Abstract
Basosquamous carcinoma of the skin is a relatively rare cutaneous neoplasm that has significant metastatic potential and a metastatic rate greater than that of basal cell and squamous cell carcinoma. We describe the use of lymphatic mapping and sentinel lymph node biopsy in a 63-year-old man after identification of basosquamous carcinoma. Sentinel lymph node biopsy, which is a standard tool to detect regional lymphatic metastasis in cutaneous melanoma, has been rarely employed to detect lymphatic metastasis of basosquamous carcinoma. The approach was successful in detecting a regional lymphatic metastasis of two nodal basins with minor morbidity. Sentinel lymph node biopsy may be useful for certain high-risk lesions of basosquamous carcinoma.
Keywords: Basosquamous carcinoma, Metastasis, Sentinel lymph node biopsy
INTRODUCTION
Lymphatic mapping and sentinel lymph node biopsy(SLNB) is able to detect occult nodal metastasis with low morbidity rates in patients with cutaneous malignant melanoma1. SLNB provides a minimally invasive way of assessing the metastatic status of draining the nodal basin. The use of SLNB in high-risk cutaneous squamous cell carcinoma (SCC) is at an early stage; only several case reports and case series have been published. Much work remains to define which patients with SCC have a high enough risk of nodal metastasis to warrant SLN examination2.
Basosquamous carcinoma (BSC) is an entity that has been classified under basal cell carcinoma (BCC) for a long time. Clinically, however, the lesions are more akin to SCC3. Several studies have revealed the striking metastatic potential of BSC, prompting increasing interest in its pathophysiology4,5. BSCs have been associated with a high overall rate of metastases, which is much higher than BCC and even higher than ordinary SCC types3. Unfortunately, the rarity and disputed histopathology of this lesion have historically made categorization difficult and have contributed to a lack of awareness of its pathology and behavior. As surgeons play an integral role in the treatment of skin cancer, it is important that they be well-versed in the recognition of the risks and prognosis associated with BSC, to ensure proper management of this important entity6. We herein report our experience of SLNB for a large primary BSC to highlight the prognostic importance of SLNB to detect lymphatic metastatic in this aggressive variant of skin cancer.
CASE REPORT
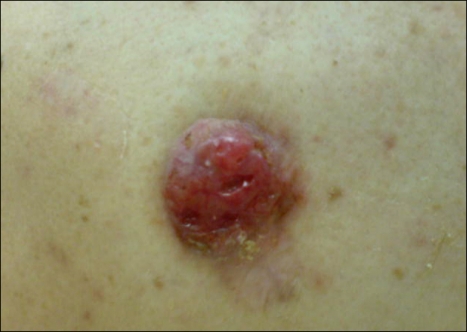
A 63-year-old male presented with a 6-month history of a rapidly growing, raised lesion in the middle of the back. The lesion measured 4 cm in maximal diameter (Fig. 1). There was no palpable axillary and inguinofemoral lymphadenopathy.
Fig. 1.
Photograph of the lesion.
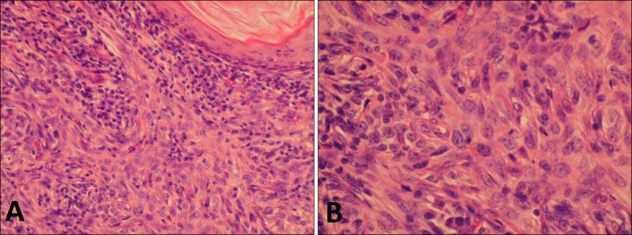
The tumor was biopsied. In the lesion, conventional basaloid tumor islands were admixed with more atypical tumor islands demonstrating higher grade cytologic atypia, an increased nuclear to cytoplasmic ratio, prominent nucleoli and variable pockets of keratinisation. A diagnosis of BSC was made (Fig. 2).
Fig. 2.
Histopathological examination of the primary tumor. (A) Conventional basaloid tumor islands were admixed with more atypical tumor cells demonstrating higher grade cytologic atypia with squamous differentiation (H&E stain, ×200). (B) A high power view revealing BCC cells with squamous differentiation (H&E stain, ×400).
