Background: The ubiquitin ligase membrane-associated RING-CH-1 (MARCH1) regulates expression of key immune molecules.
Results: MARCH1 induces endocytosis and degradation of CD86 by ubiquitination of multiple lysine residues in CD86.
Conclusion: Functional MARCH1-CD86 interaction occurs despite poor sequence conservation in the relevant domains of CD86.
Significance: MARCH1 substrates are recruited via transmembrane domain-mediated interactions, permitting substrate ubiquitination despite poor conservation in cytosolic domains.
Keywords: Antigen Presentation, Dendritic Cells, E3 Ubiquitin Ligase, Endocytosis, Ubiquitination
Abstract
The activation of naïve T cells requires antigen presentation by dendritic cells (DCs), and the process of antigen presentation is regulated over the course of DC maturation. One key aspect of this regulation is the cell surface up-regulation upon DC maturation of peptide·MHC-II complexes and the costimulatory molecule CD86. It is now clear that these critical induction events involve changes in ubiquitin-dependent trafficking of MHC-II and CD86 by the E3 ligase membrane-associated RING-CH-1 (MARCH1). Although ubiquitin-dependent trafficking of MHC-II has been well characterized, much less is known regarding the post-transcriptional regulation of CD86 expression. Here, we examined the physical and functional interaction between CD86 and MARCH1. We observed that CD86 is rapidly endocytosed in the presence of MARCH1 followed by lysosome-dependent degradation. Furthermore, we found that the association between CD86 and MARCH1 was conferred primarily by the transmembrane domains of the respective proteins. In contrast to MHC-II, which has a single, conserved ubiquitin acceptor site in the cytosolic domain, we found that multiple lysine residues in the cytosolic tail of CD86 could support ubiquitination consistent with the relative lack of sequence conservation across species within the CD86 cytosolic domain. These findings suggest that MARCH1 recruits multiple substrates via transmembrane domain-mediated interactions to permit substrate ubiquitination in the face of diverse cytosolic domain sequences.
Introduction
DCs3 play a central role in the priming of naïve CD4 and CD8 T cells during adaptive immune responses (1–4). Optimal activation of naïve T cells is dependent on the maturation state of DCs with mature DC (mDCs) being the most potent stimulators of T cells (2, 5). The maturation of DCs is regulated by many factors, including pathogen-associated molecular patterns, detected primarily by receptors of the Toll-like receptor and the nucleotide-binding domain, leucine-rich repeat-containing protein families (6, 7). Immature DC (iDCs) have poor T cell-stimulating capacity because of low surface expression of MHC-I and -II as well as the costimulatory molecules CD80 and CD86 relative to mDCs (4, 8). It is now apparent that ubiquitination has a major role in regulating antigen presentation by affecting the trafficking of MHC-II and CD86 in iDCs. Indeed, the E3 ubiquitin ligase MARCH1 suppresses the cell surface display of these proteins (9–11), and MARCH1 expression is negatively regulated during DC maturation (9, 12, 13).
DCs from MARCH1-deficient mice are defective at antigen presentation and cytokine production (11). Although clearly important, much remains to be learned about the properties and functions of MARCH1, including its impact on costimulation through CD86. Many assumptions regarding the function of MARCH1 have been inferred from studies on its homologs, virus-encoded E3 ligases of the K3 family. In fact, it was early studies on these E3 ligases of the herpesvirus and poxvirus families (e.g. mK3, kK3, kK5, M153R, etc.) that led to the identification of MARCH genes in mammalian genomes (14, 15). The viral K3 molecules mediate immune evasion by affecting the cell surface expression of multiple immune regulatory molecules through ubiquitin-dependent mechanisms; these substrates include MHC-I and -II, ICAM-1, and CD86 among others. K3/MARCH family members are structurally similar as each is membrane-anchored and possesses an N-terminal RING-CH (C4HC3) domain that is necessary for E2 recruitment and thus ubiquitination (16–20). In terms of domain organization, substrate recruitment, and overall function, the viral K3 molecules are relatively well understood, whereas much less is known about the 11 members of the MARCH family (18–20).
A distinctive feature of viral K3 family molecules is their ability to recruit diverse substrates. The murine γ-herpesvirus-68 protein mK3, an endoplasmic reticulum-resident E3 ligase (21–25), utilizes the MHC-I peptide-loading complex to recruit MHC-I to the RING-CH domain of mK3 for ubiquitination on the MHC-I cytosolic tail in a manner that is largely independent of primary amino acid sequence (22, 26). Kaposi sarcoma-associated herpesvirus E3 ligases kK3 and kK5 mediate ubiquitin-dependent internalization of MHC-I (and other molecules) from the cell surface for sorting to lysosomes for degradation (27–31). No clear recognition motif has been found that is directly responsible for substrate targeting by kK3 and kK5, although substrate specificity partly depends on the location of the ubiquitin acceptor residues along the cytosolic tail of the substrate relative to the E3 ligase (32, 33). This might explain the large substrate repertoire of kK3 and kK5 (34–37). For MHC-II, the single, conserved lysine residue within the cytosolic tail is necessary for MARCH1-mediated targeting (38–40). By contrast, CD86 shows relatively poor sequence conservation in its cytosolic domain.
Despite the unique requirement for CD86 in many immune responses (41, 42), its biogenesis is not well described in contrast to the wealth of information on MHC-II trafficking. The sorting motifs within the invariant chain direct newly synthesized MHC-II molecules from the trans-Golgi network to luminal vesicles of late endosomes either directly or indirectly through transient trafficking to the plasma membrane (8, 43, 44). In iDCs, MHC-II is ubiquitinated via MARCH1 after invariant-chain processing, and this ubiquitination causes degradation of MHC-II within lysosomes (39, 40, 45). DC maturation with Toll-like receptor ligands results in a decrease in MARCH1 mRNA and protein levels (9, 12, 13) and a corresponding decrease in ubiquitination of MHC-II (10, 39, 40).
Much like MHC-II, CD86 is expressed at low levels on the surface of iDCs (46), is promptly induced at the surface following Toll-like receptor signaling (47), and is up-regulated in MARCH1-deficient mice (11). Here, we examined the impact of MARCH1 on CD86 biogenesis and explored the mechanisms that determine specificity of MARCH1 for CD86. Our data indicate that CD86 is rapidly internalized in cells expressing MARCH1, leading to lysosome-dependent degradation. Furthermore, detailed mapping studies revealed that simple sequence determinants were insufficient to explain specificity in the functional interaction between ligase and substrate. Rather our findings suggest that MARCH1 can functionally interact with multiple diverse substrates.
EXPERIMENTAL PROCEDURES
Mice
4–6-week old B7.2−/− (CD86) mice (C57BL/6 background) were obtained from The Jackson Laboratory and were housed in the animal facility at the University of Arizona. All animal experiments were done under the approval and supervision of the Institutional Animal Care and Use Committee.
Antibodies and Reagents
Mouse anti-HA antibody (clone 6E2) and rabbit anti-HA antibody (clone C29F4) were purchased from Cell Signaling Technology. Fluorochrome-conjugated rat anti-CD86 (clone GL1) and hamster anti-mouse CD80 (clone 16-10A1) were obtained from R&D Systems. GL1 and 16-10A1 hybridomas were obtained from the American Type Culture Collection (ATCC), and culture supernatants were used as a source of unconjugated antibodies. Mouse anti-H2-Ld mAb 30-5-7 hybridoma was a gift from Dr. Ted Hansen (Washington University School of Medicine). Anti-β-actin antibody (ACTN05) was obtained from Abcam. Anti-mouse CD11c (clone N418), rat anti-MHC-II (M5/114.15.2), and rat anti-CD86 (PO.3) antibodies were purchased from BD Biosciences. Donkey anti-rat DyLight 488 and donkey anti-rat Cy3 secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. Goat anti-rat Alexa Fluor 488 secondary antibody was purchased from Invitrogen. Mouse anti-ubiquitin antibody (clone P4D1) was purchased from Santa Cruz Biotechnology. Bafilomycin A (used at 0.1 μm) was obtained from Alexis Biochemicals (now part of Enzo Life Sciences). N-Glycosidase F and endoglycosidase H were purchased from New England Biolabs. GM-CSF and IL-4 (used at 10 ng/ml each) and IFN-γ (used at 100 units/ml) were purchased from Peprotech. Lipopolysaccharide (LPS) O26:B6 was purchased from Sigma and used at 100 ng/ml.
