensitive electrogenic Ca2+ uptake in lipid bilayers and reconstituted liposomes. Again, however, also this potential candidate soon disappeared from the major journals (Saris et al, 1993).
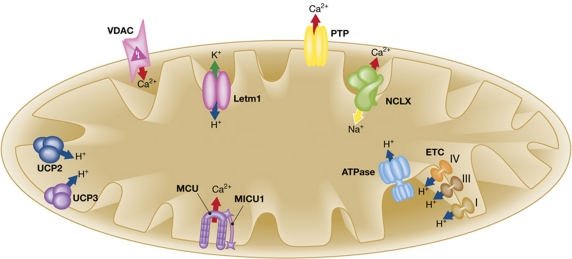
Figure 1.
Schematic representation of the mitochondrial Ca2+, Na+ and H+ handling machinery. Ion fluxes are indicated by arrows. Red arrow, Ca2+; blue arrow, H+; green arrow, K+; yellow arrow, Na+. ETC, electron transport chain; Letm1, Leucine-zipper EF-hand containing transmembrane protein 1; NCLX, Na+/Ca2+ exchanger; PTP, permeability transition pore; UCP2/3, uncoupling protein 2/3; VDAC, voltage-dependent anion channel. See text for details.
The purification of the Na+/Ca2+ or the H+/Ca2+ antiporters has been even more frustrating. In spite of a few remarkable reports identifying the stoichiometry of the Na+/Ca2+ exchanger (3 or 4 Na+ ions per Ca2+) (Brierley et al, 1994), their molecular identity remained, until very recently, completely mysterious. Two papers (in 1992 and 2004) reported the partial purification of a 110-kDa protein capable of reconstituting a Na+/Ca2+ exchange process in liposomes (Li et al, 1992; Paucek and Jaburek, 2004). Similarly, another protein of 66 kDa with H+/Ca2+ exchanger characteristics in reconstituted liposomes was partially purified by the group of Satrustegui (Villa et al, 1998). Surprisingly, none of these observations was successfully pursued, leading to the cloning of the relevant genes.
In very recent years, the search for the molecular identity of the MCU was intensified. A key innovative finding was published in 2004 by Kirichok et al (2004): by patch clamping, the authors provided the direct electrophysiological demonstration that the MCU is a gated, Ca2+ selective, ion channel. It is noteworthy that the channel nature of the MCU had been originally proposed 25 years before by one of us (TP), on the basis of indirect data (Bragadin et al, 1979), though it was not followed by more direct evidence. In 2007, Graier and co-workers proposed that an essential component of the uniporter-mediated Ca2+ uptake system, though not the MCU itself, is represented by the isoforms 2 and 3 of the uncoupling protein, UCP, but these data have not been confirmed by other investigators and still await clarification (Trenker et al, 2007). In 2009, Clapham and co-workers, using a siRNA genome-wide screen in Drosophila, concluded that the ubiquitously expressed mitochondrial protein Letm1 fulfils the criteria for being a 1 H+/Ca2+ antiport (Jiang et al, 2009). Because of this stoichiometry, however, Letm1 should allow Ca2+ uptake in coupled mitochondria, unlike the classical view of H+ (or Na+)/Ca2+ antiporters that, in coupled mitochondria, catalyse Ca2+ efflux from the matrix. It is also noteworthy that the proposal by Clapham's group is somehow a rediscovery of the original idea of P Mitchell (Moyle and Mitchell, 1977), that is, that Ca2+ uptake by mitochondria depends not on the MCU (with the net translocation of two positive charges per Ca2+ taken up), but rather by an antiport with the net transfer of only one positive charge per Ca2+. Clapham and co-workers did not go as far as claiming that all Ca2+ uptake depends on Letm1, but suggested that Letm1 allows Ca2+ uptake only at low cytosolic [Ca2+], while for higher Ca2+ levels they admitted that the still unknown MCU is the responsible channel. A similar conclusion was very recently reached also by Waldeck-Weiermair et al (2011). Jiang et al (2009) also showed that Letm1, when overexpressed, caused a substantial augmentation of agonist-dependent mitochondrial Ca2+ accumulation, though this finding was not confirmed in endothelial cells (Waldeck-Weiermair et al, 2011). Most importantly, they reported that Ca2+ accumulation, when the purified protein is incorporated in liposomes, is favoured by the generation of a K+ diffusion potential and it is completely blocked by RR, as expected for the classical MCU. In our biased opinion, a number of experimental and conceptual considerations make it unlikely that Letm1 is a component of the Ca2+ uptake system, in particular: (i) the H+/Ca2+ stoichiometry in respiring mitochondria has been firmly and unequivocally established many years ago (2H+ per Ca2+) and, when Ca2+ uptake is driven by K+ diffusion potential, no H+ extrusion has ever been measured; (ii) importantly, Letm1 is