Pre-operative computed tomography of the chest and abdomen did not reveal evidence of distant metastasis or regional lymphadenopathy. We elected to perform a SLNB for regional pathologic staging of this high-risk lesion. Pre-operative lymphoscintigraphy documented the presence of discrete hot foci at both axillary sites. In the operating room, we injected 0.5 ml of isosulfan blue dye into the dermis around the primary tumor 10~20 min prior to the operation.
Wider excision margins are used when biopsy findings and lesion size indicate that a tumor is aggressive. In the present case, we excised the primary ulcerated lesion with a 2 cm radial skin margin and down to the muscle fascia, and then directly closed. Then, we first explored the left axilla through a short transverse incision over the hot spot in the mid axilla found by a handheld gamma probe. Deep to the clavipectoral fascia, we found and removed hot, blue SLN. After that, the right axilla was explored. We found and removed a discrete hot and blue stained node. The two nodes were sent for permanent pathology.
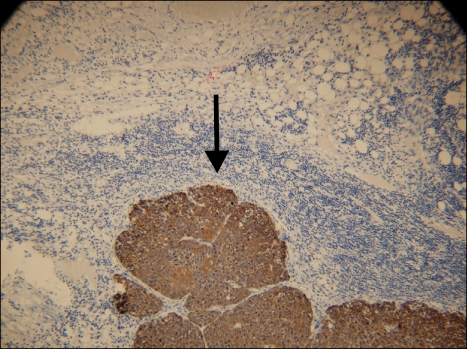
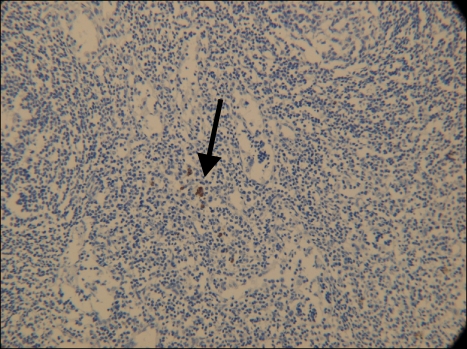
Permanent histologic evaluation demonstrated a BSC with negative margins on the primary skin specimen. Immunohistochemical staining with cytokeratins AE1 and AE3 confirmed the presence of SCC metastases in SLN of the left axilla (Fig. 3) and isolated tumor cells in SLN of the right axilla (Fig. 4).
Fig. 3.
SLN of the left axilla with metastatic BSC (CK AE1/AE3, ×10).
Fig. 4.
SLN of the right axilla with isolated tumor cells of SCC(CK AE1/AE3, ×20).
Complete lymphadenectomy of the left axilla was performed 1 week later. Five of 14 LNs were positive for metastatic BSC in the therapeutic LN dissection. The patient's recovery was uncomplicated. Oncology consultation led to the recommendation of close follow-up examinations without adjuvant chemotherapy or radiation. At a follow-up 16 months later found that the wounds wellhealed, with no evidence of local recurrence or identifiable metastases.
DISCUSSION
The incidence of BSC is ill-defined. However, two of the larger studies reported an incidence of BSC of 1.5~2.7% of all skin carcinomas4,5. Some common characteristics have emerged when many patients with BSC are compared. BSC is most commonly found in the head and neck region, and less common in the trunk and limbs. Additionally, there is a male predominance and a mean age distribution in the 7th decade of life4,5.
Albeit relatively rare, it is the metastatic potential of this lesion that is most concerning. To date, studies of BSC have been hindered by small cohorts and debates over histology, but there is an overall trend toward greater metastases than with BCC, and possibly even SCC. In a cohort of 28 cases of BSC, five patients developed LN metastases, nine had recurrences and one had pulmonary metastases4. In a study of 1,000 consecutive Mohs surgery cases, two of 228 cases (0.87%) of patients with SCC had pulmonary metastases, compared to two of 27 patients with BSC5. In comparison, SCC has a reported incidence of regional nodal metastases of 2~6%7, BCC has a metastatic rate of less than 0.1%8, while the reported BCS metastatic rate of 7.4% is much higher than BCC and even higher than ordinary SCC types5.