Cell Lines
Mouse-derived embryonic fibroblast cell lines WT3 (H2b) or 3KO (β2m−/−, Kb−/−, Db−/−) have been described (22). The mouse-derived DC line DC2.4 was obtained from Dr. Kenneth Rock (University of Massachusetts Medical Center), and the mouse DC-derived line MJDC was generated in our laboratory (48). Bone marrow-derived DCs (BMDCs) were generated from CD86-deficient mice (C57BL/6 background) by culturing bone marrow cells with GM-CSF and IL-4 (10 ng/ml each) for 6 days as described (48). All cell lines and primary cells were cultured in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum (FBS; Hyclone), 1 mm HEPES (Invitrogen), 2 mm l-glutamine, 0.1 mm nonessential amino acids, 1 mm sodium pyruvate, and 100 units/ml penicillin/streptomycin (all from Mediatech).
DNA Constructs
Bicistronic retroviral or lentiviral vectors were used to express all cDNA constructs in this study. These vectors have all been described elsewhere (26, 48). Murine MARCH1 cDNA (corresponding to isoform 2; accession number NM_001166375.1 in GenBank) was cloned from C57BL/6 spleen cDNA and tagged at the N terminus with the HA epitope (48). MARCH1 mutants used in this study have also been described (48). The murine CD80 and CD86 cDNAs were cloned by RT-PCR from C57BL/6 BMDC cDNA and confirmed by DNA sequence analysis. Site-directed mutagenesis using a QuikChange XL mutagenesis kit (Stratagene) was used to generate all of the point mutants in this study. Chimeras between CD86 and CD80 were generated by standard PCR or fusion PCR. The amino acid numbering for the domains of the chimeras is given in the legend to Fig. 5. Chimeras comprising human β2m fused to the murine CD86 transmembrane and cytosolic regions, chimeras generated from H2-Ld ectodomain and human CD86 transmembrane and cytosolic domains, and chimeras consisting of the human β2m ectodomain plus the tapasin transmembrane region and cytosolic tail have been described elsewhere (24, 26).
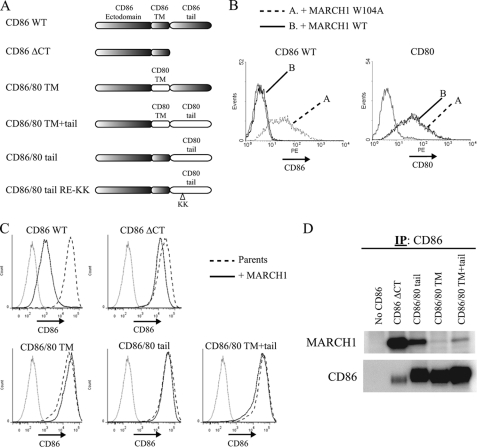
FIGURE 5.
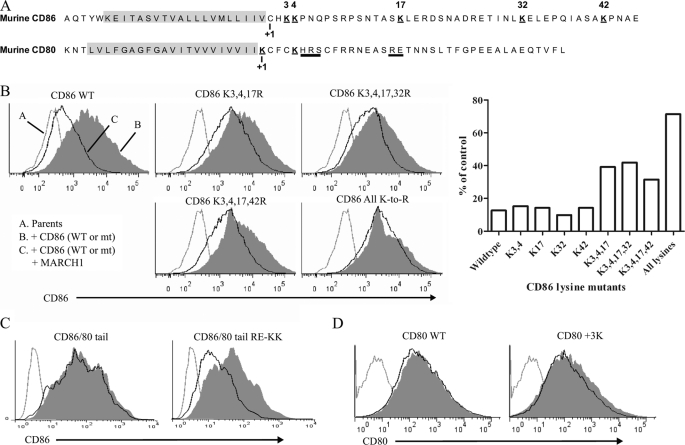
Mapping domains of CD86 required for sensitivity to MARCH1. A, map indicating the domains of various CD86/CD80 chimeric molecules. CD86.ΔCT corresponds to residues 1–245; CD86/80 TM corresponds to residues 1–222 of CD86, 212–233 of CD80, and 241–286 of CD86; CD86/80 tail corresponds to residues 1–241 of CD86 and 233–270 of CD80; CD86/80 TM+tail corresponds to residues 1–222 of CD86 and 212–270 of CD80. B, fibroblast cells (WT3) were co-transfected with bicistronic vectors co-expressing MARCH1 (WT or W104A) and GFP as a reporter and either CD86 or CD80. Gray histograms represent CD86 staining (background) of the parental WT3 cell line. Histograms are gated on GFP+ (MARCH1-expressing) cells. C, flow cytometry was used to analyze the surface expression of CD86 on the panel of CD86/CD80 chimeras after stable expression of each construct following transduction of WT3 cells with retroviral vectors. Gray-colored histograms represent isotype control staining. D, the indicated chimeric molecules were precipitated from WT3 cells co-expressing MARCH1 and blotted as indicated. IP, immunoprecipitation. PE, phycoerythrin.
Transfections and Transductions
Transfection was done using FuGENE 6 (Roche Applied Science) according to the manufacturer's protocol. To generate stable lines expressing MARCH1 and its mutant forms or substrate molecules (such as CD86), PlatE ecotropic packaging cells (49) were seeded in a 6-well plate and transfected with retroviral vectors. 12–24 h post-transfection, the medium was replaced with fresh, complete RPMI 1640 medium, and 48 h post-transfection, supernatants containing virus particles were harvested, passed through 0.45-μm filters, and used to infect target cells (DC2.4, MJDC, WT3, or 3KO) as described (26). 48 h postinfection, drug selection was performed for at least 1 week. Stable cell lines were maintained in complete RPMI 1640 medium along with their corresponding selection drug.
Expression of CD86 or CD86 with a deletion of the cytosolic domain (ΔCT) in BMDCs was accomplished using lentiviral bicistronic vectors as described previously (48). Briefly, packaging cells (293T cells) were transfected with ViraPower packaging plasmids (Invitrogen) and our lentiviral vectors. 48 h post-transfection, virus supernatants were collected in Optiseal tubes (Beckman Coulter) and concentrated by ultracentrifugation at 50,000 × g for 2 h at 4 °C (48). Virus pellets were resuspended in complete medium at of the starting volume to generate 10× virus stocks, which were used fresh or stored at −80 °C. BMDCs were infected for 3 days using concentrated virus, left untreated, or treated with 100 ng/ml LPS for 18 h and stained with anti-CD86 antibody for flow cytometry.
Immunoprecipitation, SDS-PAGE, and Immunoblotting
Cell lysates were prepared using 1% IGEPAL CA-630 (Nonidet P-40) (from Sigma) dissolved in either Dulbecco's phosphate-buffered saline (D-PBS) or in 50 mm Tris, 150 mm NaCl (TBS), supplemented with 0.3–1 mm PMSF, 20 mm iodoacetamide, 10 μm MG132 (all from Sigma), and protease mixture inhibitor set III (Calbiochem). Following centrifugation to remove nuclei, supernatants were collected, and protein concentrations were quantified using the BCA assay (Thermo Scientific). For co-immunoprecipitation, cell lysates were prepared using 1% digitonin (Wako) or 1% IGEPAL in either D-PBS or TBS. Prior to immunoprecipitation, preclearing of lysates on protein A- (GE Healthcare) or protein G (Sigma)-Sepharose beads was performed for at least 1 h. Precleared lysates were incubated with protein A or protein G beads prebound to antibody for at least 4 h. Beads were washed with 0.1% digitonin or 0.1% IGEPAL, and samples were boiled in LDS sample buffer (Invitrogen) with or without 2-mercaptoethanol (1% final concentration). For endoglycosidase H treatment, immunoprecipitates were eluted by boiling in 10 mm Tris-Cl (pH 6.8), 0.5% SDS. Eluted samples were mixed with an equal volume of 100 mm sodium acetate (pH 5.4), separated (equal volumes) into two samples that were left undigested or digested with 1 milliunit of endoglycosidase H at 37 °C for 1 h. N-Glycosidase F treatment was performed according to the supplied protocol from New England Biolabs. Briefly, immunoprecipitates were eluted in 1× Glycoprotein Denaturing Buffer. Eluted samples were digested in the presence of 50 mm sodium phosphate (pH 7.5) and 1% Nonidet P-40 buffer with 15 milliunits (International Union of Biochemistry) of N-glycosidase F.
