Background: Cholix toxin (Cholix) is a novel ADP-ribosyl transferase cytotoxin produced by Vibrio cholerae.
Results: Cholix-induced apoptosis is dependent on caspase activation, which is regulated by both mitochondria-dependent and -independent pathways.
Conclusion: Inflammatory caspases and caspase-8 are responsible for both mitochondrial signals and other caspase activation.
Significance: The cell death pathway induced by eEF2-ADP-ribosylation might differ in various cell types.
Keywords: ADP-ribosylation, Apoptosis, Caspase, Mitochondrial Apoptosis, Toxins, Cholix Toxin
Abstract
Cholix toxin (Cholix) is a novel ADP-ribosylating cytotoxin produced by Vibrio cholerae, which utilizes eukaryotic elongation factor 2 as a substrate and acts by a mechanism similar to that of diphtheria toxin and Pseudomonas exotoxin A. First it was found that Cholix-treated HeLa cells exhibited caspase-dependent apoptosis, whereas intestinal cells such as Caco-2, HCT116, and RKO did not. Here we investigated Cholix-induced cell death signaling pathways in HeLa cells. Cholix-induced cytochrome c release into cytosol was initiated by specific conformational changes of pro-apoptotic Bak associated with Bax. Silencing of bak/bax genes or bak gene alone using siRNA significantly suppressed cytochrome c release and caspase-7 activation, but not activation of caspases-3 and -9. Although pretreatment with a caspase-8 inhibitor (Z-IETD-FMK) reduced Cholix-induced cytochrome c release and activation of caspases-3, -7, and -9, cytotoxicity was not decreased. Pretreatment with Z-YVAD-FMK, which inhibits caspase-1, -4, and -5, suppressed not only cytochrome c release, activation of caspase-3, -7, -8, or -9, and PARP cleavage, but also cytotoxicity, indicating that caspase-1, -4, and -5 activation is initiated at an early stage of Cholix-induced apoptosis and promotes caspase-8 activation. These results show that the inflammatory caspases (caspase-1, -4, and -5) and caspase-8 are responsible for both mitochondrial signals and other caspase activation. In conclusion, we showed that Cholix-induced caspase activation plays an essential role in generation of apoptotic signals, which are mediated by both mitochondria-dependent and -independent pathways.
Introduction
Bacterial virulence is often caused by exotoxins that are secreted by pathogenic organisms such as Clostridium botulinum, enterohemorrhagic Escherichia coli, and Vibrio cholerae. Cholera toxin (CT),3 secreted by V. cholerae, is an ADP-ribosyltransferase, which modifies the Gsα subunit in a heterotrimeric G protein, which is required for GTP hydrolysis. This ADP-ribosylation inhibits GTP hydrolysis by the G protein and maintains activation of adenylyl cyclase, which increases cAMP that keeps ion channels open, and thereby stimulates secretion of electrolytes and water, leading to the rice-water stools characteristic of the disease (1). Among more than 200 serogroups of V. cholerae known today, only the O1 and O139 groups produce CTs (2). Although non-O1/non-O139 V. cholerae do not produce CT and are not associated with epidemic diarrhea, some of these organisms are isolated from patients with a variety of extra-intestinal infections (3, 4). According to a recent report, non-O1/non-O139 V. cholerae was revealed to cause bacteremia in cirrhotic patients (5). These reports show involvement of toxins other than CT in V. cholerae disease.
Detailed genomic analysis of V. cholerae diversity shows the presence of the chxA gene encoding Cholix toxin (Cholix) (6, 7). Unlike CT, Cholix catalyzes ADP-ribosylation of eukaryotic elongation factor 2 (eEF2) (8). In addition to Cholix, toxins that ADP-ribosylate eEF2 include diphtheria toxin and exotoxin A (ETA) from Corynebacterium diphtheriae and Pseudomonas aeruginosa, respectively. Diphtheria toxin, ETA, and Cholix ADP-ribosylate diphthamide of eEF2, which is synthesized from a histidine residue by the diphthamide biosynthesis protein (Dph) family in host cells (9). ADP-ribosylation of diphthamide on eEF2 results in inhibition of protein synthesis (10).
