Background: Prostate cancer (PCa) growth is promoted by the androgen receptor (AR). Castration-resistant PCa is associated with activated signaling pathways.
Results: LCoR represses human PCa growth in vivo. Src kinase inactivates the corepressive function of LCoR in vivo.
Conclusion: LCoR, a novel corepressor for AR, inhibits PCa cell growth in vivo. LCoR is inactivated by Src kinase in castration-resistant PCa.
Significance: As a ligand-dependent CoR, LCoR is able to inhibit AR activity in the presence of agonists in comparison to SMRT, Alien, or NCoR and thus could offer new therapies without applying antagonists, which result in androgen-independent growth. For this, the target could be the here-described Src pathway.
Keywords: Androgen, Androgen Receptor, Corepressor Transcription, Prostate Cancer, Src
Abstract
The activated androgen receptor (AR) promotes prostate cancer (PCa) growth. AR antagonists repress the AR by recruitment of corepressors. Not much is known about the inactivation of AR by corepressors in the presence of agonists (androgens). Here we show that the corepressor LCoR acts as an androgen-dependent corepressor that represses human PCa growth in vivo. In line with this, progressive decrease of ligand-dependent corepressor expression was observed in the PCa TRAMP mouse model with increasing age. LCoR interacts with AR and is recruited to chromatin in an androgen-induced manner. Unexpectedly, the LXXLL motif of LCoR is dispensable for interaction with the AR. Rather, the data indicate that LCoR interacts with the AR DNA binding domain on DNA. Interestingly, the interaction of LCoR with AR is inhibited by signaling pathways that are associated with androgen-independent PCa. Here we also show that the Src kinase inactivates the corepressive function of LCoR. Interfering with endogenous Src function by a dominant negative Src mutant, the growth inhibitory activity of LCoR is enhanced in vivo in a xenograft mouse model system. Thus, our studies indicate a role of LCoR as an AR corepressor and a tumor suppressor. Further, the decreased expression or inactivation of LCoR is as an important step toward PCa carcinogenesis in vivo.
Introduction
Prostate cancer (PCa)4 in men is a serious health problem worldwide and the second leading cause of cancer-related male deaths (1). The growth of the normal prostate and PCa is regulated by the androgen receptor (AR) (2, 3), a ligand-activated transcription factor and a member of nuclear hormone receptor (NHR) superfamily. Its modular structure comprises an N-terminally located transcription activation function (AF1), a central DNA binding domain (DBD), and a C-terminal ligand binding domain (LBD). Binding of natural agonist dihydrotestosterone (DHT) to the LBD induces conformational changes in the AR, leading to its shuttling into the nucleus and subsequent binding to the regulatory elements in the target genes, thereby modulating their expression. The AR is vital for proper prostate function, and its mutations have been linked to benign prostatic hyperplasia (BPH) and PCa. Further, increased AR-mediated transactivation has been linked to progression of PCa. Therefore, the AR is a key factor and drug target for PCa.
The transcriptional activity of AR is regulated by interacting proteins termed “coactivators” that positively modulate receptor function and by “corepressors” that inhibit AR function (4, 5) such as Alien (6), silencing mediator of retinoid and thyroid receptors (SMRT) (7), and NCoR (8). Binding of the agonist to the AR allows a preferential functional interaction of coactivators through their NR-box containing the LXXLL motif, which in turn promotes gene activation (9). Conversely, we have shown previously that corepressors are mostly recruited to antagonist-bound AR and lead to suppression of AR target gene expression (6, 10, 11). LCoR was first identified as a ligand-dependent interaction partner for the LBD of estrogen receptor (ER) requiring the LXXLL of the LCoR motif for binding to the ER (12).
Development of hormone therapy-resistant or castration-resistant PCa is associated with increased AR mRNA and protein levels that lead to reduced corepressor recruitment to AR target genes in the presence of AR-specific antagonists (2, 3). Moreover, various signaling pathways, including the Src kinase pathway, are activated in androgen-independent/castration-resistant PCa, leading to activated AR (13). Evidence also suggests that in this situation, binding of corepressors to the AR is reduced in the presence of AR antagonists as one underlying molecular mechanism (10, 11).
This study focused on the inhibition of the AR in the presence of AR agonists and identifies LCoR as a novel androgen-dependent corepressor for the human AR that inhibits the growth of PCa. Further, LCoR itself is in turn functionally inactivated by the Src kinase pathway.
MATERIALS AND METHODS
Reagents
AR antibody, salmon sperm, and DNA/protein A-agarose 50% slurry were obtained from Upstate Biotechnology. LCoR antibody was obtained from Genway Biotechnology. The myc- and nonspecific IgG antibodies were from Santa Cruz Biotechnology, Inc.. Methyltrienolone or R1881 were from PerkinElmer Life Sciences. DHT, CPA, proteinase K, RNase A, and guanidium HCl were from Sigma. Casodex and OHF were from Schering AG. The RNA purification kit was from Qiagen. PP2, U0126, and LY294002 were procured from Calbiochem. The complete mini protease inhibitor and the protease inhibitor mixture were from Roche. Matrigel was purchased from BD Biosciences. Three-week-old athymic male nude mice were obtained from NxGen Biosciences, and mice studies were permitted by the Animal Ethics Committee of the University of Wisconsin.
Plasmid Constructs
Mammalian expression vectors for various ARs and luciferase reporters have been described previously (11). pSG5-LCoR and pSG5-LCoR-mut were a kind gift from Dr. John White. The pABΔgal, pAB-gal-NCoR, pABgal-Alien, pETE-Hyg and pCMX-VP16, and Src-wt and Src mutants were described previously (6, 11, 13). The pCS2-MT-LCoR, pAB-VP-DBD, and VP-LCoR fusions were generated by standard cloning techniques.
Cell Culture and Transient Transfection
CV1 and C4–2 cells were grown and transfected as described previously (11, 13). Unless stated otherwise, 1 μg of each luciferase reporter, 0.2 μg of AR expression plasmids, 1 μg of pSG5/pSG5-LcoR, and 0.4 μg of pCMX-LacZ were used in transfections, and 16 h post-transfection they were treated with hormone R1881 at concentrations of 10−10 m, DHT 10−7 m, Casodex 10−7 m, CPA 10−7 m and OH-F 10−7 m and all signaling inhibitors at 1 μm. 72 h post-transfection, cells were harvested and measured for luciferase and β-galactosidase activities as described previously (11). Independent triplicate experiments were performed each time and were repeated three times. The error bars represent mean ± S.D. Student's t test was used to calculate the p values. A p value below 0.05 was considered statistically significant.