Early treatment of subclinical nodal disease could lead to fewer deaths from SCC. The optimal surgical management of LNs remains controversial in clinically node negative(N0) patients. SLNB has recently started to be used in selected cases of high-risk cutaneous SCC. In patients with localized disease, metastasis to the regional LN basin is the strongest predictor of recurrence and survival; therefore, detecting subclinical metastatic disease is extremely important for staging certain skin cancers2. Indeed, it may prove to be more sensitive in the detection of nodal micrometastatic disease than conventional nodal dissection9. So, early diagnostic method like SLNB should be employed in BSC.
In the present case, we used a standard dual identity approach using both radiotracer and blue colloid dye, as is our current practice for melanoma.
The most appropriate treatment regimen for BSC remains to be established. Current standard of care is wide local excision, evaluation for metastasis to nodal basins and distant sites, and careful follow-up for recurrence and metastasis. The high rate of LN metastases on presentation of BSC should prompt consideration of SLNB in the absence of palpable lymphadenopathy5.
Although uncommon among skin carcinomas, BSC commands special attention for its diagnostic challenges and metastatic potential. It is vital that the treating physician appreciate the importance of early resection with free margins and a full workup for LN and distant metastases. He or she must also recognize the increased risk of metastases associated with lymphatic or perineural invasion, male gender and large size; the latter also confers a potential risk of misdiagnosis. Familiarity with the peculiar histology and pathophysiology of these lesions will assist the dermatologist, surgeon and pathologist in the correct diagnosis, planning and treatment regimen, and give the patient the best chances for cure6.
Future efforts should be directed at evaluating the utility of SLNB and to define patient populations most likely to benefit from this technique.
References
- 1.Lee SJ, Lim HJ, Kim HY, Song CH, Kim BS, Lee WJ, et al. The feasibility of sentinel lymph node biopsy with a multidisciplinary cooperative team approach for the management of koreans with cutaneous malignant melanoma. Ann Dermatol. 2010;22:26–34. doi: 10.5021/ad.2010.22.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross AS, Schmults CD. Sentinel lymph node biopsy in cutaneous squamous cell carcinoma: a systematic review of the English literature. Dermatol Surg. 2006;32:1309–1321. doi: 10.1111/j.1524-4725.2006.32300.x. [DOI] [PubMed] [Google Scholar]
- 3.Anadolu-Brasie R, Patel AR, Patel SS, Singh A, Nouri K. Squamous Cell Carcinoma of the Skin. In: Nouri K, editor. Skin cancer. New York: McGraw-Hill; 2008. p. 104. [Google Scholar]
- 4.Martin RC, 2nd, Edwards MJ, Cawte TG, Sewell CL, McMasters KM. Basosquamous carcinoma: analysis of prognostic factors influencing recurrence. Cancer. 2000;88:1365–1369. doi: 10.1002/(sici)1097-0142(20000315)88:6<1365::aid-cncr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Bowman PH, Ratz JL, Knoepp TG, Barnes CJ, Finley EM. Basosquamous carcinoma. Dermatol Surg. 2003;29:830–833. doi: 10.1046/j.1524-4725.2003.29217.x. [DOI] [PubMed] [Google Scholar]
- 6.Costantino D, Lowe L, Brown DL. Basosquamous carcinoma-an under-recognized, high-risk cutaneous neoplasm: case study and review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:424–428. doi: 10.1016/j.bjps.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 8.von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043–1060. doi: 10.1016/s0190-9622(84)80334-5. [DOI] [PubMed] [Google Scholar]
- 9.Doubrovsky A, De Wilt JH, Scolyer RA, McCarthy WH, Thompson JF. Sentinel node biopsy provides more accurate staging than elective lymph node dissection in patients with cutaneous melanoma. Ann Surg Oncol. 2004;11:829–836. doi: 10.1245/ASO.2004.01.026. [DOI] [PubMed] [Google Scholar]