Abstract
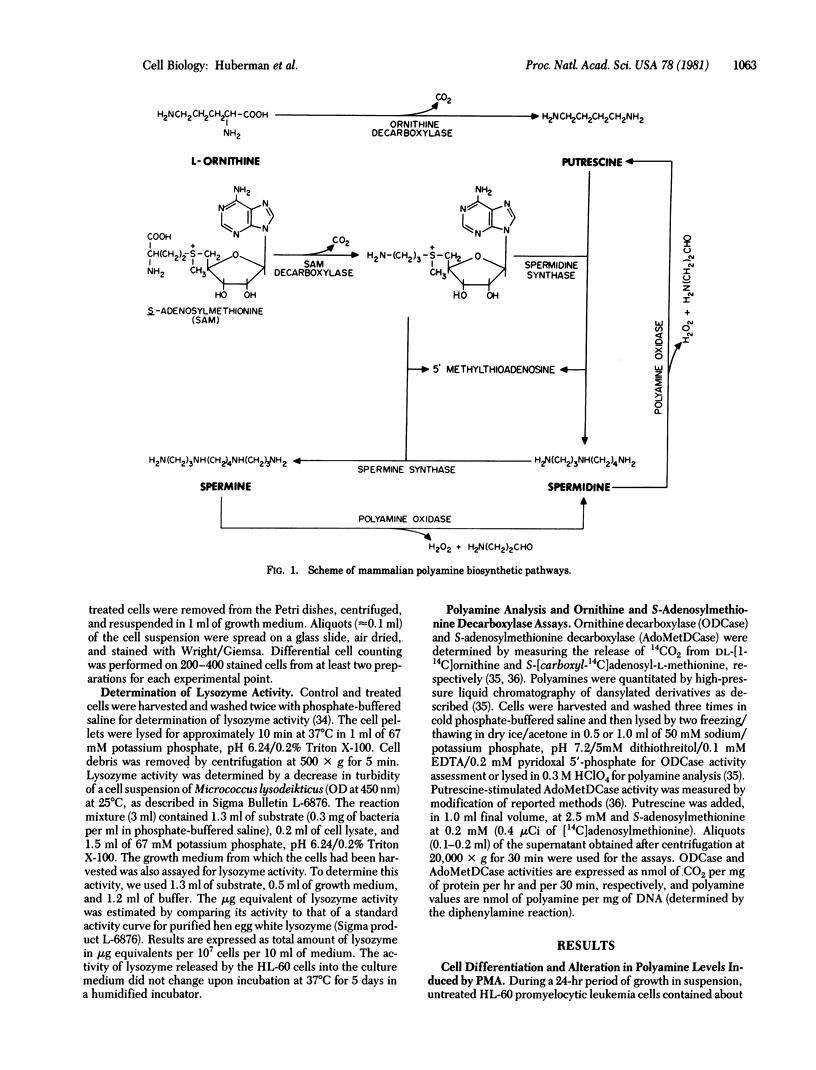
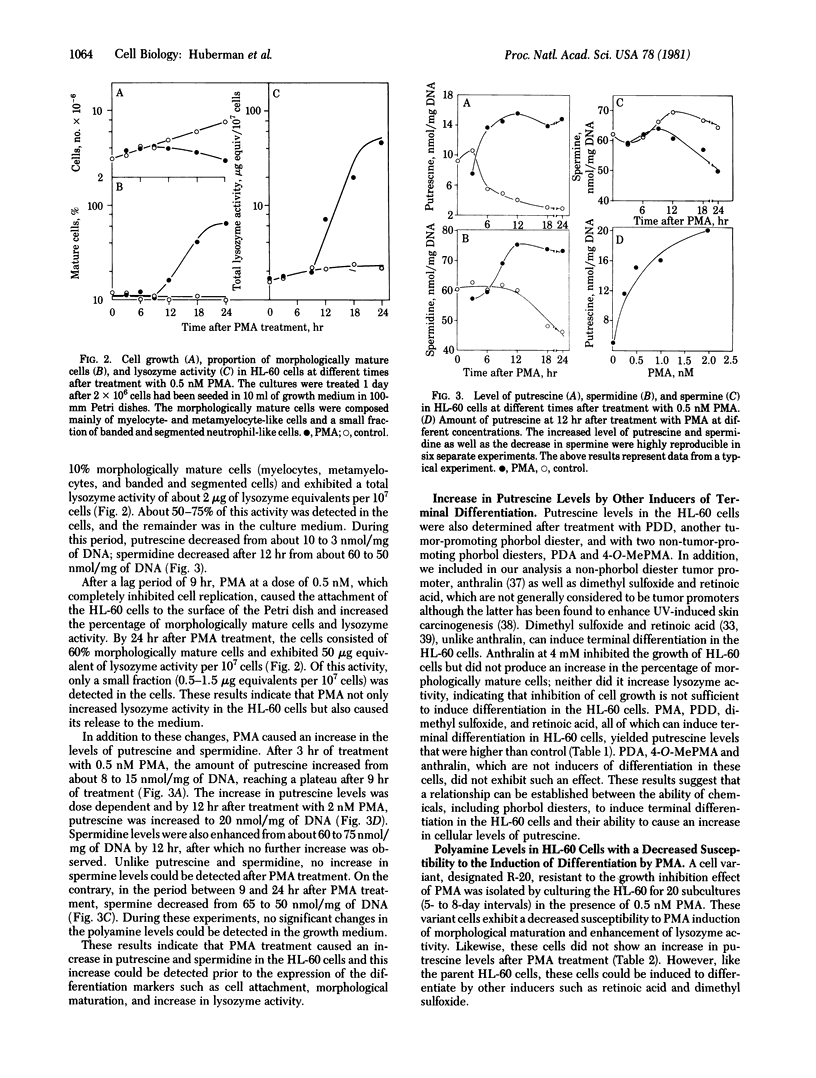
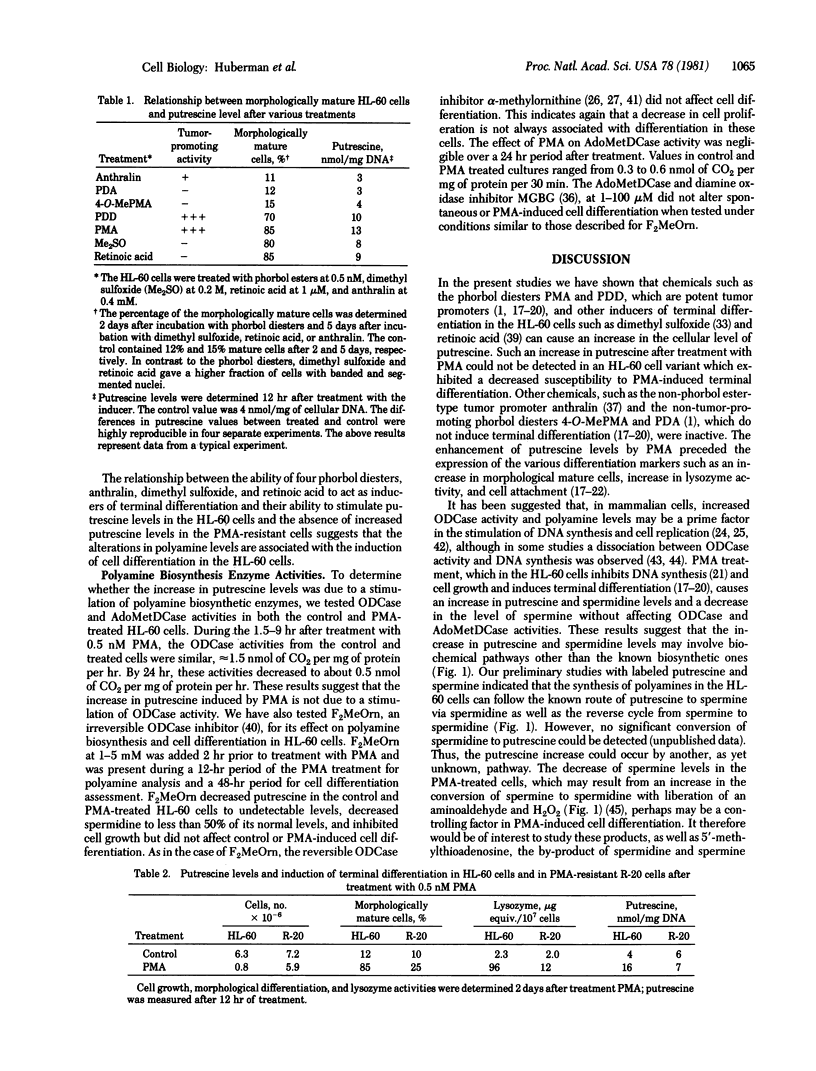
Polyamine levels were evaluated in human HL-60 promyelocytic leukemia cells after treatment with inducers of terminal differentiation. Differentiation in these cells was determined by increases in the percentage of morphologically mature cells and in lysozyme activity. Treatment of the HL-60 cells with phorbol 12-myristate-13-acetate (PMA), phorbol 12,13-didecanoate or other inducers of terminal differentiation such as dimethylsulfoxide and retinoic acid resulted in increased levels of putrescine. However, no increase in putrescine could be detected after PMA treatment of a HL-60 cell variant that exhibited a decreased susceptibility to PMA-induced terminal differentiation. Similarly, no increase in putrescine was observed with two non-tumor-promoters (phorbol 12,13-diacetate and 4-O-methyl-PMA) or with anthralin, a non-phorbol tumor promoter. In addition to enhancing putrescine levels, PMA also increased the amount of spermidine and decreased the amount of spermine. The increase in putrescine and spermidine preceded the expression of the various differentiation markers. Unlike the changes observed in the polyamine levels after PMA treatment, the activities of ornithine and S-adenosylmethionine decarboxylases, which are polyamine biosynthetic enzymes, did not significantly change. α-Methylornithine and α-difluoromethylornithine and methylglyoxal bis(guanylhydrazone), which are inhibitors of the polyamine biosynthetic enzymes, did not affect differentiation in control or PMA-treated cells. Because of these observations, we suggest that the change in polyamine levels involve biochemical pathways other than the known biosynthetic ones. By-products of these pathways may perhaps be the controlling factors involved in the induction of terminal differentiation in the HL-60 and other cell types as well.
Keywords: tumor promoters, putrescine, retinoic acid, anthralin, ornithine decarboxylase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M. M., Newton N. E., Weeks C. E. Inhibitors of polyamine biosynthesis. 1. Alpha-methyl-(plus or minus)-ornithine, an inhibitor of ornithine decarboxylase. J Med Chem. 1974 Apr;17(4):447–451. doi: 10.1021/jm00250a016. [DOI] [PubMed] [Google Scholar]