Semiquantitative PCR
PCR reactions were carried out using a forward and reverse primer for LcoR (forward, 5′-tgcaactactcagaaccctgtgct-3′; reverse 5′-tggcagctgtggacaattggtttc-3′) and GAPDH (forward, 5′-aatcccatcaccatcttccaggag-3; reverse 5′-gcattgctgatgatcttgaggctg-3). The PCR reaction standardization kit was obtained from Epicenter Biotechnologies (Madison, WI).
ChIP Assay
ChIP experiments involving the PSA enhancer region (ARE III) were performed essentially as described previously (6). ChIP experiments were repeated three times with similar results.
Real-time RT-PCR
Isolation of mRNA and the real-time PCR was performed as described earlier (6). A total of 200,000 C4-2 cells/well were seeded out in charcoal-stripped serum containing T media in six-well tissue culture dishes. After 24 h, cells were treated with R1881 (10−10 m) for 48 h, total cellular RNA was isolated, and 1 mg RNA was reverse-transcribed to cDNA and subjected to amplification by light cycler using specific primers and control primers against actin.
GST Pull-down
GST and GST-AR-DBD were expressed in Escherichia coli strain HB101 overnight at 16 °C after induction with 0.1 mm isopropyl-β-D-thiogalactopyranosid (Sigma). After bacterial extraction, GST proteins were affinity-purified via glutathione beads that were either incubated with 0.5 mg LNCaP whole cell extract as positive control for full-length LCoR binding to AR-DBD or with 10 μg of His-tagged purified LCoR 101–218 or 219–433. His-tagged proteins were expressed in E. coli strain BL21 under the same conditions. Affinity purification was performed with nickel-nitrilotriacetic acid beads (Invitrogen). The binding to GST/-AR-DBD was analyzed via LCoR-Western blotting (LCoR antibody was obtained from Cell Signaling Technology, Inc.). Ponceau staining of proteins served as loading control.
Generation of Stable Clones
A total of 200,000 C4–2 cells were transfected with Src or mutant Src along with the pETE-Hyg plasmid in 5:1 molar ratio (total amount being 10 μg) using the calcium phosphate method. The medium was replaced with fresh T media 18 h post-transfection. Stable clones were selected as described previously (6).
In Vivo Tumor Xenograft Model
1 × 106 C4-2 stable clones were suspended in 100 μl of complete cell culture media and 100 μl of matrigel. Cells were subcutaneously implanted in the left and right flanks of five athymic male nude mice in every experimental group. At different time points, tumors were measured by using a digital Vernier caliper.
Immunohistochemistry
Paraffin-embedded prostate tissues arrays (4 mm) were obtained from US Biomax. Immunostaining was performed using LCoR antibody (dilution 1:50) essentially as described previously (13).
RESULTS
LCoR Functionally Represses the AR Transactivation Function
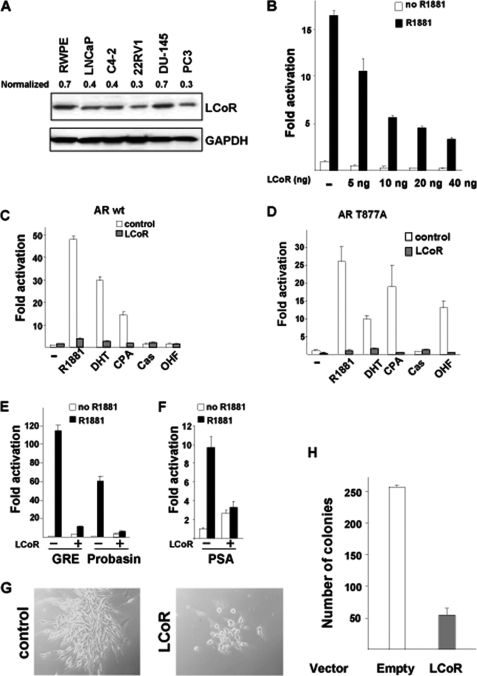
A varying degree of LCoR protein expression was observed in a panel of prostate epithelial cells, both normal and tumorigenic (Fig. 1A). RWPE1 non-tumorigenic normal prostate epithelial cells exhibited higher LCoR protein levels compared with human prostate carcinoma cells such as LNCaP, C4–2, 22RV1, and PC3, except DU145, indicating a decreased LCoR expression in most PCa carcinoma cells compared with normal prostate epithelial cells.
FIGURE 1.
Functional repression of AR by LCoR. A, Western blot analysis showing LCoR proteins in a panel of prostate cell lines. β-actin was used as a loading control. B, CV1 cells were transfected with pMMTV-Luc, WT hAR, and increasing amounts of pSG5-LCoR (5–40 ng). Cells were transfected with 1 μg of WT hAR (C) or T877A mutant (D). E, CV1 cells were transfected with GRE-Luc, pARR3-Luc (probasin), or PSA-Luc (F) along with WT hAR and pSG5-LCoR. The graph represents the fold hormone induction with S.D. between triplicates. LCoR-mediated AR repression was significant in C–F (Student's t test, p < 0.005). LCoR-mut represents the NR-box mutant of LCoR (12). AR ligands (agonists): DHT, R1881. AR ligands (antagonists): OHF, Casodex, CPA. G, stable clones of C4-2 cells overexpressing either the empty vector control (left panel) or overexpressing LCoR (right panel) and the number of colonies obtained from the corresponding stable clones (H).
To examine the transcriptional effect of LCoR on AR transactivation function, reporter assays employing androgen-responsive promoters were performed in CV1 cells lacking endogenous AR expression. Ectopic expression of LCoR (5–40 ng) repressed agonist R1881-activated WT AR transactivation in a dose-dependent manner (Fig. 1B). Also, LCoR repressed WT AR activated by the natural agonist DHT and the partial antagonist CPA (Fig. 1C). Because WT AR was not activated either by Casodex or OHF, a repressive effect was not observed.