structurally analogous to the yeast protein Mdm38p (Schlickum et al, 2004) and, when transfected, it can rescue the phenotype of Mdm38p knockout yeasts cells (Nowikovsky et al, 2004); (iii) it is notorious that yeast do not possess a RR-sensitive mitochondrial Ca2+ uptake system (Carafoli et al, 1970); (iv) in mammalian cells, the phenotype of Letm1 knockout cells can be rescued by addition of the H+/K+ ionophore nigericin (Dimmer et al, 2008). This latter evidence represents a very strong—and, in our opinion, conclusive—argument in favour of Letm1 being itself a K+/H+ antiporter. The data of the mitochondrial Ca2+ uptake inhibition by Letm1 knockout can be explained by an effect on the ΔΨ. Although the authors claimed that they found no appreciable difference in ΔΨ between control and Letm1 KO cells, we suspect that the methodology used, rhodamine 123 uptake, is insufficiently sensitive to reveal such drop. On the contrary, the increase, upon Letm1 overexpression, of mitochondrial Ca2+ uptake can be easily explained by a H+/K+ antiport catalysed by Letm1. Indeed, an artificial H+/K+ antiport, nigericin, causes a drop in ΔpH and an increase in ΔΨ, thus augmenting the capacity of mitochondria to take up Ca2+ via the classical MCU. The reported RR sensitivity of Ca2+ uptake in liposomes reconstituted with Letm1 isolated from mitochondria could be, on the other hand, due to a contaminant protein in the preparation. Clearly, the problem of Letm1 and Ca2+ uptake is at the moment unsolved and more experiments need to be performed in order to clarify the issue. The last data on MCU (see below), however, have somehow reduced the interest for this topic.
The discovery of the mitochondrial Na+/Ca2+ antiporter and of an MCU component
In the last year, good news finally emerged regarding the molecular identities of the major players in mitochondrial Ca2+ handling. In January 2010, Sekler and co-workers published a paper where they demonstrated that NCLX (until then considered an isoform of the PM Na+/Ca2+ exchanger family) fulfils the criteria to be the elusive mitochondrial Ca2+/Na+ antiport (Palty et al, 2010). NCLX had been previously identified by the same group and by others (Cai and Lytton, 2004; Palty et al, 2004) and had been considered a novel PM Na+/Ca2+ exchanger, the only mammalian member of a phylogenetically ancestral branch of the Na+/Ca2+ exchanger superfamily. Two spliced variants of the NCLX (a long and a short isoform) are known to be ubiquitously expressed and the different roles of these isoforms are not presently known: indeed, the discovery was a serendipitous bonus, as the authors were looking for a specific Zn2+ transporter (Sekler, personal communication). The interest was triggered by the observation that NCLX, when overexpressed, was partially mis-targeted to the PM and could mediate not only a Na+/Ca2+ exchange, but also an efficient Li+/Ca2+ exchange, a feature that is known to be a unique characteristic of the (at that time) unidentified mitochondrial Na+/Ca2+ exchanger. They could also show that (i) practically all endogenous NCLX is recovered in the mitochondrial fraction; (ii) NCLX is sensitive to the classical mitochondrial Na+/Ca2+ exchanger inhibitor CGP-37157; (iii) KO of NCLX drastically reduced Na+-dependent Ca2+ efflux in isolated mitochondria; (iv) a catalytically inactive mutant of NCLX transfected in cells blocks the Na+/Ca2+ exchange in isolated mitochondria, thus acting as dominant negative; and (v) last, but not least, the PM mis-targeted NCLX appears to mediate an electrogenic transport of 3–4 Na+ ions per Ca2+ transported. Admittedly, the data obtained by Sekler's group have yet to be reproduced by others and the other team that initially identified NCLX concluded that the protein is expressed in both the PM and ER/SR. We do not know the reasons for this important discrepancy that still awaits clarification. However, it is again our biased opinion that the data reported by Sekler's team (and some of their more recent unpublished results) are very convincing and strongly support the idea that NCLX is indeed the Na+/Ca2+ antiporter of the mitochondrial inner membrane. In addition, the mitochondrial NCLX is remarkably similar in size to the mitochondrial protein that, when purified and reconstituted, exhibited Na+/Ca2+ exchange activity, see above (Li et al, 1992; Paucek and Jaburek, 2004).