Samples were separated by SDS-PAGE using either 7 or 3–8% NuPAGE Tris acetate or 10 or 4–12% Bis-Tris polyacrylamide gels (Invitrogen) and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore). Membranes were blocked for 30 min in 5% milk dissolved in D-PBS with 0.1% Tween, 0.01% SDS. Membranes were incubated in primary antibody solutions at 4 °C with rocking overnight. Membranes were washed three times in 0.1% Tween, 0.01% SDS in D-PBS and then incubated for 1 h in the appropriate secondary antibodies (biotinylated or HRP-conjugated). For biotinylated secondary antibodies, HRP-conjugated streptavidin (Zymed Laboratories Inc.) was added to the membrane for 1 h. Membranes were then incubated in SuperSignal West Femto ECL reagent (Thermo Scientific) and visualized using a ChemiDoc XRS (Bio-Rad) digital imaging system.
Pulse-Chase Labeling
DC2.4 cells with or without MARCH1 constructs (107 cells/ml) were preincubated for 30 min in Cys- and Met-free medium (Sigma) containing 5% dialyzed fetal calf serum (Invitrogen). Cells were then labeled with Expre35S35S [35S]Cys/Met labeling mixture (PerkinElmer Life Sciences) at 200 μCi/ml for 30 min. The chase was initiated with the addition of medium containing label-free Met and Cys (5 mm), and samples were taken at appropriate time points. Where indicated, bafilomycin A was added at 0.1 μm. Immunoprecipitation and N-glycosidase F treatment were performed from lysates of labeled cells as described previously (26). Samples were then separated by SDS-PAGE and transferred to Immobilon-P PVDF membranes. After a 10-min methanol fixation, membranes were dried and exposed to BioMAX-MR film (Eastman Kodak Co.). Quantitation of the band intensities from autoradiographs was done using ImageJ software, and plots were generated using GraphPad Prism software.
Flow Cytometry
After harvesting cells, staining was done in staining buffer (1% BSA, 0.1% sodium azide in D-PBS). Fc receptor blocking was performed in some cases (primarily for DCs) using anti-CD16/32 antibody (clone 2.4G2; BD Biosciences or as hybridoma supernatants). Cells were incubated on ice for 30 min in primary antibody diluted in staining buffer. For unlabeled primary antibody, appropriate fluorochrome-conjugated secondary antibody was added, and cells were incubated on ice for at least 30 min. Cells were then washed, resuspended in 1× D-PBS, and fixed with an equal volume of 1% paraformaldehyde (in D-PBS). Stained cells were analyzed using a FACSCalibur cytometer or an LSRII cytometer (BD Biosciences), and data were analyzed using WinMidi (The Scripps Research Institute) or FlowJo (Treestar).
Immunofluorescence and Confocal Microscopy
DC2.4 cells +/− HA-tagged MARCH1 were plated on poly-d-lysine Cellware (BD Biosciences) and incubated at 37 °C with 5% CO2 overnight. Where indicated, cells were treated with 0.1 μm bafilomycin A. Cells were then washed with PBS and fixed in 4% paraformaldehyde for 20 min. Free aldehydes were quenched with 50 mm ammonium chloride for 12 min and rinsed with PBS. Blocking and permeabilization were completed using 10% FBS in PBS with 0.05% saponin (Sigma) for 1 h. Primary antibody was diluted in blocking solution and incubated with the cells overnight at 4 °C with rocking. Cells were washed with PBS for 1 h. Secondary antibodies were diluted in blocking solution and incubated with cells at room temperature with rocking. Coverslips were mounted on slides using ProLong Gold (Invitrogen). Cells were examined using z-sectioning on a Zeiss 510 Meta confocal microscope at 63× with frame averaging of 4.
Statistical Analysis
Where indicated in the figure legends, two-tailed Student's t-tests were performed to determine statistical significance (p < 0.05).
RESULTS
MARCH1 Induces Rapid Cell Surface Loss of CD86
Previous studies have shown that overexpression of MARCH1 leads to the down-regulation of surface CD86 (15). In addition, knock-down of MARCH1 in human monocyte-derived DCs or knock-out of MARCH1 in mice resulted in an increase in cell surface CD86 levels on resting antigen-presenting cells (9, 11). Although these studies clearly demonstrate that the surface expression of CD86 is affected by MARCH1, the mechanisms by which MARCH1 affects this key costimulator are unknown. Therefore, we examined the regulation of CD86 expression by MARCH1.
The surface expression of CD86, MHC-II, and CD80 was analyzed using flow cytometry on two DC cell lines (MJDC and DC2.4 (48, 50)). As shown in Fig. 1A, MARCH1 expression decreased the steady-state surface levels of MHC-II and CD86, whereas CD80 levels were unaffected. To explore the mechanism for this down-regulation, we next asked whether the expression of MARCH1 induced rapid internalization of CD86. Cell surface CD86 on DC2.4 cells +/− MARCH1 was labeled with unconjugated anti-CD86 antibody at 4 °C. Labeled cells were then incubated at 37 °C for various times. Cells from each time point were placed directly on ice, then labeled with fluorochrome-conjugated secondary antibody, fixed, and analyzed by flow cytometry. As shown in Fig. 1B, CD86 was progressively lost from the surface of the MARCH1-expressing cells over the course of 2 h. To determine whether MARCH1 affected the initial endocytosis of CD86 from the cell membrane, a similar assay was performed but with short (3-min) time intervals. In the presence of MARCH1, CD86 decreased from the surface within the first 3 min of the assay (Fig. 1C) consistent with rapid endocytosis of CD86 induced by MARCH1.
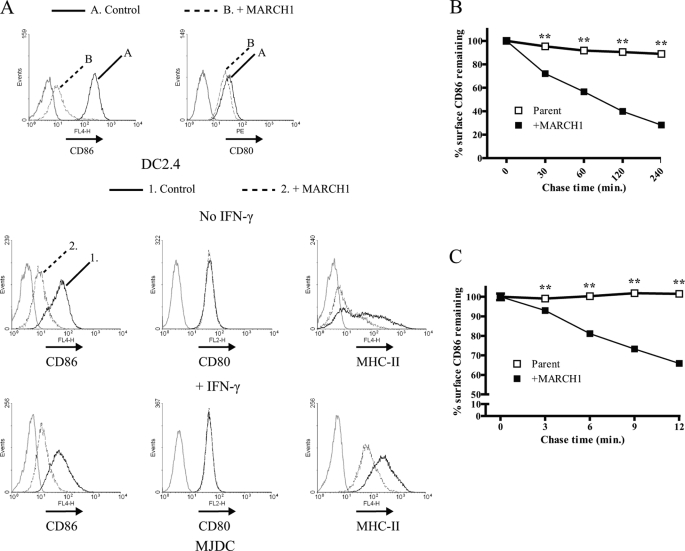
FIGURE 1.
Rapid loss of cell surface CD86 in presence of MARCH1. A, flow cytometry was used to examine the surface levels of CD80 and CD86 on DC2.4 cells +/− MARCH1 (top row) and CD80, CD86, and MHC-II on MJDC cells +/− MARCH1 (middle and bottom rows). In some cases, MJDC cells were treated with 100 units/ml IFN-γ for 18 h prior to staining to increase MHC-II synthesis. Gray histograms represent staining using an isotype control antibody. Control shown for DC2.4 cells represents staining of the parental cell line, whereas Control for MJDC cells represents staining of cells transduced with the empty retroviral vector. All histograms for MJDC were gated on GFP+ (either vector-only or MARCH1-expressing) cells. B and C, DC2.4 cells +/− MARCH1 were stained with unlabeled CD86 antibody (GL1) at 4 °C for 30 min. After washing, cells were incubated in culture medium at 37 °C for the indicated time points and then transferred to ice. Cells were then incubated with a fluorochrome-conjugated secondary antibody, washed with D-PBS, fixed with 1% paraformaldehyde, and analyzed by flow cytometry. For each time point, the mean fluorescence intensity (MFI) value for CD86 surface expression (of triplicate samples) was normalized relative to the mean fluorescence intensity value for time 0 and plotted as percent (%) surface CD86 remaining ±S.E. (**, p < 0.01). Internalization data are representative of at least three independent experiments each for B and C; error bars are not apparent on the graphs because their ranges are smaller than the symbols used. PE, phycoerythrin.