Apoptosis, which toxins often induce in targeted cells, is regulated by many factors like caspase activation and mitochondrial pore formation. Caspases play key roles in apoptosis, proliferation, differentiation, and inflammation (11). To date, 11 caspases have been identified (12). Mitochondrial outer membrane permeabilization (MOMP), a major event involved in pore formation, is regulated by Bcl-2 family proteins (13). Bcl-2, Bcl-XL, Bcl-w, and Mcl-1 have anti-apoptotic roles that maintain the integrity of mitochondrial membranes, resulting in cell survival (14). On the other hand, Bak and Bax initiate MOMP in response to some apoptotic stimuli through specific conformational changes, leading to oligomerization on the mitochondrial outer membrane (15). MOMP results in a release into cytosol of pro-apoptotic factors such as cytochrome c, Apaf-1, and Smac.
ETA shows homology to Cholix with a primary structural identity of ∼35% and causes inflammatory responses especially in mouse liver (16). Du et al. reported that in mouse embryo fibroblasts (MEF) ETA inhibits synthesis of anti-apoptotic Bcl-2 family protein Mcl-1 and induces apoptosis, a process dependent on MOMP initiated by pro-apoptotic Bcl-2 family protein Bak (17). The cholix gene is present in many strains of V. cholerae independent of serogroup (7), and Cholix shows cytotoxicity in MEF cells (8). Although Cholix is a potent virulence factor of non-O1/non-O139 V. cholerae disease, little is known about cytotoxicity for human cells. In this study, we show, in HeLa cells, that Cholix-induced cell death was dependent on caspase activation, which is regulated by both mitochondria-dependent and -independent pathways.
EXPERIMENTAL PROCEDURES
Cells and Reagents
Caco-2, HCT116, and RKO cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Sigma) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (FBS-PCSM). HeLa cells were maintained in minimum essential medium Eagle (Sigma) supplemented with FBS-PCSM (FBS-PCSM-EMEM). Cells were grown at 37 °C in a humidified 5% CO2 atmosphere. Non-targeting control siRNA was purchased from Invitrogen, siRNA for Bak (SI00299376) and Bax (SI02661897) from Qiagen, a general caspase inhibitor (Z-VAD-FMK) from BD Biosciences, and caspase-3-specific inhibitor (Z-DEVD-FMK) from Sigma. The other specific inhibitors Z-YVAD-FMK (inhibitor of caspase-1, -4, and -5), Z-IETD-FMK (caspse-8), and Z-LEHD-FMK (caspase-9) were purchased from R&D Systems. For Western blot analysis, anti-cleaved caspase-3 (9661), anti-caspase-6 (9762), anti-cleaved caspase-7 (9491S), anti-cleaved caspase-8 (9496S), anti-cleaved caspase-9 (9501), anti-Bak (3814S), anti-Bax (2772), anti-Bcl-2 (2870), anti-Bcl-XL (2764), anti-Mcl-1 (4572), and anti-cleaved PARP (9542) antibodies were purchased from Cell Signaling Technology. These studies also utilized anti-GAPDH (sc-25778) and anti-cytochrome c (sc-13560) antibodies (Santa Cruz Biotechnology); HRP-conjugated anti-rabbit IgG (7074) and anti-mouse IgG (7076) antibodies (Cell Signaling Technology); anti-Bak (Ab2) (AM04) antibody (Calbiochem); and anti-Bax (clone 3) (OP-43–100UG) antibody (Oncogene).
Preparation of Cholix and Catalytically Inactivated Mutant Cholix(E581A)
To construct an E. coli expression system for Cholix, the cholix gene (1998 bp) from V. cholerae O236 was inserted into pGEX-6P-1 (GE Healthcare) vector encoding glutathione S-transferase (GST) and the PreScission Protease (GE Healthcare) digestion sequence. The cholix gene was amplified by PCR with ExTaq DNA polymerase (Takara Bio) and primer pairs (forward, 5′-GGGAATCCATGGTCGAAGATGAGTTAAACA-3′; reverse, 5′-GCGGCCGCTTATTTCAGTTCATCTTTTCGC-3′), which contain EcoRI and NotI digestion sites in the underlined sequences. The amplified products of the expected size were subcloned into pCR-TOPO vector (Invitrogen). The cholix gene fragment was obtained through digestion by EcoRI and NotI, and ligated into EcoRI-NotI-digested pGEX6P-1 vector using Rapid DNA Ligation Kit (Roche Applied Science). The resultant plasmid, designated as pGEX-6P-1/Cholix, was used for transformation of E. coli strain TOP10 (Invitrogen), sequenced, and then used for transformation of E. coli strain BL21(DE3). Expressed recombinant GST-tagged protein was applied onto glutathione-Sepharose 4B (GE Healthcare), which had been equilibrated with phosphate-buffered saline (PBS). The GST-Cholix fusion protein was retained on the gel after washing with PBS. The purified Cholix proteins were isolated from a flow-through fraction after digestion by PreScission Protease at 4 °C overnight. To replace a catalytic residue Glu-581 with alanine in Cholix, site-directed mutagenesis was conducted with a QuikChange site-directed mutagenesis kit (Stratagene) using primer pairs (sense, GGAGGGGAAGACGCGACTGTCATTGGCTGG; antisense, CCAGCCAATGACAGTCGCGTCTTCCCCTCC) in which underlines show the mutation site. The mutant was designated as Cholix(E581A). After sequence analysis, Cholix(E581A) was expressed and purified using the same method as described above.