A few months after the publication of the NCLX paper, another new protein involved in mitochondrial Ca2+ handling, this time the MCU, was identified by V Mootha's group. In this case, the protein was named MICU1, acronym for mitochondrial Ca2+ uptake 1 (Perocchi et al, 2010). The identification of MICU1 came from the establishment of the so-called MitoCarta in which about 1000 proteins have been identified (many of them with unknown functions) that are specifically present in mitochondria (Zhang et al, 2010). Using bioinformatics followed by a selective siRNA screening, Perocchi et al (2010) identified a protein of unknown function, ubiquitously expressed in mammalian cells that possess two classical EF-hand Ca2+-binding domains. When MICU1 is down-regulated, it results in a drastic reduction of the mitochondrial Ca2+ uptake of intact cells challenged with IP3-generating agonists. Most relevant, MICU1 does not have an orthologue in yeast (see above). MICU1 is a 54-kDa protein, with only one putative transmembrane domain, which makes it unlikely that it can function as a Ca2+ channel. MICU1 down-regulation did not affect ΔΨ, O2 consumption or ATP synthesis by mitochondria, indicating that the inhibition of its activity does not grossly compromise the overall functionality of the organelle, but rather specifically affects the Ca2+ uptake mechanism. Two observations, which suggest an ancillary role of MICU1 in the MCU functions, need to be stressed: (i) when overexpressed it does not increase the Ca2+ uptake of mitochondria in intact cells (Rizzuto, personal communication) and (ii) when studied in MICU1 down-regulated permeabilized cells, apparently mitochondria could initially take up Ca2+, but this capacity was soon lost upon further Ca2+ additions to the medium (Perocchi et al, 2010). The authors did not exclude that this residual Ca2+ uptake was independent of mitochondria, but taken together the above-mentioned characteristics suggest that MICU1 is not the channel-forming subunit of MCU itself, but rather an associated key subunit. The identification of MICU1 appears, however, a fundamental step in the molecular understanding of mitochondrial Ca2+ uptake machinery, but the channel itself still managed to escape molecular identification. Fortunately, we did not have to wait long for the final discovery, carried out in parallel by the groups of Mootha and Rizzuto on opposite sides of the Atlantic Ocean.
The Ca2+ uniporter, at last!
Two papers came out, back to back, in the same issue of Nature a few weeks ago reporting the identification of another protein, this time called MCU, that possesses all the characteristics expected by the elusive Ca2+ uniporter of the mitochondrial membrane (Baughman et al, 2011; De Stefani et al, 2011). The two papers addressed the problem with slightly different approaches, but their conclusions are remarkably similar and, accordingly, they will be discussed together. The differences and the few remaining discrepancies between the two groups will be also pointed out and discussed. The key characteristics of MCU, agreed upon by both groups, are as follows: (i) MCU is a 40-kDa protein (previously known as NP_001028431, coiled-coil domain-containing protein 109A) ubiquitously expressed in all mammalian tissues and in most eukaryotes, but missing a yeast orthologue; (ii) MCU possesses two transmembrane domains and this characteristic makes it reasonable that it forms (through oligomerization) a gated ion channel; (iii) down-regulation of MCU drastically reduces mitochondrial Ca2+ uptake both in living cells treated with Ca2+ mobilizing agonists, in permeabilized cells perfused with buffered Ca2+ and in isolated mitochondria; (iv) transfection with the native channel rescues the phenotype of the specific siRNA-treated cells; and (v) the other classical properties of mitochondria are not affected by MCU down-regulation, that is, organelle shape and ER–mitochondrial interactions, O2 consumption, ATP synthesis and ΔΨ. Whether or not complete knockdown of MCU had deleterious effects on cell or organ functions is still unknown. The group of Rizzuto also showed that overexpression of MCU drastically increases the mitochondrial Ca2+ accumulation in intact cells while, contemporarily, reducing the amplitude of the cytosolic Ca2+ peaks (due to mitochondrial Ca2+ buffering); on the opposite site, down-regulation of MCU slightly increases the cytosolic Ca2+ peaks. Most important of all, De Stefani et al showed that bacterial expressed MCU reconstituted in lipid bilayers results in the appearance of a Ca2+ current; the single channel activity not only has electrophysiological characteristics similar to those reported by Clapham's group in patched clamped mitoplasts (Kirichok et al, 2004), but the channel activity is blocked by well-known inhibitors of the MCU, such as RR and La3+. Finally, Mootha's group showed that infecting in vivo the liver with an adenoviral vector encoding a siRNA against MCU results in an almost complete block of Ca2+ uptake by mitochondria isolated from the organ.