CD86 Ubiquitination and Association with MARCH1
Studies have shown that MARCH1 associates with and ubiquitinates MHC-II (9, 10, 51). Therefore, we examined the potential physical and functional interaction between MARCH1 and CD86. We began by analyzing the effects of MARCH1 on the steady-state levels of CD86 in DC2.4 cells +/− MARCH1 or a mutant of MARCH1 (W104A) incapable of E2 recruitment (30, 48, 52). In Fig. 2A, we show that the steady-state levels of glycosylated CD86 (with a molecular mass range between 60 and 80 kDa) were decreased significantly in the presence of wild type MARCH1, yet the levels were unchanged in the presence of the MARCH1 W104A mutant. Where indicated, cells were treated with bafilomycin A (Baf A), an inhibitor of lysosome acidification, which is known to stabilize MARCH1 (48). We observed that in the presence of Baf A the steady-state levels of MARCH1 wild type (WT) do increase, but the CD86 levels are not appreciably changed in this time frame. The fact that an intact RING-CH domain is required for the drop in CD86 levels strongly suggests a ubiquitin-dependent effect of MARCH1 on CD86.
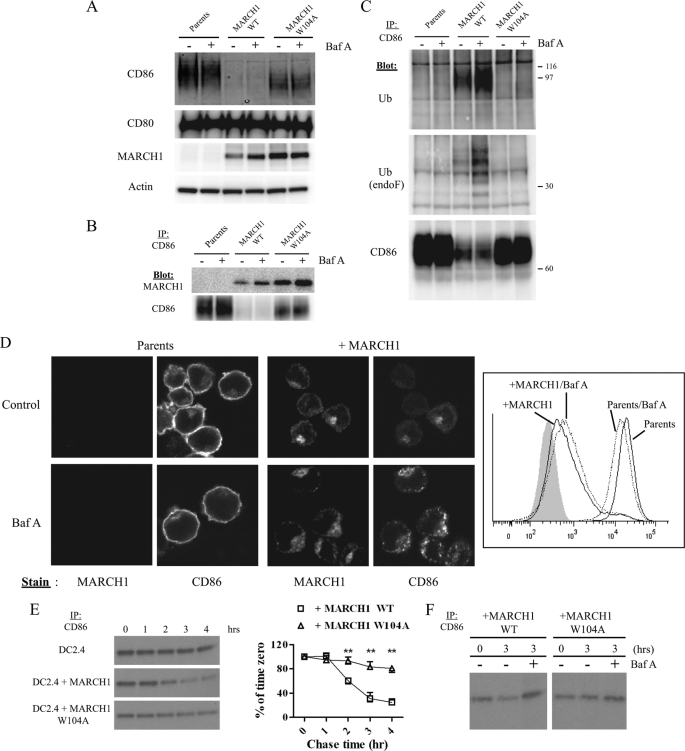
FIGURE 2.
MARCH1 associates with CD86 and promotes ubiquitination and turnover. A, whole cell lysates from DC2.4 cells +/− MARCH1 WT or MARCH1 W104A were immunoblotted for MARCH1, CD86, CD80, and actin (loading control). Baf A treatment (0.1 μm) for 3 h was also performed where indicated. B, CD86 was immunoprecipitated from digitonin lysates of DC2.4 cells (+/− MARCH1 WT or W104A), and samples were resolved by SDS-PAGE and blotted for MARCH1 and CD86. C, CD86 was immunoprecipitated (IP) from cell lysates of DC2.4 cells +/− MARCH1 WT or W104A. Precipitates were untreated (upper panel) or N-glycosidase F (endoF)-treated (middle panel) and then immunoblotted for ubiquitin (Ub) and CD86 (lower panel; non-treated samples). D, DC2.4 cells +/− MARCH1-HA were either left untreated or treated with 0.1 μm Baf A for 3 h. cells were then stained using indirect double label immunofluorescence with rabbit anti-HA tag (6E2) and rat anti-CD86 (GL-1). Antigens were visualized by confocal microscopy, and images shown represent a single optical section (left panels). Detector settings were identical for all images, and images shown are representative of three independent experiments. The right panel shows flow cytometric analysis of surface CD86 expression of cells treated as above. E, DC2.4 cells +/− MARCH1 WT or W104A were pulse-labeled with [35S]Met/Cys and chased for the indicated time points with unlabeled Cys/Met. CD86 immunoprecipitation was performed from cell lysates followed by N-glycosidase F treatment, SDS-PAGE, and autoradiography. The signal intensity for each band at each chase time point was determined and normalized to the signal intensity value of the band corresponding to time 0 and plotted as percentage (%) of time 0. The graph represents data from three replicates (±S.E.; **, p < 0.01). F, pulse-chase was performed as in D with the exception that cells were also treated with Baf A (0.1 μm) during the chase period. The results shown are representative of three independent experiments.
Next, we performed co-immunoprecipitation experiments to determine whether MARCH1 associates with CD86, and we observed that MARCH1 WT does associate with CD86. Additionally, the W104A mutant also associated with CD86 (Fig. 2B). Note that the association between MARCH1 W104A and CD86 was more pronounced because MARCH1 W104A is somewhat more stable than MARCH1 WT, and CD86 levels are much higher in the presence of this mutant (48). Finally, as shown in Fig. 2C, CD86 is ubiquitinated in the presence of MARCH1 WT as indicated by the anti-ubiquitin blot of the CD86 immunoprecipitates. The presence of extensive N-linked glycosylation makes it difficult to clearly resolve the ubiquitinated CD86 molecules. Therefore, we treated CD86 immunoprecipitates with N-glycosidase F (endoglycosidase F) to remove all N-linked sugars. The N-glycosidase F treatment revealed a distinct polyubiquitin ladder on CD86 uniquely in the presence of MARCH1 WT (Fig. 2C). Additionally, CD86 immunoprecipitates were treated with endoglycosidase H, which removes high mannose N-glycans from endoplasmic reticulum-resident molecules; we found no significant downward shift in the ubiquitinated CD86 signal, indicating that CD86 ubiquitination by MARCH1 occurs post-endoplasmic reticulum (data not shown). Consistent with this finding, treatment of cells with the inhibitor of lysosome acidification Baf A increased the extent of CD86 ubiquitination (Fig. 2C). It is noteworthy that the cell surface levels of CD86 were not restored in MARCH1-expressing cells treated with Baf A. Instead, confocal microscopy revealed that such treatment resulted in an accumulation of intracellular CD86 (Fig. 2D). These data suggest that ubiquitination of CD86 leads to its rapid endocytosis (Fig. 1) followed by lysosomal degradation; however, inhibiting degradation of ubiquitin-tagged CD86 does not rescue its surface expression. Rather these molecules appear to be retained within endocytic compartments.
Kinetics of CD86 Turnover in Presence of MARCH1
To determine the effect of MARCH1-mediated ubiquitination on CD86, we analyzed the degradation kinetics of CD86 in DC2.4 cells +/− MARCH1 and MARCH1 W104A. Cells were pulsed for 30 min with [35S]Met/Cys and then chased with unlabeled Met/Cys for the indicated times. CD86 was immunoprecipitated, precipitates were treated with N-glycosidase F, and samples were resolved by SDS-PAGE followed by radiography. As shown in Fig. 2E (left panels), CD86 was degraded in the presence of MARCH1 WT but not in cells expressing MARCH1 W104A. Loss of the CD86 signal is apparent at time points >1 h of chase consistent with a role for lysosomes in degradation. Therefore, we performed pulse-chase experiments similar to those above except that Baf A was added during the chase in some cases, and samples were collected after 3 h. In untreated cells expressing MARCH1, CD86 levels decreased as expected, but CD86 levels persisted at 3 h in Baf A-treated cells (Fig. 2F). As a control for these experiments, we examined the effect of MARCH1 on CD80 biogenesis, and in contrast to CD86, the stability of CD80 was similar in the presence or absence of MARCH1 (data not shown). Overall, these experiments indicate that MARCH1 affects the biogenesis of CD86 via ubiquitin-dependent lysosomal degradation.
