Abstract
Atopic dermatitis (AD) has numerous trigger factors. The question of whether foods can aggravate AD remains open to debate. Although a number of published papers have detailed the relationship between food allergies and AD, little research has examined the question of how food intolerance affects AD. For the purposes of this study, a six-year-old Korean boy with AD was admitted to the hospital for evaluation of the possibility of food, particularly pork, as a triggering factor in his skin disease. He had a history of worsening of symptoms when eating pork. Total serum IgE concentration was 157 IU/ml. House dust was class 2.2 (1.5 IU/ml) in MAST. All other MAST items were negative. In an oral food challenge test, he showed a positive result after eating 200 g of pork, but did not show a positive result after eating 60 g of pork. After discharge, we attempted to keep him on a balanced diet that included various types of food and prohibited him from eating food that contains a high level of histamine. After keeping the patient on a balanced and low-histamine dietary regimen, his AD symptoms showed improvement and have not worsened for more than seven months. A low-histamine, balanced diet could be helpful for AD patients having symptoms that resemble histamine intolerance in which their AD symptoms worsened after intake of histamine-rich foods, but in which food allergy tests are negative.
Keywords: Atopic dermatitis, Histamine, Oral food challenge
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin diseasethat commonly starts in early infancy, runs a course of remissions and exacerbations, and is associated with acharacteristic distribution and morphology of skin lesions. Pruritus and subsequent sleeplessness are hallmarks of AD1. Although the etiology of AD remains unknown, numerous trigger factors could be involved2. Foods that trigger AD have long been a subject of debate. Regarding the relationship between food and AD, some studies have reported that food challenge tests could exacerbate symptoms and a strict food elimination diet could result in improvement of symptoms3,4. In this article, we report on our experience with AD patients whom we treated with a balanced and low-histamine diet.
CASE REPORT
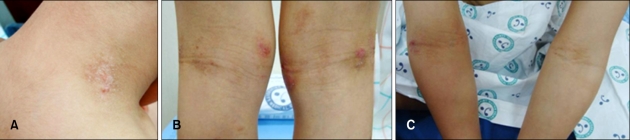
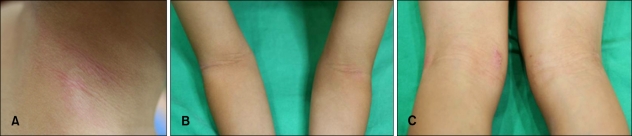
A six-year-old boy had a history of itchy, erythematous patches on his face and extremities since he was three months old. His symptoms were more severe in winter than in other seasons. By the age of five years old, his symptoms had been in remission through application of topical corticosteroid medication. However, in spite of all of the antihistamine, corticosteroid, and even immunosuppressant(cyclosporine) medicine he was prescribed, his symptoms began to worsen and showed frequent exacerbation thereafter. His parents complained that his symptoms worsened when he ate pork, and he was admitted to the hospital to undergo an oral food challenge test in order to determine whether pork could be the aggravating factor of his symptoms. He had asthma and allergic rhinitis, and his mother had AD. Cutaneous examination revealed small erythematous, excoriated papules on his neck, and erythematous scaly patches on both cubital and popliteal fossae (Fig. 1). On physical examination, everything was normal, except for his skin lesions. Routine hematological, liver, kidney, and urine examinations were normal. Total serum IgE was 157 IU/ml. House dust tested as class 2.2 (1.5 IU/ml) in MAST. All other MAST items were negative.
Fig. 1.
Immediately after being hospitalized, cutaneous examination revealed small erythematous papules and excoriations on the neck (A) and erythematous scaly patches on both popliteal (B) and cubital fossae (C).
We evaluated the patient's levels of AD symptoms using the eczema area and severity index (EASI) score5 and the visual analog scale (VAS)6, which represents the patient's subjective pruritus. He showed an EASI score of 15.6 and a VAS score of 1.2.
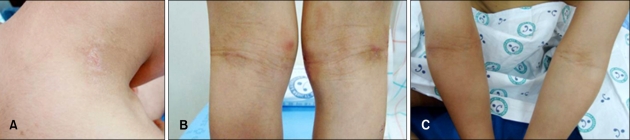
On days 1 and 2, we applied a Vaseline® occlusive dressing. After Vaseline® treatment, the patient's EASI and VAS scores were 0.7 and 0, respectively (Fig. 2).
Fig. 2.