Either group carried out site-directed mutagenesis of MCU, the group of Mootha demonstrating that mutation of S259 resulted in a functional Ca2+ accumulation, while RR sensitivity was lost; De Stefani et al showed that a mutation in the region of the putative pore results not only in an inactive channel, but the mutant protein behaves as dominant negative when expressed in HeLa cells. This latter property suggests (together with the existence of only two putative transmembrane domains) that the active uniporter is made by oligomers of MCU. Modelling the MCU structure with classical algorithms suggests indeed that the channel is a tetramer (Rizzuto et al, unpublished data). Mootha's group also showed that MCU co-precipitated with MICU1 in a supra-molecular complex. The only real discrepancy between the two groups concerns the topology of MCU: according to Baughman et al (2011) the C- and N-terminal domains face the mitochondrial matrix and the linker between the two transmembrane domains faces the intermembrane space, while the opposite is true in the model of Rizzuto, C- and N-terminal in the intermembrane space and the linker facing the matrix (Figure 2). This contrasting conclusions were reached by different approaches: the data of the former group result from a biochemical study of the endogenous MCU in isolated mitochondria and its sensitivity to proteases; the model of the second group was based on evidence obtained using a N-terminal GFP-tagged version of MCU. This latter, when transiently expressed in cells, is perfectly functional in terms of Ca2+ uptake capacity. De Stefani et al showed that the GFP fluorescence of MCU, in PM permeabilized cells, is insensitive to proteinase K (unlike a GFP expressed on the cytosolic side of the outer mitochondrial membrane), but it is rapidly quenched by Trypan Blue that permeates the outer mitochondrial membrane (in fact Trypan Blue quenches the fluorescence of a GFP-cyt.C, Pozzan, unpublished data), but does not quench a GFP localized in the matrix. We are confident that this remaining discrepancy can be rapidly solved.
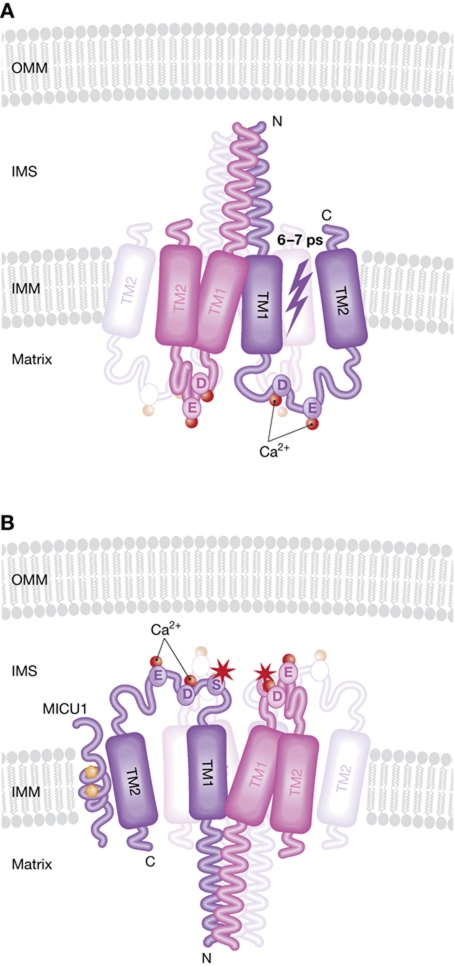
Figure 2.