Domains of MARCH1 Required for Association with CD86
We used a structure-function approach to illuminate the interaction between MARCH1 and CD86. Previously, we generated several mutants of MARCH1 (including ΔN1–121, ΔC257–279, and ΔC229–279; schematized in Fig. 3A) and analyzed the effects of each mutant on the surface expression of CD86. In cells expressing MARCH1-ΔC257–279, the surface expression of CD86 showed a modest decrease relative to the decrease observed with MARCH1 WT. Conversely, in cells expressing either MARCH1-ΔN1–121 or MARCH1-ΔC229–279, MARCH1 function was almost completely abolished with respect to surface expression of CD86 (48). Here, we examined whether these effects are due to a lack of association between CD86 and the MARCH1 mutants. To begin, we confirmed the expression and reported functional capabilities of these mutants in DC2.4 stable cell lines by immunoblot for steady-state levels of CD86 and MARCH1. The effect of each mutant on the steady-state levels of CD86 was consistent with its reported effects on the surface expression of CD86 (Fig. 3A, Ref. 48, and described above). Next, we utilized the same MARCH1 mutants to define the domains necessary for MARCH1 association with CD86 by co-immunoprecipitation. Analysis of cells expressing MARCH1-ΔN1–121 and MARCH1-ΔC257–279 showed that these mutants do associate with CD86. In addition, the association of MARCH1-ΔC229–279 with CD86 was weaker but still detectable; this MARCH1 mutant has the lowest steady-state levels of the mutants used here. These data suggest that MARCH1 association with CD86 relies primarily on the transmembrane (TM) domains of MARCH1.
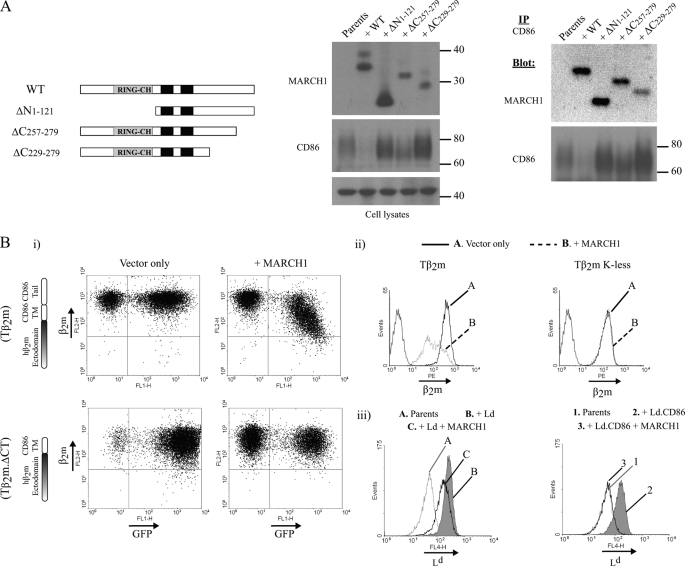
FIGURE 3.
Domains of MARCH1 and CD86 that mediate their interaction. A, co-immunoprecipitation (IP) of CD86 was performed using lysates from DC2.4 parent cells or cells stably expressing MARCH1 WT, ΔN1–121, ΔC229–279, or ΔC257–279. Whole cell lysates (middle panels) and CD86 precipitates (right panels) were blotted for CD86 and MARCH1. Note that for MARCH1 ΔN1–121, one-third of the CD86 precipitate was loaded relative to MARCH1 WT to achieve comparable signal intensity; this mutant is significantly more stable than WT. B, flow cytometry was used to examine the surface expression of the indicated chimeras in the presence of MARCH1. Panel i, a fibroblast cell line derived from β2m−/− Kb−/− Db−/− mice (3KO cells) +/− MARCH1 (GFP+) were transduced with the chimeras consisting of the human β2m (ectodomain) fused to the transmembrane and cytosolic regions of murine CD86 (Tβ2m) or with a β2m chimera lacking the cytosolic tail of CD86 (Tβ2m.ΔCT). Panel ii, 3KO cells expressing MARCH1 were transduced with either Tβ2m or Tβ2m lysineless (K-less) (lysine-to-arginine mutation of all five lysines in the tail of mouse CD86). Panel iii, DC2.4 cells were transduced with MARCH1 and either with H2-Ld or a chimera comprising the H2-Ld ectodomain fused to the transmembrane and cytosolic regions of human CD86. PE, phycoerythrin.
CD86 Domains Involved in MARCH1 Interaction
We next explored the determinants of the substrate, CD86, that are required for sensitivity to MARCH1. We made use of a chimeric molecule, named transmembrane human β2m (Tβ2m), which comprises soluble human β2m appended to the TM and cytosolic domains of murine CD86. We initially developed Tβ2m for the study of substrate selection by the viral E3 ligase mK3, and these studies showed that this molecule was readily expressed at the cell surface (26). Furthermore, we previously generated a mutant of Tβ2m lacking the cytosolic tail, Tβ2m.ΔCT. Here, we used these molecules to determine whether the TM and cytosolic domains of CD86 would confer sensitivity to MARCH1 when both proteins were expressed in β2m-deficient fibroblasts (3KO). 3KO cells stably expressing either Tβ2m or Tβ2m.ΔCT were transduced with a MARCH1-expressing retrovirus (with a bicistronic GFP reporter). As shown in Fig. 3B, panel i, the surface expression of Tβ2m was decreased in cells expressing MARCH1; this down-regulation by MARCH1 required the CD86-derived cytosolic tail of Tβ2m.
It has been shown that many viral E3 ligases require lysine residues as ubiquitin acceptor sites in the cytosolic tail of the substrate. However, E3 ligases such as kK3 and kK5 are capable of mediating ubiquitination on cysteine residues, and mK3 can utilize serine and threonine residues as ubiquitin acceptor sites (32, 53). To test the sequence requirements for MARCH1-mediated ubiquitination of CD86, we examined whether lysine residues in the tail of Tβ2m (corresponding to the tail of CD86) affected sensitivity to MARCH1. As shown in Fig. 3B, panel ii, Tβ2m lacking all five lysines in the cytosolic tail was resistant to MARCH1 despite the presence of other non-canonical acceptor residues (see sequences in Fig. 6A). These data confirm the findings of Cadwell and Coscoy (32), indicating that MARCH1-mediated targeting relies on the presence of lysines exclusively. In addition, we determined whether MARCH1 was able to down-regulate a chimeric substrate containing human CD86 sequences. Here, we used Ld.hCD86 comprising the H2-Ld (MHC-I) ectodomain fused to the TM and cytosolic domains of human CD86 (24). Both Ld.hCD86 and full-length H2-Ld were expressed in DC2.4 +/− MARCH1 and analyzed by flow cytometry to determine the effect of MARCH1 on surface expression of each molecule. MARCH1 had minimal effect on full-length Ld surface expression, whereas Ld.hCD86 surface expression was reduced (Fig. 3B, panel iii). These results show that the transmembrane and tail regions of CD86 are sufficient to confer sensitivity to heterologous substrates consistent with other data that have been reported for CD86 and MHC-II (32, 35, 38). Interestingly, human and mouse CD86 exhibit poor sequence conservation in the TM and tail regions (supplemental Fig. S1), but both are targeted by mouse MARCH1. This suggests that MARCH1 substrate recruitment is not based on simple recognition motifs.
FIGURE 6.
Multiple lysine residues in cytosolic domain of CD86 can support MARCH1-mediated ubiquitination. A, single letter representation of the TM (highlighted in gray) and the cytosolic domain sequences of murine CD80 and CD86. Numbers below each sequence represent the predicted start of the cytosolic region. Lysine residues are indicated (and numbered for CD86), and bars under the CD80 sequence show the location for two blocks of residues that were mutated to lysines (HRS in CD80 3K and RE in CD86/CD80 tail RE-KK). B, comparison of CD86 cytosolic domain lysine mutants for sensitivity to MARCH1. The numbering indicated above each histogram represents the position of lysine residues mutated in the CD86 cytosolic tail relative to the predicted end of the CD86 transmembrane domain. WT3 cells were co-transfected with the indicated CD86 constructs and a bicistronic GFP reporter vector (+/− MARCH1). Flow cytometry was used to examine the surface expression of each CD86 construct +/− MARCH1. All histograms are gated on GFP-expressing cells. The most relevant mutants are shown; plots from all mutants tested are shown in supplemental Fig. S2. The right panel represents a graph of the data from the plots shown here and in supplemental Fig. S2. The geometric mean fluorescence intensity of CD86 staining for each sample was used to determine the percentage (%) of control. This was calculated for each CD86 mutant by comparing the mean fluorescence intensity (MFI) of that mutant +/− MARCH1 as follows: ((+MARCH1 MFI)/(no MARCH1 MFI)) × 100. Data are representative of three independent experiments. C, similar to B with CD86/80 tail construct (see diagram in Fig. 5) with RE mutated to KK (see sequence map in A). D, similar to B with CD80 WT or CD80 construct with three lysine residues added to its cytosolic tail at positions 6, 7, and 8 (underlined in A). In C and D, constructs were co-transfected into WT3 cells +/− MARCH1. Histograms are gated on GFP+ (MARCH1-expressing) cells. mt, mutant.