Further, the endogenous mutant AR (AR-T877A, bearing a mutation in the LBD), often found in clinically relapsed disease (14) and shown to positively stimulate the growth of PCa (15), was also repressed by LCoR in an agonist (R1881 and DHT) dependent fashion (Fig. 1D). This AR mutant was also activated by the partial agonist CPA, whereas LCoR expression led to its down-regulation. Notably, unlike WT AR, the T877A mutant was activated by the AR antagonist OHF, and cotransfection of LCoR also repressed AR transactivation, suggesting that LCoR could overcome the T877A-mediated activation through OHF. However, no significant activation of AR was seen by Casodex. Thus, this suggests that LCoR down-regulates the transactivation of both activated WT and AR mutant T877A.
We also tested a double point mutant of AR (termed AR-SUMO, small ubiquitin modifier) lacking the SUMOylation sites of AR and devoid of interaction sites for the corepressors SMRT and Alien (6, 11, 16). LCoR repressed transactivation of the androgen-induced AR-SUMO mutant (supplemental Fig. 1), indicating that LCoR acts on AR in a distinct manner from the corepressors SMRT or Alien.
Similar to repression of the AR-mediated transactivation function on the MMTV promoter, ectopically expressed LCoR efficiently repressed AR transactivation on GRE (glucocorticoid response element), probasin, and PSA promoter reporters containing androgen response elements (Fig. 1, E and F) in an agonist-dependent manner, indicating the versatility of LCoR in its repression function in a wide androgen-responsive promoter context.
Because AR regulates the growth of PCa and interference with AR function leads to growth inhibition of PCa, we tested whether interference with its function by LCoR has an influence on cellular growth. For that purpose, stable clones of the human castration-resistant PCa C4–2 cells overexpressing LCoR were generated, and colony formation assays were performed. Using the empty expression vector, numerous colonies were formed with more cells per colonies. However, cells stably transfected with LCoR exhibited a marked decrease in both their colony-forming potential and cell number per colony (Fig. 1, G and H), indicating that LCoR functionally interferes with the growth of PCa cells.
The C Terminus of LCoR Is Sufficient to Repress AR Function via Targeting Its DBD
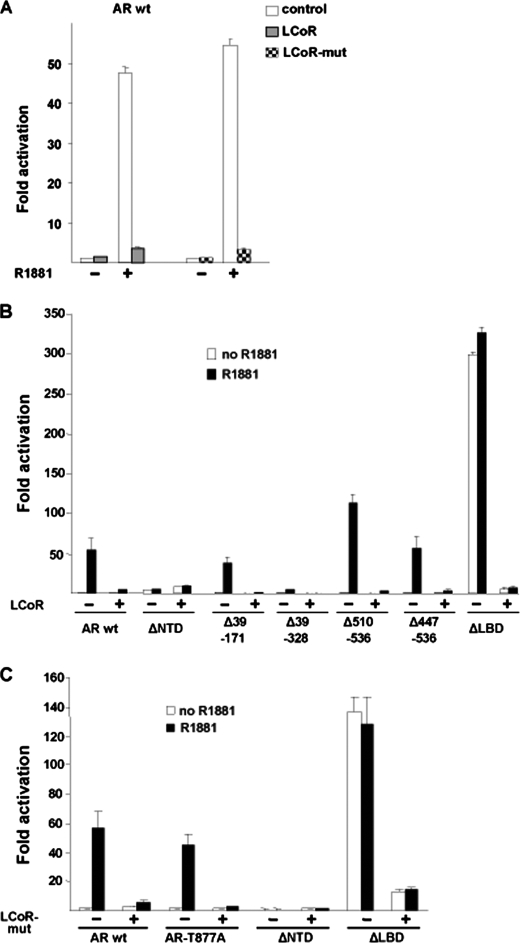
First, we analyzed whether LCoR requires the previously identified LXXLL motif necessary for interaction with ER to also repress AR in the presence of the AR agonist. Interestingly, however, the LCoR mutant, having NR-box mutated (LCoR mut), also repressed the WT AR-mediated transactivation in an agonist-dependent manner (Fig. 2A). This indicates that the NR-box of LCoR is dispensable for AR repression and suggests a different mode of interaction.
FIGURE 2.
Mutational analysis of AR-LCoR-mediated repression. A, CV1 cells were transfected with the pMMTV-Luc reporter along with the pSG5 or pSG5-LCoR expression vectors and treated with agonist R1881. LCoR-mediated AR repression was significant (Student's t test, p < 0.001). B, various AR deletion plasmids that lead to truncated AR proteins were cotransfected with LCoR and later treated with agonist R1881. LCoR-mediated AR repression was significant (Student's t test, p < 0.01). C, CV1 cells were transfected with pMMTV-Luc along with pSG5-LCoR-mut. In addition, various AR mutant expression plasmids were also transfected, and cells were treated with R1881. The graph represents the fold hormone induction. LCoR mutant-mediated AR repression was significant (Student's t test, p < 0.05).
To map down the region(s) of AR that is targeted by LCoR and to define whether a functional interaction between AR and LCoR takes place, various deletion mutants of the AR NTD were analyzed. The N-terminal of AR harbors the major transactivation function. Deleting AF-1 (ΔNTD) therefore renders the intact C terminus AR transcriptionally incompetent, and no repressive effect of LCoR on this AR mutant was observed. Ectopically expressed LCoR repressed various N-terminal AR truncations (Δ39–171, Δ39–328, Δ510–536, and Δ447–536) in an androgen-dependent manner (Fig. 2B), indicating that NTD is also dispensable for LCoR-mediated repression of AR.
The LBD deletion (ΔLBD) of AR was activated ligand-independently and was also repressed by LCoR. This suggests that in contrast to many other NHRs, where intact LBD is required for LCoR-mediated repression, the LBD of AR is dispensable for LCoR-mediated repression.
Employing the LCoR mut, devoid of the LXXLL motif, revealed similar pattern to repress the AR mutants, AR deletions repressing the T877A, and AR ΔLBD mutant well (Fig. 2C). Taken together, these experiments rule out the involvement of either the NR-box of LCoR or of the LBD of the AR in repression by LCoR. The involvement of DBD-LBD cannot be entirely ruled out because deletion of AF-1 bearing the N terminus results in transcriptionally incompetent AR, and, therefore, repressive effects of LCoR could not be tested in these experiments.