Main features of the recently identified MCU. The figure schematizes the main properties of the MCU according to De Stefani et al (A) and Baughman et al (B). MCU has two transmembrane domains (TM1 and 2) that spans the inner mitochondrial membrane (IMM) with the N- and C-termini facing the intermembrane space (IMS), according to De Stefani et al (A) or the matrix (Baughman et al (B)). When reconstituted in lipid bylayers, MCU can mediate a Ca2+ current of 6–7 ps (A). The amino acids that, when mutated, drasticaly reduce Ca2+ uptake (D and E) or the one that confers RR sensitivity to the MCU (S, red star in panel B) are also shown. MICU1 that is reported to physically interact with MCU is also shown in (B). Its two EF-hand domains are indicated by the orange pentagons. MCU most likely oligomerizes in the IMM, and evidence from De Stefani et al (personal communication) suggests that it forms a tetramer (shadowed subunits in panels (A, B)).
Conclusions
After 50 years of intense and frustrating experimentation, two of the major players in the mitochondrial Ca2+ saga—the MCU and the Na+/Ca2+ antiport—have now been molecularly identified. We can now expect a strong acceleration in the search for the functional role of this property of mitochondria, in both physiology and physiopathology. The key molecular targets have been revealed and new tools, such as siRNA and dominant-negative constructs to inhibit their functions in a highly specific ways, are available. It is easy to predict that we will soon have KO mice for both proteins. With the identification of the molecular nature of the proteins, we have also more promising ways for starting a journey in search of pharmacological inhibitors with important medical applications in a variety of cell processes. Mitochondrial Ca2+ handling, from its humble state of ‘interesting laboratory artefact’ (as it was labelled in the 1980s), has evolved in the last decade—and even more now—into a process of major interest for a large group of investigators with a plethora of promising medical applications.
Footnotes
The authors declare that they have no conflict of interest.
References
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155 [DOI] [PubMed] [Google Scholar]
- Bragadin M, Pozzan T, Azzone GF (1979) Activation energies and enthalpies during Ca2+ transport in rat liver mitochondria. FEBS Lett 104: 347–351 [DOI] [PubMed] [Google Scholar]
- Brand MD, Chen CH, Lehninger AL (1976) Stoichiometry of H+ ejection during respiration-dependent accumulation of Ca2+ by rat liver mitochondria. J Biol Chem 251: 968–974 [PubMed] [Google Scholar]
- Brierley GP, Baysal K, Jung DW (1994) Cation transport systems in mitochondria: Na+ and K+ uniports and exchangers. J Bioenerg Biomembr 26: 519–526 [DOI] [PubMed] [Google Scholar]
- Cai X, Lytton J (2004) Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J Biol Chem 279: 5867–5876 [DOI] [PubMed] [Google Scholar]
- Carafoli E (2010) The fateful encounter of mitochondria with calcium: how did it happen? Biochim Biophys Acta 1797: 595–606 [DOI] [PubMed] [Google Scholar]
- Carafoli E, Balcavage WX, Lehninger AL, Mattoon JR (1970) Ca2+ metabolism in yeast cells and mitochondria. Biochim Biophys Acta 205: 18–26 [DOI] [PubMed] [Google Scholar]
- Chance B (1965) The energy-linked reaction of calcium with mitochondria. J Biol Chem 240: 2729–2748 [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T (2010) Mitochondria: the calcium connection. Biochim Biophys Acta 1797: 607–618 [DOI] [PubMed] [Google Scholar]
- Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G (2010) Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39: 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca HF, Engstrom GW (1961) Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci USA 47: 1744–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM (2009) Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316 [DOI] [PubMed] [Google Scholar]
- Denton RM, McCormack JG (1980) On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett 119: 1–8 [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L (2008) LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet 17: 201–214 [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T (2010) Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38: 280–290 [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T (2007) Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ 14: 1267–1274 [DOI] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE (2009) Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326: 144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364 [DOI] [PubMed] [Google Scholar]