Transmembrane and Cytosolic Regions of CD86 Are Necessary for Association with and Ubiquitination by MARCH1
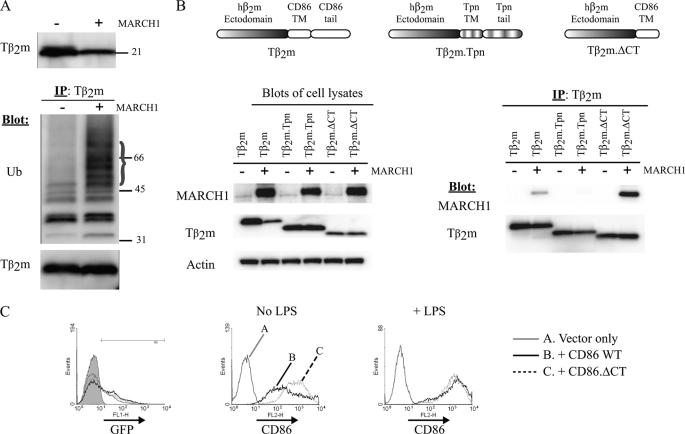
We tested whether the decrease in the surface expression of Tβ2m correlates with MARCH1-dependent ubiquitination. The steady-state levels of Tβ2m were decreased in the presence of MARCH1, and immunoprecipitation of Tβ2m followed by immunoblotting with an anti-ubiquitin antibody revealed a clear increase in ubiquitinated Tβ2m in the presence of MARCH1 (Fig. 4A). Next, we examined the association of MARCH1 with Tβ2m. As a control for the association experiments, we utilized a variant of Tβ2m called Tβ2m.Tpn (tapasin). This latter chimera comprises soluble human β2m appended to the TM and cytosolic domains of murine tapasin (26), which permits association with the Transporter associated with Antigen Processing complex (due to the tapasin TM domain) and also contains a potent endoplasmic reticulum retention signal in the tail (26). As shown in Fig. 4B, co-immunoprecipitation data revealed an association between MARCH1 and Tβ2m but not between MARCH1 and Tβ2m.Tpn. Thus, association with MARCH1 can be conferred by the TM and tail domains of CD86. To determine whether the cytosolic tail of CD86 was required for association with MARCH1, we utilized the Tβ2m.ΔCT molecule and found that it associated strongly with MARCH1 (Fig. 4B). These data indicate that the TM domain alone is sufficient to confer association with MARCH1.
FIGURE 4.
Transmembrane and cytosolic regions of CD86 confer association with MARCH1, leading to ubiquitination. A, top panel, cell lysates from 3KO cells expressing MARCH1 and Tβ2m were blotted to reveal the steady-state levels of Tβ2m. Bottom panel, Tβ2m precipitates were resolved by SDS-PAGE and blotted for ubiquitin (Ub) and Tβ2m. B, blots of lysates from 3KO cells expressing Tβ2m, Tβ2m.ΔCT, and Tβ2m.Tpn were probed with the indicated antibodies (left panel). Right panel, the indicated chimeras were precipitated from 3KO cells co-expressing MARCH1 and blotted as indicated. C, bone marrow-derived DCs generated from CD86−/− mice were infected using lentivirus vectors expressing either GFP (vector only), GFP and CD86 WT, or GFP and CD86.ΔCT (lacking the cytosolic region of CD86). 3 days postinfection, cells were harvested and either left untreated (No LPS) or treated with 100 ng/ml LPS for 18 h. Flow cytometry was used to analyze the surface expression of CD86. The histogram on the left represents GFP expression upon which the CD86 histograms are gated. Data are representative of two independent experiments. IP, immunoprecipitation.
To extend these findings to a setting of physiological expression of MARCH1, we examined the expression levels of wild type CD86 and CD86 lacking the cytosolic tail (CD86.ΔCT) in BMDCs from CD86−/− mice. Lentivirus vectors were used to transduce CD86−/− BMDCs with either CD86 or CD86.ΔCT expression constructs. Following transduction, cells were left untreated or treated with LPS for 18 h to induce DC maturation. We reasoned that in immature cells CD86.ΔCT surface levels should be higher than CD86 WT because the cytosolic tail is required for targeting by MARCH1. Furthermore, after maturation, the surface levels of both substrates should be similar because MARCH1 levels are greatly decreased after LPS treatment (9, 12, 13). As expected, CD86.ΔCT was expressed at higher levels in iDCs than CD86 WT, and this difference was abolished in cells matured with LPS (Fig. 4C). Although this experiment does not formally prove that MARCH1 was responsible for the lower expression of wild type CD86 in iDCs, these results are consistent with that interpretation.
Additional Substrate Sequence Requirements for MARCH1 Sensitivity
To further define the relative roles of the TM versus cytosolic domains of CD86 necessary for a functional interaction with MARCH1, we generated various chimeras between CD86 and CD80; although homologs, only CD86 is down-regulated by MARCH1. The chimeras generated are summarized in Fig. 5A. All of them contain the CD86 ectodomain, which was appended in various combinations with TM or tail domains from either CD80 or CD86 as indicated. As a control, we first analyzed the effects of MARCH1 on wild type CD86 and CD80 by flow cytometry after transient transfection of the respective molecules in fibroblasts. As expected, CD86 expression was significantly decreased in the presence of MARCH1 WT, whereas CD80 was resistant; both molecules were resistant to MARCH1 W104A (Fig. 5B, upper panels).
To compare the CD86/CD80 chimeras, each was stably expressed in fibroblasts and analyzed for the effects of MARCH1 on cell surface expression. Replacement of the TM and/or cytosolic domains of CD86 with those from CD80 abolished sensitivity to MARCH1 as did complete deletion of the cytosolic domain (Fig. 5C). When we examined the association of the CD86/CD80 chimeras with MARCH1, we found notable differences among the constructs. The chimeras that retained the CD86 TM domain displayed a relatively robust association with MARCH1 (Fig. 5D). The chimeras that contained the CD80 TM or CD80 TM+tail displayed very weak association with MARCH1, especially considering that these CD80 TM-containing molecules were expressed at the highest levels of all the chimeras (see CD86 blot). Nonetheless, we tested the possibility that CD80 may interact with MARCH1 by co-immunoprecipitation. In some experiments, a weak interaction could be observed, but the results were inconsistent (not shown). Thus, we conclude that MARCH1 associates much more strongly with CD86 than CD80, and this interaction is principally dependent on the CD86 TM domain. It should be noted that CD86 WT was omitted from these comparisons because the amount of MARCH1 that co-precipitates with WT CD86 is low due to the very low amounts of CD86 in these cells as a consequence of CD86 degradation. However, MARCH1 can clearly be co-isolated with WT CD86 (Figs. 2C and 3A for example).
One possible explanation for the inability of the CD80 tail to support ubiquitination when appended to the CD86 TM domain could be the issue of lysine presence and location. The location of lysine residues on substrates of RING-CH E3 ligases has been shown to be critical for targeting (33). An alignment of murine CD80 and CD86 revealed a notable lack of lysine residues in the tail of CD80 relative to CD86 as well as overall low similarity (Fig. 6A and supplemental Fig. S1). To determine the importance of lysine location in the tail of CD86 for MARCH1 recognition, we mutated lysine residues in the tail of wild type CD86 singly and in combination. Unlike MHC-II, which has a single, conserved lysine in the tail (38–40) (supplemental Fig. S1), there are multiple lysine residues in the tail of CD86 (five in murine CD86; Fig. 6A and supplemental Fig. S1). Site-directed mutagenesis was used to generate lysine-to-arginine mutations within the CD86 tail domain, and then each mutant was tested for sensitivity to cell surface down-regulation by MARCH1 following transient transfection. Mutation of any single lysine residue in the CD86 tail did not affect sensitivity to MARCH1. In fact, only when we combined various lysine mutations did we observe some resistance to MARCH1 (Fig. 6B and supplemental Fig. S2), suggesting that MARCH1 can ubiquitinate lysine residues at multiple positions within the tail of CD86. Simultaneous mutation of lysines 3, 4, and 17 of the CD86 tail either alone or with lysine 32 or 42 resulted in molecules that were less sensitive to MARCH1. This indicates that MARCH1 has a preference for lysine residues more proximal to the TM domain.