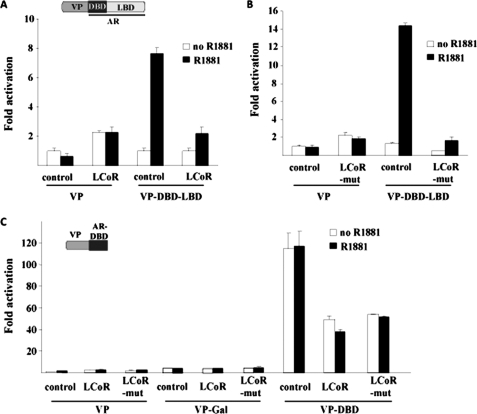
To analyze the involvement of the DBD-LBD of the AR in LCoR-mediated repression, the NTD was exchanged with the potent transactivation domain of VP16 to generate VP-DBD-LBD. This AR mutant was activated ligand-dependently, demonstrating that specificity and cotransfection of either LCoR-WT or LCoR-mut repressed the activation of this AR mutant (Fig. 3, A and B), ruling out the possibility of the involvement of the NTD of the AR in LCoR-mediated repression. All these experiments deleting or replacing one or both activation functions suggest that to repress AR function, LCoR does not require the NTD or LBD of the AR.
FIGURE 3.
Interaction analysis in mammalian cells: NTD and DBD are required. A and B, CV1 cells were transfected with pMMTV-Luc, VP16, or VP-DBD-LBD (0.1 μg) and 1 μg each of either pSG5, pSG5-LCoR, or pSG5-LCoR-mut and were incubated with R1881. The graph represents fold hormone activity. C, cells were transfected with MMTV-Luc, VP16 empty, and VP16-Gal-DBD (called VP-Gal) as controls or VP-DBD (1 μg), pSG5 alone, LCoR, or LCoR-mut and treated with R1881. LCoR-mediated repression of the VP-AR deletion mutants was significant (Student's t test, p < 0.005). The graph represents the fold hormone induction.
Therefore, the involvement of the DBD of the AR in repression by LCoR was tested. The AR chimera (VP-DBD), deleting both the NTD and the LBD, was generated, possessing only the intact DBD of AR. This fusion protein was strongly activated ligand-independently compared with the empty vector VP16 control and VP16-Gal-DBD (VP16-Gal) (Fig. 3C). Interestingly, cotransfection of either LCoR or LCoR-mut led to the repression of this AR mutant, strongly implicating that LCoR specifically targets the DBD of the AR and not of Gal to mediate repression. A strong repressive action of LCoR was not observed with this AR fusion as compared with full-length AR, which may be due to the exchange of the NTD with VP16.
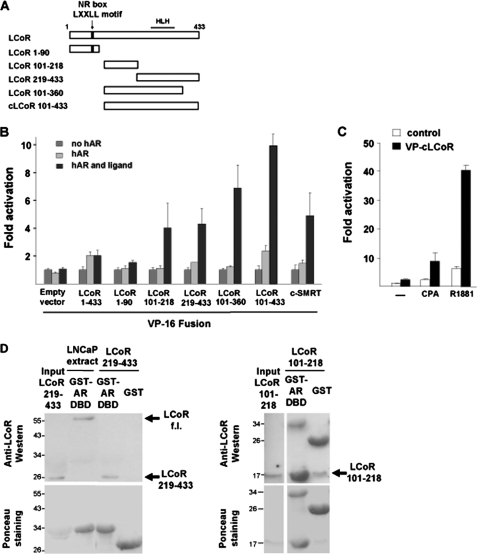
On the other hand, to map down the region of LCoR that interacts with the AR, various chimeras of LCoR deletions fused to the VP16 transactivator were generated (Fig. 4A) and employed in the mammalian one-hybrid assay. This assay is based on the interaction of VP16-LCoR fusion to the full-length AR. The activation of the reporter by the empty vector VP16 control was therefore set as 1. Further activation of the reporter is possible if VP16 is brought into close proximity of the promoter. The full-length LCoR (LCoR f.l.), because of the intact C-terminal binding protein and histone deacetylase interaction domain (12) fused to VP16, led to a weak induction (Fig. 4B), indicating a possible interaction of LCoR with the AR that was further strengthened using LCoR deletions that lack the N-terminal domain. The LCoR 1–90 fusion did not reveal an activation of the reporter, presumably because of the presence of two repressive CtBP interaction domains that may counterbalance the transactivation function of VP16. Notably, however, both LCoR 101–218 and the non-overlapping LCoR 219–433 fragments exhibited a ligand-dependent interaction with the AR, suggesting that LCoR potentially uses two independent interaction domains to bind to the AR. This was confirmed by using bacterially expressed and affinity-purified LCoR fragments and the AR-DBD (Fig. 4D). The data suggest that each of the two LCoR fragments interact independently and directly with the AR-DBD in vitro. Interestingly, the C termini of LCoR 101–360 and LCoR 101–433 (VP-cLCoR), both of which harbor the helix-loop-helix interaction motif, were found to functionally interact with WT hAR in a ligand-dependent manner. VP-cLCoR was able to interact with both CPA- and R1881-bound WT hAR and exhibited a 4- and 7-fold higher activity over the empty vector VP16, respectively (Fig. 4C). Taken together, these results indicate that to repress AR function, LCoR uses two independent domains to interact with the AR-DBD and that this interaction is independent of the NR-box of LCoR.
FIGURE 4.
LCoR deletion analysis: Requirement of two interaction domains. A, LCoR is a 433-amino acid protein with a single NR-box for interaction with selected members of the nuclear hormone receptor superfamily. The C-terminal part of LCoR harbors an HLH interaction motif. VP16-cLCoR was constructed by cloning the last 332 C terminus amino acids into the pCMX-VP16 empty vector. B, CV1 cells were transfected with MMTV-Luc, VP16, or various VP16-LCoR fusions (1 μg) and treated with CPA. Data were plotted setting each empty vector control VP16 in the absence of hormone arbitrarily as 1 and represent fold reporter activation. C, CV1 cells were transfected with pMMTV-Luc, pCMX-VP16-cLCoR (2 μg), and hAR (50 ng) treated with R1881 or CPA. The graph depicts the fold hormone induction. LCoR binding to AR was significant for B and C (Student's t test, p < 0.05). D, bacterially expressed GST or GST-AR-DBD were affinity-purified and incubated with either 0.5 mg LNCaP whole cell extract, as positive control for full-length LCoR (f.l.) binding to the AR-DBD, or with 10 μg of His-tagged bacterially expressed and purified LCoR 219–433 or 101–218 fragments that were detected by Western blotting. Ponceau staining served as a loading control. Both LCoR minimal domains show significant binding to the AR-DBD.