Gene Transfection
HeLa cells (1.0 × 105 cells) in a 12-well dish were cultivated overnight and transfected with the indicated concentrations of siRNA for non-targeting control (NC), Bak, or Bax in Lipofectamine RNAiMAX (Invitrogen) transfection reagent for 48 h, following the company's instructions.
Western Blot Analysis
Cells (1 × 105 cell/well) were plated in a 12-well dish 24 h prior to treatment with Cholix or Cholix(E581A) for the indicated times. After treatment, cells were washed with PBS and lysed with lysis buffer (50 mm Tris, pH 7.5, 0.1 m NaCl, 10% glycerol, 1% Triton X-100) on ice for 20 min. The supernatants after centrifugation (15,000 × g for 10 min) were heated at 100 °C for 5 min in SDS-sample buffer (62.5 mm Tris, pH 6.8, 1% SDS, 10% glycerol, 5 mm dithiothreitol, 0.001% bromphenol blue) and then analyzed by SDS-PAGE. The separated proteins were transferred to PVDF membranes. After blocking with 5% skim milk-TTBS (10 mm Tris, pH 7.6, 0.14 m NaCl, 0.1% Tween 20) for 30 min and washing with TTBS three times for 5 min, the membranes were incubated with primary antibodies for 1 h at room temperature or overnight at 4 °C, washed with TTBS three times, incubated with corresponding HRP-linked secondary antibodies (anti-rabbit or -mouse IgG) at room temperature for 1 h, washed with TTBS three times, and finally incubated in Super Signal West Pico mixture (Thermo Scientific). HRP-bound protein bands were visualized by using Las-1000 (Fuji Film).
Cell Viability Assay
Cells (1 × 104 cells/well) were treated with the indicated concentrations of Cholix or Cholix(E581A) for 24 h. Cell viability was determined by Cell Counting Kit (Dojindo), which detects living cells based on colorimetric quantification of NADH, according to the manufacturer's instructions.
Detection of Cytochrome c Release from Mitochondria
To evaluate cytochrome c release from mitochondria into cytosol, cytosolic fraction was obtained as described in the previous report (18). Cytochrome c in cytosolic fractions was detected by Western blotting.
Immunoprecipitation
Cholix-induced conformational changes of Bak or Bax were detected by immunoprecipitation using anti-Bak (Ab2) or anti-Bax (clone 3) antibodies according to the previous report (19). Immunocomplexes were collected by incubation with rProtein G Agarose (Invitrogen) for 1 h, followed by centrifugation (15,000 × g at 4 °C for 1 min). After the immunocomplexes were washed with lysis buffer three times, proteins were dissolved in SDS-sample buffer, applied to polyacrylamide gels, transferred to PVDF membranes, and then analyzed by Western blotting.