After application of a Vaseline® occlusive dressing, small erythematous papules and excoriations on the neck and erythematous scaly patches on popliteal and cubital fossae showed improvement (A~C).
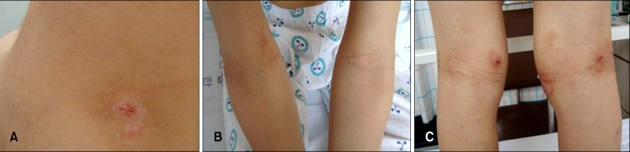
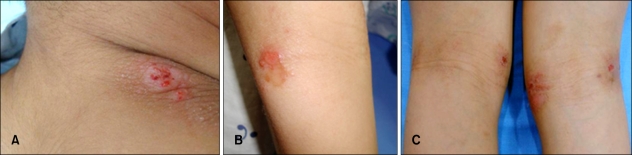
On day 3, we conducted an oral food challenge test withpork. The patient ate 200 g of boiled pork for his first meal of the day. For lunch and dinner, he ate kimchi, beef boiled in soy sauce, dried seaweed, and boiled rice. After he had eaten the pork, we evaluated his skin and symptoms each hour, looking for exacerbation. Ninety minutes after ingestion of 200 g of boiled pork for breakfast, the patient began to complain of pruritus in the flexural areas of his upper and lower limbs and neck. His VAS score was 2.5. Seven hours after intake of pork, the number of papules on the patient's neck increased, and the central area of the lesion became intensely erythematous. Lesions on his arms showed no changes; however, erythematous scaly lesions on the flexural areas of his legs became more exacerbated (Fig. 3). Nine hours after intake of pork, the number of erythematous papules had increased and oozing occurred in some areas of the neck lesion. Severe oozing also occurred on the lateral flexural part of the right arm, and erythematous patches and plaques in the flexural areas of the lower limbs had become darkened (Fig. 4).
Fig. 3.
Seven hours after ingestion of 200 g of boiled pork for breakfast, the number of papules on the patient's neck increased, and the central area of the lesion became intensely erythematous (A). Lesions on the patient's arms did not showed any change (B), however, erythematous scaly lesions on the flexural areas of his legs became more exacerbated (C).
Fig. 4.
Nine hours after ingestion of 200 g of boiled pork for breakfast, the number of erythematous papules increased and oozing occurred in some areas of the neck lesion (A). Severe oozing also occurred on the lateral flexural part of the patient's right arm (B), and erythematous patches and plaques in the flexural areas of the lower limbs became darkened (C).
On day 4, the patient was treated with application of topical corticosteroid for the exacerbated lesions and was administered a Vaseline® occlusive dressing.
After the symptoms had improved on day 5, we conducted a second oral food challenge test, having the patient ingest 60 g of pork. However, he failed to show any signs of exacerbation of AD throughout the entire day. A history of the patient's diet revealed that he typically ate fruits and vegetables, such as oranges and spinach, and fish, such as mackerel. He also had a habit of eating large amounts of one kind of food every time he ate a meal. Therefore, we suggested a balanced diet for him and recommended that he avoid eating foods that contain high levels of histamine7,8.
After being discharged from the hospital, the patient maintained a balanced and low-histamine dietary regimen, and, one month later, we were able to stop administration of oral antihistamine and topical immunomodulator (Protopic®, Fujisawa, Osaka, Japan). Three months after discharge, he had only slight erythematous patches on flexural areas of limbs and neck without pruritus (Fig. 5). His EASI and VAS score were 0.3 and 0, respectively. After the patient had been on a balanced and low-histamine dietary regimen for seven months, we did not find any skin signs or any symptoms of AD.
Fig. 5.
Three months after maintaining a balanced and low-histamine dietary regimen, the patient had only slight erythematous patches on his neck (A) and the flexural areas of his limbs (B, C).
DISCUSSION
AD is a chronic inflammatory skin disease that shows a wide variety of clinical pictures and often is complicated by relapse caused by different kinds of food. In many patients with AD, skin prick tests, analysis of allergen specific IgE against food allergens in sera, atopy patch tests, or oral allergen challenge can confirm IgE- mediated food hypersensitivities9,10. In a subgroup of patients with AD, patients displayed allergy-like symptoms; however, the results of their allergy tests were negative and their symptoms could not be linked to food allergies. Nevertheless, these patients reported that worsening of AD skin lesions frequently coincided with intake of certain types of food, and their symptoms resembled those of histamine intolerance11.