Src Activity Opposes Repressive Action of LCoR on the AR
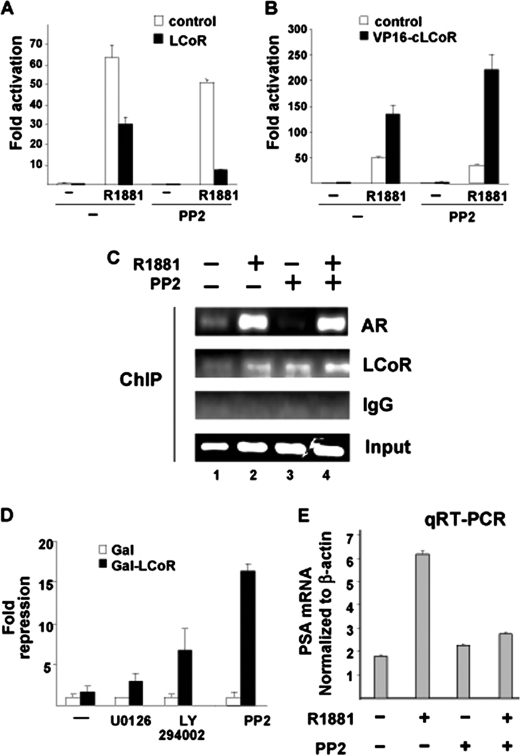
To test the functional relevance of LCoR expression in human PCa cells, the ability of LCoR to repress endogenous T877A mutant AR in PCa C4–2 cells was tested. LCoR was ectopically overexpressed, leading to only a slight (2-fold) repression of AR-mediated transactivation (Fig. 5A) in a ligand-dependent manner. The degree of repression was lower compared with robust repression observed in CV1 cells (Fig. 1, A and B), suggesting that even with forced overexpression, LCoR-mediated repression is weaker compared with that observed in CV1 cells. Our hypothesis was that this marginal decrease could be due to the activation of signal transduction pathways in PCa cells that weaken the repressive effect of LCoR on AR. To test this hypothesis, we employed a battery of signaling cascade inhibitors to block specific signal transduction pathways that are overexpressed in PCa, such as Src kinase (13). Interestingly, blocking Src function by a specific inhibitor (PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo(3,4-d) pyrimidine) enhanced LCoR-mediated AR repression in the presence of the agonist (Fig. 5A, right panel). This suggests that the Src inhibitor reduced agonist-induced AR transactivation in the presence of LCoR. It is noteworthy that agonist-induced empty vector-transfected cells (in the absence of LCoR expression) treated with PP2 also yielded a decreased ligand-induced induction. This effect is presumably due to the activation of endogenous LCoR (Fig. 5A). Thus, the potency to inhibit the AR is enhanced by treatment with the Src kinase inhibitor PP2.
FIGURE 5.
Involvement of Src kinase pathway in LCoR-mediated repression of AR. A, C4-2 cells were transfected with MMTV-Luc, pSG5, or pSG5-LCoR and treated with R1881 and Src kinase inhibitor PP2. The graph represents the fold hormone induction. B, C4-2 cells transfected with MMTV-Luc, VP16, or VP16-cLCoR (1 μg) were treated with R1881 and PP2. The graph represents the fold hormone induction. The influence of PP2 on LCoR-mediated AR repression (A) and on LCoR binding to AR (B) was significant (Student's t test, p < 0.001). C, a ChIP analysis was performed with C4-2 cells. Before lysis, samples were treated with PP2 for 48 h and with 10−8 m R1881 for 1 h. The harvested lysate was diluted and immunoprecipitated with antibodies against AR, anti-LCoR, or nonspecific anti IgG antibody. The DNA was eluted from the immunoprecipitates and amplified by primers spanning the PSA enhancer. As equal starting material prior to immunoprecipitation, the input is shown. D, C4-2 cells were transfected with the p(UAS)4TATA-Luc reporter along with 1 μg of the Gal-LCoR plasmid and treated with U0126, rapamycin, LY294002, and PP2, and data were plotted with respect to values obtained for each empty vector and represent fold reporter repression over galactosidase empty vector control. E, in total, 200,000 C4-2 cells/well were seeded out in hormone-depleted FBS containing T media in six-well tissue culture dishes. After 24 h, cells were treated with R1881 (10−10 m) for 48 h. Then, total cellular RNA was isolated, reverse-transcribed to cDNA, and amplified by light cycler using specific primers and control primers for actin. The graph represents the actin-normalized values of the PSA transcript.
To explain the restoration of the strong repressive effect of LCoR on AR in the presence of PP2, we analyzed the influence of blocking Src kinase function on the interaction of LCoR with endogenous AR employing the VP16-cLCoR chimera. Treatment of C4-2 PCa cells with PP2 in the presence of the AR-specific agonist suggests that the interaction of endogenous LCoR with endogenous AR is enhanced (Fig. 5B). In line with this, ChIP assays revealed that endogenous LCoR is corecruited to the enhancer of the PSA gene in presence of agonist R1881 in C4-2 cells and that the recruitment was enhanced by PP2 (Fig. 5C, compare lane 1 with lane 3 and lane 2 with lane 4). Further, to test for the expression of the endogenous AR target gene PSA, real-time RT-PCR (quantitative RT-PCR) experiments were performed with and without PP2 treatment. The data suggest that the androgen-induced PSA mRNA levels are decreased by PP2 treatment (Fig. 5E). Taken together, the SRC kinase pathway inhibits LCoR-mediated repression of AR, presumably by inhibiting the LCoR-AR interaction.