Previous studies on Kaposi sarcoma-associated herpesvirus-encoded kK5 showed that although wild type CD80 is resistant to kK5 function addition of lysine residues to the tail of CD80 led to kK5-mediated targeting of CD80 (35). Accordingly, we added two lysine residues to the CD80 tail region of the CD86/CD80 tail chimeric molecule, which was resistant to MARCH1 (see Fig. 5B). The resulting mutant, denoted as CD86/CD80 tail RE-KK, exhibited some sensitivity to MARCH1, indicating that the presence of these lysine residues could support ubiquitination by MARCH1 in the context of the CD86 TM and CD80 cytosolic tail sequences (Fig. 6C). This result along with the association data in Fig. 5 raised the question of whether the resistance of wild type CD80 to MARCH1 could be explained by lysine placement in its cytosolic domain. Because lysine residues close to the TM domain of CD86 are preferentially targeted by MARCH1 (positions 3, 4, and 17 for example), we added lysine residues near the TM domain of wild type CD80 (at positions 6, 7, and 8). This mutant version of CD80 remained resistant to MARCH1 (Fig. 6D), indicating that lysine placement alone is not the determinant by which MARCH1 is able to discriminate between CD80 and CD86.
DISCUSSION
It is important to understand the properties and functions of MARCH1 because it is critical to DC biology (11). MARCH1 down-regulates the surface expression of MHC-II and CD86, but most of our knowledge of MARCH1-substrate interactions comes from studies of MHC-II (9, 10, 12, 38–40, 51). Here, we demonstrate that MARCH1 associates with and ubiquitinates CD86, promoting its down-regulation from the cell surface. The rate of CD86 loss from the cell surface in the presence of MARCH1 is most consistent with a role for ubiquitin in the initial endocytic event with CD86. Subsequently, ubiquitinated CD86 is routed to lysosomes and degraded. This differs somewhat from MHC-II where its initial internalization from the cell surface was found to be independent of MARCH1 and ubiquitination (45). Indeed, in the absence of MARCH1, MHC-II was still rapidly internalized in iDCs (45); a similar observation was made in B cells (10). In the case of MHC-II, however, the β-chain contains its own endocytosis-promoting sequence motif (54) as does the invariant chain (55). These signals may obviate the need for ubiquitin-driven endocytosis with MHC-II.
For CD86, ubiquitination appears to promote endocytosis and direct CD86 to lysosomes where it cannot be efficiently retrieved to the cell surface even when lysosomal degradation is inhibited. In regard to the mechanism of CD86 endocytosis, we did not observe a consistent effect of the drug dynasore, a dynamin inhibitor (56), on this rapid endocytosis (data not shown). Dynasore was reported to affect the ability of MARCH1 to down-regulate MHC-II expression, although the drug-induced rescue was relatively modest (9). Thus, the steps in the endocytosis of CD86 subsequent to ubiquitination are unclear, but our findings suggest that CD86 and MHC-II have distinct requirements for endocytosis.
We have examined the interaction between MARCH1 and CD86 from the perspective of each molecule. MARCH1 interaction with CD86 appears to depend primarily on the MARCH1 TM domains with a possible contribution of the membrane-proximal region of the C-terminal domain. MARCH1 may either 1) bind directly to CD86 or 2) bind to unidentified cofactors that help to recruit substrates for MARCH1. In regard to the features of CD86 that are required to interact physically and functionally with MARCH1, we found that the TM region is necessary to promote association with MARCH1 and that the cytosolic tail of CD86 is necessary for ubiquitination. In fact, association and ubiquitination/down-regulation were separable as in the case of the CD86/CD80 tail chimera (Fig. 5, C and D). Conversely, weak/minimal association with MARCH1, such as in the case of the CD86/CD80 TM molecule, did not support down-regulation even though this molecule has the CD86 cytosolic tail. Our results suggest that TM interactions between MARCH1 and its substrates play an essential role in determining sensitivity similar to what has been demonstrated for the kK3 and kK5 (35, 57), mK3 (24), and MARCH9 (58).
An additional factor contributing to CD86 sensitivity to MARCH1 is the position of lysine residues in the cytosolic tail of CD86. Although lysine residues are critical for down-regulation (Figs. 4 and 6), MARCH1 is capable of utilizing multiple lysine residues. There appears to be some preference for lysine residues closer to the TM domain, which is similar to the location of the sole lysine residue in the tail of MHC-II molecules. The relatively flexible position requirement for MARCH1 targeting of CD86 fits with the lack of conservation in the cytosolic domains of CD86 molecules among species (supplemental Fig. S1).
Overall, our findings argue against a simple “recognition motif” by which MARCH1 selects its substrates, suggesting that other mechanisms impart specificity. Indeed, because MARCH family proteins have been shown to target multiple, unrelated substrates (10, 11, 14, 15, 34, 58–60), it is reasonable to postulate that substrate selectivity might depend on adaptor/accessory molecules that properly position these E3 ligases for ubiquitination of acceptor residues on substrates within cellular membranes. It is perhaps relevant that MHC-II and CD86 co-cluster at the plasma membrane of DCs prior to engagement with T cells (61), which is well known to result in clustering of immune molecules (62, 63). In the case of MHC-II and CD86, non-random distribution at the cell membrane has been shown to involve interactions with glycolipid-enriched microdomains (64) and members of the tetraspanin family (63, 65, 66). Thus, it may be that mechanisms exist to segregate MARCH1 into discrete membrane domains (at the cell surface and/or within endosomes) together with its substrates. Along these lines, we offer the following model. CD86 is recruited to MARCH1 through interactions involving the TM domains of both molecules perhaps by direct interaction or via indirect mechanisms involving accessory proteins and/or shared affinity of both molecules for the same membrane microdomains. Regardless, the interaction serves to orient the cytosolic tail of the substrate properly with respect to the RING-CH domain of MARCH1 in a manner that permits ubiquitination with a minimal requirement for specific sequence context surrounding the ubiquitin acceptor residue.
This work was supported, in whole or in part, by National Institutes of Health Grants AI060723 and AI080756.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- DC
- dendritic cell
- MARCH
- membrane-associated RING-CH
- mDC
- mature DC
- iDC
- immature DC
- BMDC
- bone marrow-derived DC
- β2m
- β2-microglobulin
- Tβ2m
- transmembrane human β2m
- D-PBS
- Dulbecco's phosphate-buffered saline
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Baf A
- bafilomycin A
- TM
- transmembrane
- Tpn
- tapasin.