- Berenblum I. A re-evaluation of the concept of cocarciongenesis. Prog Exp Tumor Res. 1969;11:21–30. doi: 10.1159/000391387. [DOI] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Topper Y. J. Relationships between spermidine, glucocorticoid and milk proteins in different mammalian species. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1131–1135. doi: 10.1016/0006-291x(79)91153-7. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- Emanuelsson H., Heby O. Inhibition of putrescine synthesis blocks development of the polychete Ophryotrocha labronica at gastrulation. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1039–1042. doi: 10.1073/pnas.75.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Forbes P. D., Urbach F., Davies R. E. Enhancement of experimental photocarcinogenesis by topical retinoic acid. Cancer Lett. 1979 Jul;7(2-3):85–90. doi: 10.1016/s0304-3835(79)80100-7. [DOI] [PubMed] [Google Scholar]
- Gazitt Y., Friend C. Polyamine biosynthesis enzymes in the induction and inhibition of differentiation in Friend erythroleukemia cells. Cancer Res. 1980 May;40(5):1727–1732. [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Heckman C., Langenbach R. Stimulation of differentiated functions in human melanoma cells by tumor-promoting agents and dimethyl sulfoxide. Cancer Res. 1979 Jul;39(7 Pt 1):2618–2624. [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Ishii D. N., Fibach E., Yamasaki H., Weinstein I. B. Tumor promoters inhibit morphological differentiation in cultured mouse neuroblastoma cells. Science. 1978 May 5;200(4341):556–559. doi: 10.1126/science.644318. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kasukabe T., Honma Y., Hozumi M. Inhibition of functional and morphological differentiation of cultured mouse myeloid leukemia cells by tumor promoters. Gan. 1979 Feb;70(1):119–123. [PubMed] [Google Scholar]
- Kluge N., Gaedicke G., Steinheider G., Dube S., Ostertag W. Globin synthesis in Friend-erythroleukemia mouse cells in protein- and lipid-free medium. Effects of dimethyl-sulfoxide, iron and erythropoietin. Exp Cell Res. 1974 Oct;88(2):257–262. doi: 10.1016/0014-4827(74)90239-0. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Sachs L. Control of lysozyme induction in the differentiation of myeloid leukemic cells. Cell. 1976 Dec;9(4 Pt 2):675–684. doi: 10.1016/0092-8674(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Willingham M., Pastan I. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1980 May;77(5):2706–2710. doi: 10.1073/pnas.77.5.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti U., Slaga T. J., Ben T., Patterson E., Hennings H., Yuspa S. H. Dissociation of tumor promoter-stimulated ornithine decarboxylase activity and DNA synthesis in mouse epidermis in vivo and in vitro by fluocinolone acetonide, a tumor-promotion inhibitor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3908–3912. doi: 10.1073/pnas.74.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Miao R. M., Filedsteel A. H., Fodge D. W. Opposing effects of tumor promoters on erythroid differentiation. Nature. 1978 Jul 20;274(5668):271–272. doi: 10.1038/274271a0. [DOI] [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. Inhibition of induced differentiation of C3H/10T 1/2 clone 8 mouse embryo cells by tumor promoters. Cancer Res. 1980 Feb;40(2):334–338. [PubMed] [Google Scholar]
- Nagasawa K., Mak T. W. Phorbol esters induce differentiation in human malignant T lymphoblasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2964–2968. doi: 10.1073/pnas.77.5.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu M., Shoji M., Aoki N., Sato S., Miwa M., Sugimura T. Enhancing effect of phorbol esters on induction of differentiation of mouse myeloid leukemia cells by human urinary protein and lipopolysaccharide. Cancer Res. 1979 Nov;39(11):4668–4672. [PubMed] [Google Scholar]
- Newton N. E., Abdel-Monem M. M. Inhibitors of polyamine biosynthesis. 4. Effects of alpha-methyl-(+/-)-ornithine and methylglyoxal bis(guanylhydrazone) on growth and polyamine content of L1210 leukemic cells of mice. J Med Chem. 1977 Feb;20(2):249–253. doi: 10.1021/jm00212a012. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G., Lewis M. A., Diamond L. Ornithine decarboxylase activity and DNA synthesis after treatment of cells in culture with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1979 Nov;39(11):4477–4480. [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis and their specificity for tumor promotion. Cancer Res. 1975 Sep;35(9):2426–2433. [PubMed] [Google Scholar]