However, we have also further addressed the possibility that the LCoR-mediated silencing may be inhibited by the activated Src kinase pathway. For that purpose, Gal4-LCoR chimeras were generated. The Gal4-LCoR chimera repressed a cognate reporter much more strongly in CV1 cells (approximately 20-fold) as compared with repression observed in C4-2 cells (approximately 4-fold), indicating that the LCoR-mediated silencing is much weaker in the PCa C4-2 cells (supplemental Fig. 2). Similarly, the corepressor NCoR chimera exhibited a potent-silencing function in CV1 cells compared with C4-2 cells. Conversely, the AR corepressor Alien repressed the induction of the reporter to the same degree in both of these cell lines. This suggests that the repression function of different corepressors is regulated in a cell type-dependent context.
Therefore, our hypothesis was that LCoR could be functionally attenuated by activating signaling pathways, which may play a role in PCa progression (16, 17, 18). A battery of specific signaling inhibitors was therefore employed to test whether signaling kinases influence the autonomous repression function of LCoR. Inhibitors of MAPK (U0126), PI3K (LY294002), and Src kinase (PP2) signaling, all of which have been reported to be overexpressed in PCa, were therefore included. Interestingly, blocking of Src activity restored the strong repression function of LCoR in C4-2 cells (Fig. 5D). Also, blocking of PI3K activity led to a partial enhancement of LCoR repression function, suggesting that LCoR could potentially be targeted by signaling kinases or a circuit of signaling cascades. Notably, the blocking of the MAPK function did not significantly modulate the repressor function of LCoR.
The data suggest that the LCoR-mediated gene silencing is inhibited by the Src kinase pathway. Thus, taken together, inhibiting the Src kinase pathway using PP2 strongly enhances both the interaction of LCoR with AR and LCoR-mediated gene silencing.
Src Regulates LCoR-mediated Growth Repression in PCa in Vivo and in Vitro
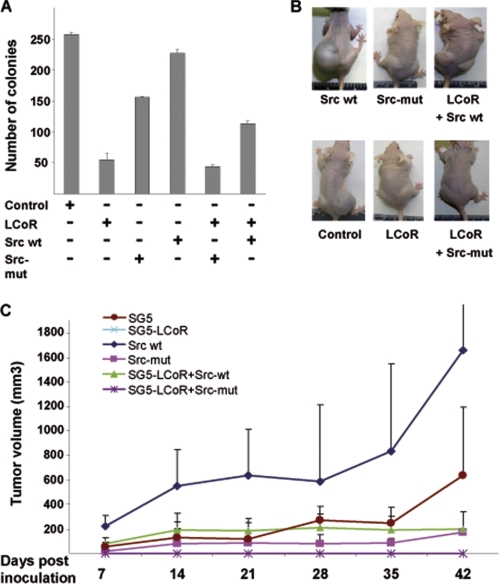
The influence of the Src kinase pathway on LCoR was further investigated by cellular growth analyses. For that purpose, stable clones of C4-2 cells overexpressing LCoR and/or Src, WT or mutant, were generated. Cells stably transfected with the LCoR expression vector showed a marked decrease (3.5-fold) in their colony-forming potential as compared with control cells transfected with the empty vector (Fig. 6A). Moreover, interference with endogenous Src function using a Src mutant (Src-mut) further potentiated the repressive effect of LCoR on cellular growth. In contrast, coexpression of Src along with LCoR resulted in an increase in colony number as compared with LCoR alone. These results further indicate that LCoR represses PCa cell growth and that its function is largely governed by Src kinase activity in PCa cells.
FIGURE 6.
LCoR represses PCa growth in vitro and in vivo and is attenuated by Src kinase signaling. A, number of stable clones obtained from C4-2 cells overexpressing either empty vector control, LCoR, or dominant negative with a mutation in the kinase cassette Src-mut, Src, LCoR+Src mut, or LCoR+Src. LCoR-mediated repression of colony formation and potentiation by the Src mutant was significant (Student's t test, p < 0.01). B, representative photograph of the athymic nude mice bearing the xenograft of the C4-2 cell clones overexpressing LCoR and/or Src. C, tumor size of xenografts of the above-mentioned stable clones of C4-2 cells in athymic nude mice representing the growth of tumor over 6 weeks (n = 5).
Moreover, the tumorigenic potential of C4-2 cells overexpressing LCoR and/or Src in vivo xenograft experiment was analyzed. Both C4-2 WT and C4-2/Src cells developed large tumors in athymic male nude mice (Figs. 6, B and C). Notably, mice bearing C4-2 cells stably overexpressing either LCoR alone or in combination with Src-mut did not develop any tumors. An interesting finding of the experiment was that cells coexpressing LCoR did not develop any tumors through the entire course of the 6-week-study, and in combination with Src, only moderate-sized tumors were developed.
This indicates on one hand the importance of functionally active corepressors to reduce tumor growth and on the other hand the negative influence of Src activity on corepressor LCoR-mediated repression of prostate tumorigenesis. In agreement with cell culture data, expression of Src-mut alone also resulted in moderate-sized tumors.
To summarize, the results indicate that LCoR acts as a corepressor for ligand-activated AR in vivo, represses the transactivation of AR and, subsequently, the growth of PCa, and that the overexpression of Src negatively regulates its corepression function and, therefore, also has a positive influence on tumor development.
Differential Expression of LCoR in Prostate Tumorigenesis
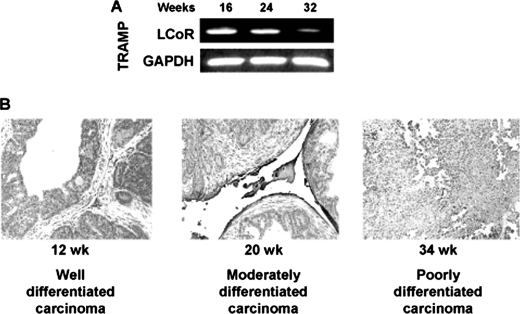
A further link between LCoR and PCa progression arose while analyzing the expression of LCoR in TRAMP mice that exhibit age-dependent progression stages of PCa similar to human disease (19). The data suggest that the expression of LCoR decreased as the tumor progressed from well differentiated carcinoma at 16 weeks to a poorly differentiated carcinoma at 32 weeks (Fig. 7A). The level of LCoR expression was also determined in situ in TRAMP mouse PCa tissue, where LCoR expression was highest in PCa tissue in 12-week-old mice, which represents well differentiated carcinoma. The LCoR levels were reduced further in moderately differentiated carcinoma derived from 20-week-old mice, and the lowest expression of LCoR was observed in the poorly differentiated carcinoma of mice at 34 weeks of age (Fig. 7B). These data strongly suggest a link between LCoR expression and PCa progression.