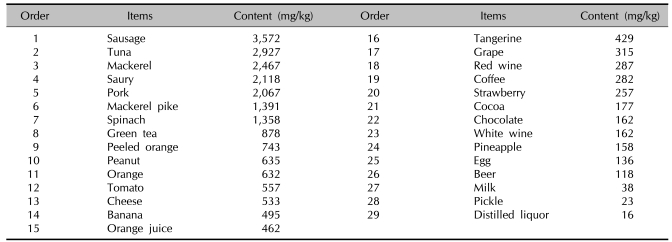
Histamine is a biogenic amine and the product of decarboxylation of the amino acid and L-histidine. Due to microbial contamination, food and beverages sometimes contain varying amounts of biogenic amines in relevant amounts. Therefore, spoiled or fermented foods can contain high levels of biogenic amines. In particular, food that undergoes microbial ripening, such as cheese, salami, sauerkraut, or red wine, can contain high levels of histamine (Table 1)7,8. Histamine intolerance belongs to the group of non-IgE-mediated hypersensitivity-like reactions, and is known as a pharmacological food intolerance. Currently, no valid in vitro tests can prove histamine intolerance; thus, a double-blind, placebo-controlled food challenge (DBPCFC) test remains the gold standard for diagnostic workup of non-IgE-mediated food intolerance12.
Table 1.
Histamine contents in foods in Korea
Cited from a study of the histamine content in food in Korea7.
Histamine intolerance results from a disequilibrium of accumulated histamine and the capacity for histamine degradation. The primary enzymes for metabolism of ingested histamine are as follows: diamine oxidase (DAO) and histamine-N-methyltransferase (HNMT). DAO expression in mammals is restricted to specific tissues; the highest activities are shown in the small bowel, colon ascendens, placenta, and kidneys. Human tissues show wide expression of HNMT; the most significant expression occurs in the kidneys and liver, followed by the spleen, colon, prostate gland, ovaries, spinal cord cells, bronchi, and trachea. Scientists regard HNMT as the key enzyme for histamine degradation in bronchial epithelium. Impaired histamine degradation is based on a reduction in the activity of histamine-degrading enzymes DAO and HNMT, and the resulting excess histamine can cause numerous symptoms that mimic an allergic reaction. Typical symptoms of histamine intolerance include the following: gastrointestinal disorders, sneezing, rhinorrhea and congestion of the nose, headache, dysmenorrhea, hypotonia, arrhythmias, urticaria, pruritus, flushing, and asthma. In contrast to an IgE-mediated food allergy, in which ingestion of even a small amount of the allergen elicits symptoms, in histamine intolerance, the cumulative amount of histamine is crucial8.
When compared to control subjects, patients with severe AD have shown higher basal plasma histamine concentrations and increased spontaneous histamine release in response to multiple stimuli, and after food challenges13,14. In addition, compared with control subjects, a subgroup of AD patients showed lower DAO activities15. A subgroup of patients with AD and low DAO serum activity, who followed a histamine-free diet for two weeks, showed reductions in both symptoms of histamine intolerance and severity score for atopic dermatitis (SCORAD)16.
Based on the low VAS score (1.2), it is possible that the patient had a mild case of AD that had undergone a naturally improving process. The VAS score of 1.2 was evaluated immediately after his admission to the hospital, when his skin lesions and symptoms were stabilized for participation in the food challenge test. Prior to hospitalization, the patient's AD symptoms were too severe (including itching symptoms) to undergo the food challenge test, which indicates that he did not have a mild case of AD. He also showed deteriorating AD symptoms from the food challenge, which included a histamine-rich diet. However, previous treatment with oral antihistamines had not resulted in significant improvement of his AD symptoms. There is a possibility that a higher dose of antihistamines was needed in order to yield effects. In addition to histamine, neuropeptide mediators, such as interleukin-31 and substance-P, are known to cause skin inflammation and itching. These mediators likely contributed to exacerbation of the patient's symptoms. Our patient showed negative results on in vitro allergy tests. In the oral food challenge test with pork, in order to eliminate intolerance to artificial and natural flavors, MSG and other food additives were not used when cooking the pork, which, for the food challenge, was simply prepared by simmering. He showed a positive reaction when eating large amounts of pork (200 g), but a negative reaction with small amounts of pork (60 g).
The patient's history revealed that he had a habit of eating large amounts of one kind of a certain type of food every time he ate a meal, as indicated by the 200 g of pork he consumed in a single meal for the provocation test and the types of foods he used to eat, such as oranges, spinach, and mackerel, which contain high levels of histamine. Pork is known to contain high levels of histamine7,8. These findings suggest that histamine intolerance could be related to exacerbation of his AD symptoms.