- Pacifici M., Holtzer H. Effects of a tumor-promoting agent on chondrogenesis. Am J Anat. 1977 Sep;150(1):207–212. doi: 10.1002/aja.1001500116. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Olashaw N., Meo P. Terminal differentiation in human promyelocytic leukaemic cells in the absence of DNA synthesis. Nature. 1980 Mar 6;284(5751):69–70. doi: 10.1038/284069a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Soprano K. J., Baserga R. Reactivation of ribosomal RNA genes in human-mouse hybrid cells by 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1566–1569. doi: 10.1073/pnas.77.3.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Hansen G., Woodcock J., Metcalf D. Colony stimulating factor and the regulation of granulopoiesis and macrophage production. Fed Proc. 1975 Dec;34(13):2272–2278. [PubMed] [Google Scholar]
- Stuart R. K., Hamilton J. A. Tumor-promoting phorbol esters stimulate hematopoietic colony formation in vitro. Science. 1980 Apr 25;208(4442):402–404. doi: 10.1126/science.6245446. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Ishida H., Takano T., Suzuki F. Polyamine and differentiation: induction of ornithine decarboxylase by parathyroid hormone is a good marker of differentiated chondrocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1481–1485. doi: 10.1073/pnas.77.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duuren B. L., Segal A., Tseng S. S., Rusch G. M., Loewengart G., Maté U., Roth D., Smith A., Melchionne S., Seidman I. Structure and tumor-promoting activity of analogues of anthralin (1,8-dihydroxy-9-anthrone). J Med Chem. 1978 Jan;21(1):26–31. doi: 10.1021/jm00199a005. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Primary amines inhibit recycling of alpha 2M receptors in fibroblasts. Cell. 1980 May;20(1):37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- Vorbrodt A., Meo P., Rovera G. Regulation of acid phosphatase activity in human promyelocytic leukemic cells induced to differentiate in culture. J Cell Biol. 1979 Nov;83(2 Pt 1):300–307. doi: 10.1083/jcb.83.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks C. E., Slaga T. J. Inhibition of phorbol ester-induced polyamine accumulation in mouse epidermis by anti-inflammatory steroid. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1488–1496. doi: 10.1016/0006-291x(79)91233-6. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Lichti U., Ben T., Patterson E., Hennings H., Slaga T. J., Colburn N., Kelsey W. Phorbol esters stimulate DNA synthesis and ornithine decarboxylase activity in mouse epidermal cell cultures. Nature. 1976 Jul 29;262(5567):402–404. doi: 10.1038/262402a0. [DOI] [PubMed] [Google Scholar]