FIGURE 7.
Progressive decrease in LCoR expression in vivo. A, LCoR transcript expression is progressively decreased in TRAMP mice as a function of increase in age. Prostate tissue from 16-week-old mice expresses higher LCoR mRNA than 24-week- old mice and is further decreased at 24 and 32 weeks. B, immunohistochemical analysis for LCoR expression in prostate tissue of TRAMP mice of different ages (12, 20, and 34 weeks). Experiments were conducted on five randomly selected tumor tissues from TRAMP mice in each group with similar results, and only one representative result is shown.
DISCUSSION
In this study, we have characterized LCoR as a novel androgen-dependent AR corepressor that regulates the growth of PCa cells in vitro and in vivo. Our data demonstrate that the LCoR-mediated repression of AR transactivation is associated with the inhibition of PCa growth in vivo and further links Src kinase activity to LCoR function.
The corepressors SMRT and Alien act preferentially in the presence of AR antagonists and inhibit AR-mediated transactivation (6, 11). Interestingly, SMRT was shown to interact with AR in the presence of the AR agonist but did not exhibit repression, whereas in the presence of the partial antagonist CPA, SMRT binds to AR and inhibits AR-mediated transactivation. Alien, on the other hand, seems to interact with AR only in the presence of AR antagonists and not in the presence of an agonist (6). This suggests that these corepressors repress AR only in the presence of antagonists. Here, we focused on whether AR transactivation and PCa growth can be repressed in the presence of agonists.
A major activator of AR seems to be activating signaling pathways. Several underlying possibilities can be envisaged about how these signaling pathways activate the AR. One of them would be the inactivation of corepressor function. Therefore, this work focuses on one of several molecular mechanisms of how corepressor function is modulated by signal transduction pathways. Repression of AR function by LCoR highlights its physiological relevance, which may play a protective role in the onset and progression of the disease by repressing AR-mediated gene activation and, thereby, the growth of PCa.
We demonstrate that the NR-box of LCoR, although required to interact with various other NHR members, is not required to interact with AR, and our data suggest that at least the AR-DBD is targeted by LCoR. In vitro protein-protein interaction assays with purified LCoR fragments and the AR-DBD confirm that two independent sites in LCoR exist that interact with the AR-DBD. All the N-terminal AR truncations are significantly repressed by LCoR (Fig. 2B), which does not confirm but suggests that LCoR may not target the AR NTD, which it is the case in the interaction of the ligand-sensitive CoRs such as SMRT, NCoR, or Alien with the AR (6, 11). Some coactivators, like Ubc9, have been shown to target the DBD of the AR to activate its function (16). Therefore, it is possible that corepressors can also target the same domain to modulate AR transactivation and so can compete with the coactivators, such as Ubc9, to interact with AR. This kind of mechanism could help to counterbalance the activated AR to fine-tune its activation.
Rendering LCoR functionally attenuated could be beneficial for PCa growth to attain a desirable constitutive activation of AR. Posttranslational regulation by Src kinase activity indicates that, in addition to directly activating AR function (13, 20), Src kinase can repress interaction of corepressors such as LCoR and thereby indirectly activate AR function in PCa cells. This work also provides a mechanistic explanation for Src kinase family-mediated involvement in the AR pathway, where Src inhibits corepressors such as LCoR. In fact, many other signal transduction pathways are overexpressed in androgen-independently growing PCa cells, which may potentially decrease the interaction of corepressors with AR to activate AR function. In C4-2 cells, introduction of dominant-negative Ras restores sensitivity to Casodex (21). Similarly, treatment of C4-2 cells with the Her2 tyrosine kinase inhibitor AG825 leads to apoptosis in C4-2 cells, indicating that overexpression of Her2 may confer androgen independence to C4-2 cells (22). Similar to that, we have found that Src kinase is overexpressed in PCa cells, where it promotes the transactivation function of AR (13).
The rationale for using C4-2 cells in this study is that although they represent androgen-insensitive or hormone-refractory tumor cells, their growth is dependent on functional androgen receptor-mediated signaling (23). Moreover, in these cells as well, LCoR marginally interacts with AR, and this is enhanced in the presence of an Src kinase inhibitor. Therefore, these cells have been used as a model system to find out the role of LCoR on AR.
One way to promote AR transactivation by Src kinase could involve attenuation of the corepressor LCoR to repress AR function. In this study, we found that a pivotal mode of Src kinase action against LCoR is by interfering with the binding of LCoR to AR in vivo and by decreasing the autonomous repression potential of LCoR in C4-2 PCa cells. This indicates a novel mechanism that PCa can utilize to weaken specific corepressors at multiple levels to constitutively activate AR function. Both of these LCoR inactivating mechanisms may work in synergy to functionally attenuate LCoR from acting as a potent corepressor for AR in PCa cells.
In contrast to the CoR Alien (6), cells overexpressing LCoR produce fewer colonies in the presence of the AR agonist, indicating the potential growth-inhibitory effect of LCoR on cellular growth.
It is possible that the ratio of coactivators to corepressors, which has been shown to modulate NHR function (24), gives a competitive advantage to coactivators to bind to AR in PCa cells. This increase in ratio of coactivators to corepression may arise from the overexpression of coactivators or decreased expression of corepressors or from the functional attenuation of corepressors by signal transduction pathways, which are known to be activated in PCa cells.
These results are suggestive of the existence of multimodal ways where, in addition to directly activating AR function, as has been reported previously (13, 20), corepressor function can be repressed by activating signaling cascades, decreasing both its autonomous repression potential and interaction with AR, which together allow AR to become more active in PCa.
