Abstract
EMBO J 30 20, 4126–4141 (2011); published online September 20 2011
The V-ATPase, the major cellular proton pump, is comprised of the peripheral sector V1 catalysing ATP hydrolysis and the membrane integral sector V0 translocating protons. Ten years ago, Andreas Mayer's group made the surprising observation that proteolipids of the V0 transmembrane sector are implicated in membrane fusion, but deeper analysis proved to be difficult—mostly because V-ATPase disruption is lethal. Based on a screen for V0 separation-of-function mutants in yeast, a study published in this issue of The EMBO Journal now provides direct evidence that the V0 sector regulates the core fusion machinery during the lipid-mixing step of vacuolar fusion.
Cells are filled with membrane-enclosed compartments that continuously exchange materials via cargo-containing membrane vesicles that form on the donor compartment and deliver their cargo to the target compartment by membrane fusion. Membrane fusion therefore controls fundamental cellular processes, including the maintenance of intracellular compartments and membrane transport in the vacuolar apparatus of eukaryotic cells. Thus, it is not surprising that the membrane fusion machinery was studied extensively in past years, leading to a very comprehensive mechanistic view on how SNAREs (soluble NSF maleimide-sensitive factor), Rabs and the HOPS (homotypic fusion and vacuole protein sorting) complex mediate membrane fusion (Wickner, 2010). Nevertheless, the picture is not yet complete. Some time ago, a highly intriguing study by Andreas Mayer's group implicated the V0 transmembrane sector of the V-ATPase in membrane fusion (Peters et al, 2001). V0 consists of six subunits, the a, d and e subunits and three c subunits called proteolipids, which are named after their very hydrophobic property that makes them soluble in organic solvents, without carrying a lipid modification. Limitations for further analysis that arose from the severe V-ATPase disruption phenotypes have now been overcome by an elegant yeast screen for vacuole fusion by Andreas Mayer's laboratory (Strasser et al, 2011). They randomly mutagenized the proteolipid subunits of the V0 sector and screened in vivo for mutants having normal proton pump activity but fragmented vacuoles, indicative of a vacuole fusion defect. Subsequent extensive biochemical analysis on cell-free yeast vacuoles revealed that the identified mutants maintained normal SNARE interactions, but membrane fusion was impaired before lipid and content mixing. Interestingly, mutants were found in all three proteolipid subunits and the mutated residues are conserved in higher eukaryotes. Furthermore, mutations are clustered in the proteolipid transmembrane domains at the subunit interfaces, leading to the hypothesis that they might affect subunit interactions. This notion is supported by the following results: first, identified mutants stabilized interactions between the V0 and V1 ATPase sectors, a phenotype that was also observed by knockdown of the V0 interacting SNARE. Second, one of the identified mutants showed impaired proteolipid oligomerization. And third, the in-frame hybrid of two proteolipid subunits led to fragmented vacuoles in vivo and a strong vacuole fusion defect in vitro. Collectively, these results strongly suggest that the V0 subunits act as an entity requiring a specific conformation that is impaired by mutations of the transmembrane domain of proteolipids, that depends on SNARE interactions and that requires conformational freedom of the individual subunits.
Evidence of this notion, that the V-ATPase directs membrane fusion independently from the canonical proton translocation activity, has been mounting. Specifically, observations show that the V0 sector is involved in membrane fusion during synaptic vesicle exocytosis (Hiesinger et al, 2005) and phagocytosis (Peri and Nusslein-Volhard, 2008). In addition, the study by Strasser and colleagues provides a possible molecular explanation for several reports linking known constituents of the membrane traffic machinery to the V0 subunits. This includes physical interactions with synaptophysin (Klemmer et al, 2009) and synaptobrevin (Di Giovanni et al, 2010), as well as Rab7 (McCray et al, 2010). Furthermore, in Drosophila melanogaster, the V0 sector has been convincingly shown to influence membrane trafficking through physical interactions with syntaxins independent of effects mediated by the establishment of a proton gradient (Williamson et al, 2010). And recently in mice, a specific mutation in the a3 subunit of V0 has been described that uncouples proton translocation from V-ATPase-dependent aspects of osteoblast function (Ochotny et al, 2011).
But the recent work from Strasser et al also indicates that these independent functions of the V0 sector can influence each other, which is potentially quite exciting. They present persuasive data that SNARE binding to the V0 sector destabilizes V0/V1 assembly, suggesting that the V0 sector undergoes a SNARE-induced switch from a translocation-competent to a fusion-competent conformation. While more work is necessary to establish the relevance of this model in a cellular context, it is tempting to speculate that a mechanism like this may serve as a conduit for cross-talk between membrane trafficking and organellar ion homeostasis in the cell.