- Li W, Shariat-Madar Z, Powers M, Sun X, Lane RD, Garlid KD (1992) Reconstitution, identification, purification, and immunological characterization of the 110-kDa Na+/Ca2+ antiporter from beef heart mitochondria. J Biol Chem 267: 17983–17989 [PubMed] [Google Scholar]
- Mitchell P, Moyle J (1967) Chemiosmotic hypothesis of oxidative phosphorylation. Nature 213: 137–139 [DOI] [PubMed] [Google Scholar]
- Moyle J, Mitchell P (1977) Electric charge stoichiometry of calcium translocation in rat liver mitochondria. FEBS Lett 73: 131–136 [DOI] [PubMed] [Google Scholar]
- Nicholls DG (2005) Mitochondria and calcium signaling. Cell Calcium 38: 311–317 [DOI] [PubMed] [Google Scholar]
- Nowikovsky K, Froschauer EM, Zsurka G, Samaj J, Reipert S, Kolisek M, Wiesenberger G, Schweyen RJ (2004) The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J Biol Chem 279: 30307–30315 [DOI] [PubMed] [Google Scholar]
- Palty R, Ohana E, Hershfinkel M, Volokita M, Elgazar V, Beharier O, Silverman WF, Argaman M, Sekler I (2004) Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J Biol Chem 279: 25234–25240 [DOI] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I (2010) NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 107: 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfili E, Sandri G, Sottocasa GL, Lunazzi G, Liut G, Graziosi G (1976) Specific inhibition of mitochondrial Ca2+ transport by antibodies directed to the Ca2+−binding glycoprotein. Nature 264: 185–186 [DOI] [PubMed] [Google Scholar]
- Paucek P, Jaburek M (2004) Kinetics and ion specificity of Na(+)/Ca(2+) exchange mediated by the reconstituted beef heart mitochondrial Na(+)/Ca(2+) antiporter. Biochim Biophys Acta 1659: 83–91 [DOI] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK (2010) MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskin JS, Gunter TE, Gunter KK, Russell PR (1976) Evidence for more than one Ca2+ transport mechanism in mitochondria. Biochemistry 15: 3834–3842 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86: 369–408 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AW, Brini M, Pozzan T (1992) Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358: 325–327 [DOI] [PubMed] [Google Scholar]
- Saris NE, Sirota TV, Virtanen I, Niva K, Penttila T, Dolgachova LP, Mironova GD (1993) Inhibition of the mitochondrial calcium uniporter by antibodies against a 40-kDa glycoproteinT. J Bioenerg Biomembr 25: 307–312 [DOI] [PubMed] [Google Scholar]
- Scarpa A, Azzone GF (1970) The mechanism of ion translocation in mitochondria. 4. Coupling of K+ efflux with Ca2+ uptake. Eur J Biochem 12: 328–335 [DOI] [PubMed] [Google Scholar]
- Schlickum S, Moghekar A, Simpson JC, Steglich C, O'Brien RJ, Winterpacht A, Endele SU (2004) LETM1, a gene deleted in Wolf-Hirschhorn syndrome, encodes an evolutionarily conserved mitochondrial protein. Genomics 83: 254–261 [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Bond M, Somlyo AV (1985) Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature 314: 622–625 [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV, Shuman H (1979) Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol 81: 316–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G, Sandri G, Panfili E, De Bernard B, Gazzotti P, Vasington FD, Carafoli E (1972) Isolation of a soluble Ca2+ binding glycoprotein from ox liver mitochondria. Biochem Biophys Res Commun 47: 808–813 [DOI] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I (1983) Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–68 [DOI] [PubMed] [Google Scholar]
- Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF (2007) Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol 9: 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasington F, Murphy JV (1962) Ca2+ uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem 237: 2670–2677 [PubMed] [Google Scholar]
- Villa A, Garcia-Simon MI, Blanco P, Sese B, Bogonez E, Satrustegui J (1998) Affinity chromatography purification of mitochondrial inner membrane proteins with calcium transport activity. Biochim Biophys Acta 1373: 347–359 [DOI] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Jean-Quartier C, Rost R, Khan MJ, Vishnu N, Bondarenko AI, Imamura H, Malli R, Graier WF (2011) Leucine zipper EF hand-containing transmembrane protein 1 (Letm1) and uncoupling proteins 2 and 3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. J Biol Chem 286: 28444–28455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N, Kroemer G (2001) The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol 2: 67–71 [DOI] [PubMed] [Google Scholar]
- Zhang X, Cui J, Nilsson D, Gunasekera K, Chanfon A, Song X, Wang H, Xu Y, Ochsenreiter T (2010) The Trypanosoma brucei MitoCarta and its regulation and splicing pattern during development. Nucleic Acids Res 38: 7378–7387 [DOI] [PMC free article] [PubMed] [Google Scholar]