Taken together, our data characterize a novel transcriptional corepressor for AR that plays an important role in regulating PCa cell growth in vitro and tumor progression in vivo. The data suggest that the role of LCoR is to fine-tune the androgen receptor transcriptional activity in the presence of androgen agonists. Further, the data indicate that activated or overexpressed Src kinase in PCa cells inhibits LCoR binding and LCoR-mediated gene silencing. Thus, we suggest the existence of signal transduction pathways that may indirectly regulate corepressor function. Employment of chemotherapeutics on the basis of the inhibition of specific kinases that regulate corepressor action on AR could therefore prove to be beneficial in the treatment of therapy-resistant PCa.
Acknowledgments
We thank Dr. John White for providing LCoR and Dr. Sarah Courtneidge for the Src expression plasmids.
This work was supported by German Research Foundation Grant DFG BA 1457/2 (to A. B.), by United States Public Health Service Grants RO1CA78809, RO1CA101039, and RO1CA120451 (to H. M.), and by O'Brian Center Grant P50DK065303-01 (to H. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- PCa
- prostate cancer
- AR
- androgen receptor(s)
- NHR
- nuclear hormone receptor
- AF
- activation function
- DBD
- DNA binding domain
- LBD
- ligand binding domain
- DHT
- dihydrotestosterone
- SMRT
- silencing mediator of retinoid and thyroid receptors
- NCoR
- nuclear receptor corepressor
- LCoR
- ligand-dependent corepressor
- ER
- estrogen receptor
- CoR
- corepressor
- CPA
- cyproterone acetate
- NTD
- amino-terminal domain
- OHF
- hydroxy-flutamide
- VP16
- viral protein 16 activation domain
- NR
- nuclear receptors
- hAR
- human androgen receptor
- pMMTV
- plasmid mouse mammary tumor virus
- GRE
- glucocorticoid response element
- PSA
- prostate specific antigen
- SUMO
- small ubiquitin modifier.
REFERENCES
- 1. Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M. J. (2009) CA-Cancer J. Clin. 59, 225–249 [DOI] [PubMed] [Google Scholar]
- 2. Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. (2004) Nat. Med. 10, 33–39 [DOI] [PubMed] [Google Scholar]
- 3. Dehm S. M., Tindall D. J. (2007) Mol. Endocrinol. 21, 2855–2863 [DOI] [PubMed] [Google Scholar]
- 4. Heemers H. V., Tindall D. J. (2007) Endocr. Rev. 28, 778–808 [DOI] [PubMed] [Google Scholar]
- 5. Wang L., Hsu C. L., Chang C. (2005) Prostate 63, 117–130 [DOI] [PubMed] [Google Scholar]
- 6. Moehren U., Papaioannou M., Reeb C. A., Hong W., Baniahmad A. (2007) Mol. Endocrinol. 21, 1039–1048 [DOI] [PubMed] [Google Scholar]
- 7. Liao G., Chen L. Y., Zhang A., Godavarthy A., Xia F., Ghosh J. C., Li H., Chen J. D. (2003) J. Biol. Chem. 278, 5052–5061 [DOI] [PubMed] [Google Scholar]
- 8. Berrevoets C. A., Umar A., Trapman J., Brinkmann A. O. (2004) Biochem. J. 3, 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenfeld M. G., Lunyak V. V., Glass C. K. (2006) Genes Dev. 20, 1405–1428 [DOI] [PubMed] [Google Scholar]
- 10. Eisold M., Asim M., Eskelinen H., Linke T., Baniahmad A. (2009) J. Mol. Endocrinol. 42, 429–435 [DOI] [PubMed] [Google Scholar]
- 11. Dotzlaw H., Moehren U., Mink S., Cato A. C., Iñiguez Lluhí J. A., Baniahmad A. (2002) Mol. Endocrinol. 16, 661–673 [DOI] [PubMed] [Google Scholar]
- 12. Fernandes I., Bastien Y., Wai T., Nygard K., Lin R., Cormier O., Lee H. S., Eng F., Bertos N. R., Pelletier N., Mader S., Han V. K., Yang X. J., White J. H. (2003) Mol. Cell 11, 139–150 [DOI] [PubMed] [Google Scholar]
- 13. Asim M., Siddiqui I. A., Hafeez B. B., Baniahmad A., Mukhtar H. (2008) Oncogene 27, 3596–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taplin M. E., Bubley G. J., Ko Y. J., Small E. J., Upton M., Rajeshkumar B., Balk S. P. (1999) Cancer Res. 59, 2511–2515 [PubMed] [Google Scholar]
- 15. Sun C., Shi Y., Xu L. L., Nageswararao C., Davis L. D., Segawa T., Dobi A., McLeod D. G., Srivastava S. (2006) Oncogene 25, 3905–3913 [DOI] [PubMed] [Google Scholar]
- 16. Poukka H., Karvonen U., Janne O. A., Palvimo J. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 14145–14150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong S. H., Privalsky M. L. (2000) Mol. Cell. Biol. 20, 6612–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnes C. J., Vadlamudi R. K., Mishra S. K., Jacobson R. H., Li F., Kumar R. (2003) Nat. Struct. Biol. 10, 622–628 [DOI] [PubMed] [Google Scholar]
- 19. Greenberg N. M., DeMayo F., Finegold M. J., Medina D., Tilley W. D., Aspinall J. O., Cunha G. R., Donjacour A. A., Matusik R. J., Rosen J. M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo Z., Dai B., Jiang T., Xu K., Xie Y., Kim O., Nesheiwat I., Kong X., Melamed J., Handratta V. D., Njar V. C., Brodie A. M., Yu L. R., Veenstra T. D., Chen H., Qiu Y. (2006) Cancer Cell 10, 309–319 [DOI] [PubMed] [Google Scholar]
- 21. Bakin R. E., Gioeli D., Bissonette E. A., Weber M. J. (2003) Cancer Res. 63, 1975–1980 [PubMed] [Google Scholar]
- 22. Murillo H., Schmidt L. J., Tindall D. J. (2001) Cancer Res. 61, 7408–7412 [PubMed] [Google Scholar]
- 23. Snoek R., Cheng H., Margiotti K., Wafa L. A., Wong C. A., Wong E. C., Fazli L., Nelson C. C., Gleave M. E., Rennie P. S. (2009) Clin. Cancer Res. 15, 39–47 [DOI] [PubMed] [Google Scholar]
- 24. Liu Z., Auboeuf D., Wong J., Chen J. D., Tsai S. Y., Tsai M. J., O'Malley B. W. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7940–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]