Therefore, we recommended a diet balanced in quantity of foods and in foods that contain a low level of histamine. Table 1 lists foods that contain high levels of histamine. We showed Table 1 to the child and his parents and advised them that while the foods items listed did not have to be completely removed from his diet, he should only eat them in small quantities and that the types of food listed should be served in rotation. We also advised that types of food not listed in Table 1 should also be included with each meal.
After a balanced diet that included various types of food, the patient's AD symptoms showed improvement and have not worsened for more than seven months.
Accurate data regarding the serum histamine level and DAO activity would have helped us to conclude that the patient had displayed symptoms of histamine intolerance. The lack of this data was our shortcoming. Although we should take into account other factors that might have contributed to the patient's improvement, such as seasonal variation, our patient's skin has remained in an improved state for more than seven months after institution of a low-histamine and balanced diet. Some doctors have reported cases of improved AD symptoms with a histamine-free diet or strict elimination of histamine-rich foods17,18. In cases of AD showing negative results on allergy tests and worsening of AD skin lesions after intake of certain types of food, it would be reasonable to consider histamine intolerance. Therefore, a low-histamine diet and a histamine-free diet could be helpful for such cases.
Footnotes
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0004436).
References
- 1.Werfel T, Breuer K. Role of food allergy in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004;4:379–385. doi: 10.1097/00130832-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Morren MA, Przybilla B, Bamelis M, Heykants B, Reynaers A, Degreef H. Atopic dermatitis: trigerring factors. J Am Acad Dermatol. 1994;31:467–473. doi: 10.1016/s0190-9622(94)70213-6. [DOI] [PubMed] [Google Scholar]
- 3.Uenishi T, Sugiura H, Uehara M. Role of foods in irregular aggravation of atopic dermatitis. J Dermatol. 2003;30:91–97. doi: 10.1111/j.1346-8138.2003.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 4.Significance of food hypersensitivity in children with atopic dermatitis. Pediatr Dermatol. 1986;3:161–174. doi: 10.1111/j.1525-1470.1986.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M EASI Evaluator Group. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 6.Langner MD, Maibach HI. Pruritus measurement and treatment. Clin Exp Dermatol. 2009;34:285–288. doi: 10.1111/j.1365-2230.2009.03218.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Park CW, Lee CH. A study of histamine content in food in Korea. Korean J Dermatol. 2007;45:768–771. [Google Scholar]
- 8.Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 9.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–819. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr. 1985;107:669–675. doi: 10.1016/s0022-3476(85)80390-5. [DOI] [PubMed] [Google Scholar]
- 11.Agro AF, Rotilio G, Costa MT, Mondovi B. Evidence for a ping-pong mechanism in the diamine oxidase reaction. FEBS Lett. 1969;4:31–32. doi: 10.1016/0014-5793(69)80188-2. [DOI] [PubMed] [Google Scholar]
- 12.Worm M, Fiedler EM, Dölle S, Schink T, Hemmer W, Jarisch R, et al. Exogenous histamine aggravates eczema in a subgroup of patients with atopic dermatitis. Acta Derm Venereol. 2009;89:52–56. doi: 10.2340/00015555-0565. [DOI] [PubMed] [Google Scholar]
- 13.Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545–552. doi: 10.1111/j.1365-2222.1983.tb02636.x. [DOI] [PubMed] [Google Scholar]
- 14.Sampson HA, Jolie PL. Increased plasma histamine concentrations after food challenges in children with atopic dermatitis. N Engl J Med. 1984;311:372–376. doi: 10.1056/NEJM198408093110605. [DOI] [PubMed] [Google Scholar]
- 15.Ionescu G, Kiehl R. Monoamine and diamine oxidase activities in atopic eczema. Allergy. 1988;43:318–319. doi: 10.1111/j.1398-9995.1988.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 16.Maintz L, Benfadal S, Allam JP, Hagemann T, Fimmers R, Novak N. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106–1112. doi: 10.1016/j.jaci.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Broberg A, Engström R, Kalimo K, Reimers L. Elimination diet in young children with atopic dermatitis. Acta Derm Venereol. 1992;72:365–369. [PubMed] [Google Scholar]
- 18.Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy. 2009;64:258–264. doi: 10.1111/j.1398-9995.2008.01917.x. [DOI] [PubMed] [Google Scholar]