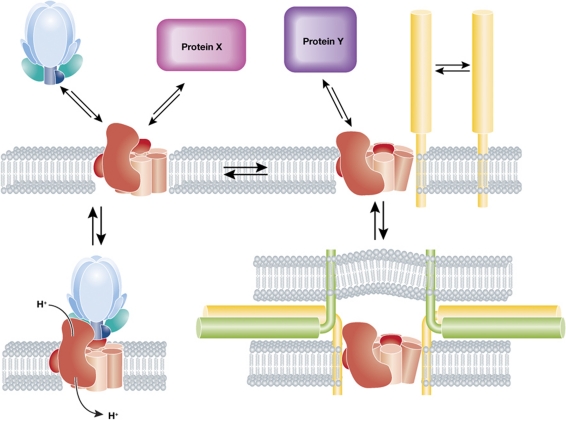
It has been shown that a proton gradient is necessary to support membrane traffic in the endocytic pathway (Clague et al, 1994), but the mechanism has been elusive (Huotari and Helenius, 2011). This pH sensing mechanism was proposed to involve two proteins that regulate transport, the small GTPase Arf6 and its guanine exchange factor ARNO. These bind the V0 sector in a pH-dependent fashion, which lead to the proposal that the V0 sector itself can indicate the presence of a pH gradient to cytosolic proteins by way of a conformational change (Hurtado-Lorenzo et al, 2006). In the light of this recent work, it is reasonable to liken this conformational change a ‘two-way street’ where allosteric alterations induced by protein–protein interactions can also influence lumenal pH. Indeed, V-ATPase functions and the resulting pH gradient can be regulated by V0/V1 assembly—and assembly in turn may be modulated by interactions with protein or lipid partners (Figure 1) (Toei et al, 2010; Huotari and Helenius, 2011).
Figure 1.
Outline of proposed V-ATPase functions and partners. The V-ATPase, which consists of the V0 sector (red), containing the proteolipid core, and the V1 ATPase sector (blue), drives proton translocation across membranes (lower left panel). In yeast, and perhaps in mammalian cells, acidification can be regulated by V0–V1 dissociation–association (upper left panel). Alternative functions presumably depend on the interactions of the V0 sector with protein partners (X or Y) that may or may not cause a conformational change (upper panels). The paper of Strasser et al shows that the V0 sector interacts with SNAREs (yellow/green) and suggests that these interactions are accompanied by a conformational change in V0 proteolipids (upper right panel), eventually leading to trans-SNARE pairing (lower right panel) and fusion.
If the V0 subunits are really a nexus where protein–protein interactions, the pH gradient and the intrinsic membrane fusion activity of the V0 sector meet, it would indicate that the conformational changes in the V0 sector may serve as a general mechanism linking membrane traffic to organellar pH. This is supported by a survey of the studies referenced here, which ascribe pH-dependent and -independent functions to the V-ATPase across all endocytic compartments from yeast to mammals. While the road may still be long and much work still needs to be done, the various mutants uncovered by the screen of Strasser and colleagues will serve as a vital tool for dissecting these emerging functions of the V0 sector in cellular trafficking and membrane dynamics.
Acknowledgments
Support to JG was from the Swiss National Science Foundation, PRISM from the EU Sixth Framework Program, the NCCR in Chemical Biology and LipidX from the Swiss SystemsX.ch initiative, evaluated by the Swiss National Science Foundation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Clague MJ, Urbe S, Aniento F, Gruenberg J (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 269: 21–24 [PubMed] [Google Scholar]
- Di Giovanni J, Boudkkazi S, Mochida S, Bialowas A, Samari N, Leveque C, Youssouf F, Brechet A, Iborra C, Maulet Y, Moutot N, Debanne D, Seagar M, El Far O (2010) V-ATPase membrane sector associates with synaptobrevin to modulate neurotransmitter release. Neuron 67: 268–279 [DOI] [PubMed] [Google Scholar]
- Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, Verstreken P, Cao Y, Zhou Y, Kunz J, Bellen HJ (2005) The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121: 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30: 3481–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8: 124–136 [DOI] [PubMed] [Google Scholar]
- Klemmer P, Smit AB, Li KW (2009) Proteomics analysis of immuno-precipitated synaptic protein complexes. J Proteomics 72: 82–90 [DOI] [PubMed] [Google Scholar]
- McCray BA, Skordalakes E, Taylor JP (2010) Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation. Hum Mol Genet 19: 1033–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochotny N, Flenniken AM, Owen C, Voronov I, Zirngibl RA, Osborne LR, Henderson JE, Adamson SL, Rossant J, Manolson MF, Aubin JE (2011) The V-ATPase a3 subunit mutation R740S is dominant negative and results in osteopetrosis in mice. J Bone Miner Res 26: 1484–1493 [DOI] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C (2008) Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133: 916–927 [DOI] [PubMed] [Google Scholar]
- Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409: 581–588 [DOI] [PubMed] [Google Scholar]
- Strasser B, Iwaszkiewicz J, Michielin O, Mayer A (2011) The V-ATPase proteolipid cylinder promotes the lipid-mixing stage of SNARE-dependent fusion of yeast vacuoles. EMBO J 30: 4126–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49: 4715–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W (2010) Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol 26: 115–136 [DOI] [PubMed] [Google Scholar]
- Williamson WR, Wang D, Haberman AS, Hiesinger PR (2010) A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J Cell Biol 189: 885–899 [DOI] [PMC free article] [PubMed] [Google Scholar]