REFERENCES
- 1. Heath W. R., Carbone F. R. (2009) Nat. Immunol. 10, 1237–1244 [DOI] [PubMed] [Google Scholar]
- 2. Steinman R. M., Witmer M. D. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 5132–5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ueno H., Klechevsky E., Morita R., Aspord C., Cao T., Matsui T., Di Pucchio T., Connolly J., Fay J. W., Pascual V., Palucka A. K., Banchereau J. (2007) Immunol. Rev. 219, 118–142 [DOI] [PubMed] [Google Scholar]
- 4. Banchereau J., Steinman R. M. (1998) Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 5. De Smedt T., Pajak B., Muraille E., Lespagnard L., Heinen E., De Baetselier P., Urbain J., Leo O., Moser M. (1996) J. Exp. Med. 184, 1413–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawai T., Akira S. (2010) Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 7. Davis B. K., Wen H., Ting J. P. (2011) Annu. Rev. Immunol. 29, 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trombetta E. S., Mellman I. (2005) Annu. Rev. Immunol. 23, 975–1028 [DOI] [PubMed] [Google Scholar]
- 9. De Gassart A., Camosseto V., Thibodeau J., Ceppi M., Catalan N., Pierre P., Gatti E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3491–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuki Y., Ohmura-Hoshino M., Goto E., Aoki M., Mito-Yoshida M., Uematsu M., Hasegawa T., Koseki H., Ohara O., Nakayama M., Toyooka K., Matsuoka K., Hotta H., Yamamoto A., Ishido S. (2007) EMBO J. 26, 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohmura-Hoshino M., Matsuki Y., Mito-Yoshida M., Goto E., Aoki-Kawasumi M., Nakayama M., Ohara O., Ishido S. (2009) J. Immunol. 183, 6893–6897 [DOI] [PubMed] [Google Scholar]
- 12. Young L. J., Wilson N. S., Schnorrer P., Proietto A., ten Broeke T., Matsuki Y., Mount A. M., Belz G. T., O'Keeffe M., Ohmura-Hoshino M., Ishido S., Stoorvogel W., Heath W. R., Shortman K., Villadangos J. A. (2008) Nat. Immunol. 9, 1244–1252 [DOI] [PubMed] [Google Scholar]
- 13. Walseng E., Furuta K., Goldszmid R. S., Weih K. A., Sher A., Roche P. A. (2010) J. Biol. Chem. 285, 41749–41754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goto E., Ishido S., Sato Y., Ohgimoto S., Ohgimoto K., Nagano-Fujii M., Hotta H. (2003) J. Biol. Chem. 278, 14657–14668 [DOI] [PubMed] [Google Scholar]
- 15. Bartee E., Mansouri M., Hovey Nerenberg B. T., Gouveia K., Früh K. (2004) J. Virol. 78, 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coscoy L., Ganem D. (2003) Trends Cell Biol. 13, 7–12 [DOI] [PubMed] [Google Scholar]
- 17. Lehner P. J., Hoer S., Dodd R., Duncan L. M. (2005) Immunol. Rev. 207, 112–125 [DOI] [PubMed] [Google Scholar]
- 18. Nathan J. A., Lehner P. J. (2009) Exp. Cell Res. 315, 1593–1600 [DOI] [PubMed] [Google Scholar]
- 19. Ohmura-Hoshino M., Goto E., Matsuki Y., Aoki M., Mito M., Uematsu M., Hotta H., Ishido S. (2006) J. Biochem. 140, 147–154 [DOI] [PubMed] [Google Scholar]
- 20. Wang X., Herr R. A., Hansen T. (2008) Semin. Cancer Biol. 18, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boname J. M., Stevenson P. G. (2001) Immunity 15, 627–636 [DOI] [PubMed] [Google Scholar]
- 22. Lybarger L., Wang X., Harris M. R., Virgin H. W., 4th, Hansen T. H. (2003) Immunity 18, 121–130 [DOI] [PubMed] [Google Scholar]
- 23. Stevenson P. G., Efstathiou S., Doherty P. C., Lehner P. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8455–8460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X., Lybarger L., Connors R., Harris M. R., Hansen T. H. (2004) J. Virol. 78, 8673–8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X., Ye Y., Lencer W., Hansen T. H. (2006) J. Biol. Chem. 281, 8636–8644 [DOI] [PubMed] [Google Scholar]
- 26. Corcoran K., Wang X., Lybarger L. (2009) J. Biol. Chem. 284, 17475–17487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boname J. M., Thomas M., Stagg H. R., Xu P., Peng J., Lehner P. J. (2010) Traffic 11, 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coscoy L., Ganem D. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8051–8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duncan L. M., Piper S., Dodd R. B., Saville M. K., Sanderson C. M., Luzio J. P., Lehner P. J. (2006) EMBO J. 25, 1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hewitt E. W., Duncan L., Mufti D., Baker J., Stevenson P. G., Lehner P. J. (2002) EMBO J. 21, 2418–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishido S., Wang C., Lee B. S., Cohen G. B., Jung J. U. (2000) J. Virol. 74, 5300–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cadwell K., Coscoy L. (2005) Science 309, 127–130 [DOI] [PubMed] [Google Scholar]
- 33. Cadwell K., Coscoy L. (2008) J. Virol. 82, 4184–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartee E., McCormack A., Früh K. (2006) PLoS Pathog. 2, e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coscoy L., Ganem D. (2001) J. Clin. Investig. 107, 1599–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Q., Means R., Lang S., Jung J. U. (2007) J. Virol. 81, 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mansouri M., Douglas J., Rose P. P., Gouveia K., Thomas G., Means R. E., Moses A. V., Früh K. (2006) Blood 108, 1932–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lapaque N., Jahnke M., Trowsdale J., Kelly A. P. (2009) J. Biol. Chem. 284, 7007–7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shin J. S., Ebersold M., Pypaert M., Delamarre L., Hartley A., Mellman I. (2006) Nature 444, 115–118 [DOI] [PubMed] [Google Scholar]
- 40. van Niel G., Wubbolts R., Ten Broeke T., Buschow S. I., Ossendorp F. A., Melief C. J., Raposo G., van Balkom B. W., Stoorvogel W. (2006) Immunity 25, 885–894 [DOI] [PubMed] [Google Scholar]
- 41. Girvin A. M., Dal Canto M. C., Rhee L., Salomon B., Sharpe A., Bluestone J. A., Miller S. D. (2000) J. Immunol. 164, 136–143 [DOI] [PubMed] [Google Scholar]
- 42. Salek-Ardakani S., Arens R., Flynn R., Sette A., Schoenberger S. P., Croft M. (2009) J. Immunol. 182, 2909–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berger A. C., Roche P. A. (2009) J. Cell Sci. 122, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonifacino J. S., Traub L. M. (2003) Annu. Rev. Biochem. 72, 395–447 [DOI] [PubMed] [Google Scholar]
- 45. Walseng E., Furuta K., Bosch B., Weih K. A., Matsuki Y., Bakke O., Ishido S., Roche P. A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 20465–20470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Inaba K., Witmer-Pack M., Inaba M., Hathcock K. S., Sakuta H., Azuma M., Yagita H., Okumura K., Linsley P. S., Ikehara S., Muramatsu S., Hodes R. J., Steinman R. M. (1994) J. Exp. Med. 180, 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Villadangos J. A., Cardoso M., Steptoe R. J., van Berkel D., Pooley J., Carbone F. R., Shortman K. (2001) Immunity 14, 739–749 [DOI] [PubMed] [Google Scholar]
- 48. Jabbour M., Campbell E. M., Fares H., Lybarger L. (2009) J. Immunol. 183, 6500–6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morita S., Kojima T., Kitamura T. (2000) Gene Ther. 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- 50. Shen Z., Reznikoff G., Dranoff G., Rock K. L. (1997) J. Immunol. 158, 2723–2730 [PubMed] [Google Scholar]
- 51. Thibodeau J., Bourgeois-Daigneault M. C., Huppé G., Tremblay J., Aumont A., Houde M., Bartee E., Brunet A., Gauvreau M. E., de Gassart A., Gatti E., Baril M., Cloutier M., Bontron S., Früh K., Lamarre D., Steimle V. (2008) Eur. J. Immunol. 38, 1225–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joazeiro C. A., Wing S. S., Huang H., Leverson J. D., Hunter T., Liu Y. C. (1999) Science 286, 309–312 [DOI] [PubMed] [Google Scholar]
- 53. Wang X., Herr R. A., Chua W. J., Lybarger L., Wiertz E. J., Hansen T. H. (2007) J. Cell Biol. 177, 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhong G., Romagnoli P., Germain R. N. (1997) J. Exp. Med. 185, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bakke O., Dobberstein B. (1990) Cell 63, 707–716 [DOI] [PubMed] [Google Scholar]
- 56. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 57. Sanchez D. J., Coscoy L., Ganem D. (2002) J. Biol. Chem. 277, 6124–6130 [DOI] [PubMed] [Google Scholar]
- 58. Hoer S., Smith L., Lehner P. J. (2007) FEBS Lett. 581, 45–51 [DOI] [PubMed] [Google Scholar]
- 59. Hör S., Ziv T., Admon A., Lehner P. J. (2009) Mol. Cell. Proteomics 8, 1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ohmura-Hoshino M., Matsuki Y., Aoki M., Goto E., Mito M., Uematsu M., Kakiuchi T., Hotta H., Ishido S. (2006) J. Immunol. 177, 341–354 [DOI] [PubMed] [Google Scholar]
- 61. Turley S. J., Inaba K., Garrett W. S., Ebersold M., Unternaehrer J., Steinman R. M., Mellman I. (2000) Science 288, 522–527 [DOI] [PubMed] [Google Scholar]
- 62. Grakoui A., Bromley S. K., Sumen C., Davis M. M., Shaw A. S., Allen P. M., Dustin M. L. (1999) Science 285, 221–227 [DOI] [PubMed] [Google Scholar]
- 63. Monks C. R., Freiberg B. A., Kupfer H., Sciaky N., Kupfer A. (1998) Nature 395, 82–86 [DOI] [PubMed] [Google Scholar]
- 64. Meyer zum Bueschenfelde C. O., Unternaehrer J., Mellman I., Bottomly K. (2004) J. Immunol. 173, 6119–6124 [DOI] [PubMed] [Google Scholar]
- 65. Engering A., Pieters J. (2001) Int. Immunol. 13, 127–134 [DOI] [PubMed] [Google Scholar]
- 66. Unternaehrer J. J., Chow A., Pypaert M., Inaba K., Mellman I. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]