RESULTS
Cytotoxicity of Cholix in Intestinal and Cervical Cells
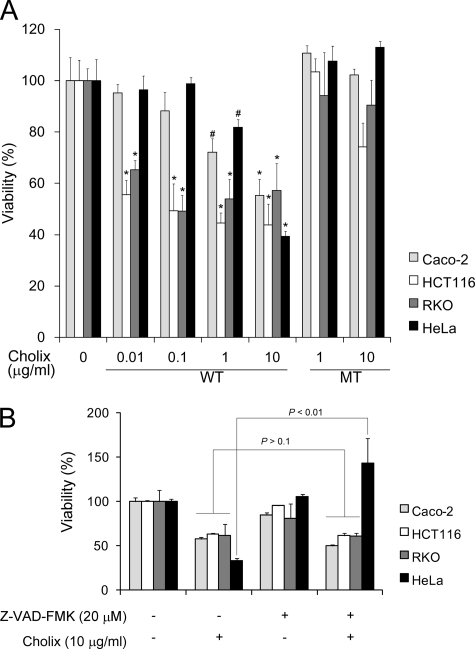
To determine cytotoxicity of Cholix, human intestinal cells (Caco-2, HCT116, and RKO) or HeLa cells were treated with Cholix or catalytically inactivated mutant Cholix(E581A) for 24 h (Fig. 1A). Cholix-treated cells showed decreased viability in a dose-dependent manner. As expected, Cholix(E581A)-treated cells behaved similarly to controls. Next, we examined whether the cytotoxicity in these cells was caspase-dependent or not (Fig. 1B). Although pretreatment with a general caspase inhibitor (Z-VAD-FMK) significantly repressed Cholix-induced HeLa cell death, pretreatment did not affect cytotoxicity for Caco-2, HCT116, or RKO cells. DNA fragmentation was observed in Cholix-treated HeLa cells by TUNEL assay (data not shown). These data suggest that Cholix induces caspase-dependent apoptosis in HeLa cells.
FIGURE 1.
Effects of Cholix on cell viability. A, cells (1 × 104 cells/well) in a 96-well dish were cultured for 48 h and then incubated with indicated concentrations of wild-type or mutant Cholix for 24 h. Cell viability was determined using a Cell Counting kit. Data are the means ± S.D. value from three separate experiments. Student's t test was used for comparisons with the untreated cells. #, p < 0.1 and *, p < 0.01, respectively. B, effects of pretreatment with a general caspase inhibitor on cytotoxicity of Cholix. Cells (1 × 104 cells/well) in a 96-well dish were grown for 48 h and then pretreated with 20 μm general caspase inhibitor (Z-VAD-FMK) for 40 min, followed by incubation with Cholix (10 μg/ml) in the presence or absence of Z-VAD-FMK. Cell viability was determined as described above. Data are the means ± S.D. value from three separate experiments.
Cholix Induces Caspase Activation and Cytochrome c Release
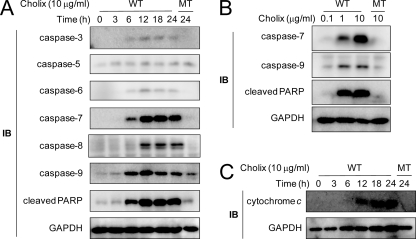
As we showed in Fig. 1, Cholix induced apoptotic HeLa cell death. We next investigated activation of caspases in Cholix-treated HeLa cells. Activation of caspase-3, -6, -7, -8, and -9 was observed after incubation with Cholix for 12–18 h (Fig. 2A). In addition, PARP cleavage was also detected after 6-h treatment. In contrast, Cholix(E581A) did not induce caspase activation. The amounts of cleaved caspases 7 and 9 and PARP increased in the presence of Cholix in a dose-dependent manner (Fig. 2B). Cholix also induced cytochrome c release from mitochondria into cytosol after 12-h incubation (Fig. 2C). Our findings show that, in HeLa cells, Cholix induced pore formation in mitochondrial membranes, leading to cytochrome c release into cytosol.
FIGURE 2.
Induction of apoptosis by Cholix. A, Cholix treatment induced cleavage of caspases and PARP. Cells (1 × 105 cells/well) in a 12-well dish were grown for 24 h and then incubated with wild-type (WT) or mutant (MT) Cholix (10 μg/ml) for the indicated times. Lysates were collected from cells and resolved by immunoblotting (IB) to detect the indicated caspase cleavage or PARP cleavage. GAPDH was used as a loading control. B, cells were incubated with WT or MT Cholix at different concentrations for 18 h and analyzed for induction of caspase-7, caspase-9, or PARP cleavage. C, HeLa cells were incubated with WT or MT Cholix (10 μg/ml) for 18 h, and then the cytosolic fraction was collected as described previously (18). Cytochrome c release into cytosol was detected using an anti-cytochrome c antibody. Data are representative of three separate experiments.
Cholix Induces Bak/Bax Conformational Changes and Loss of Mcl-1
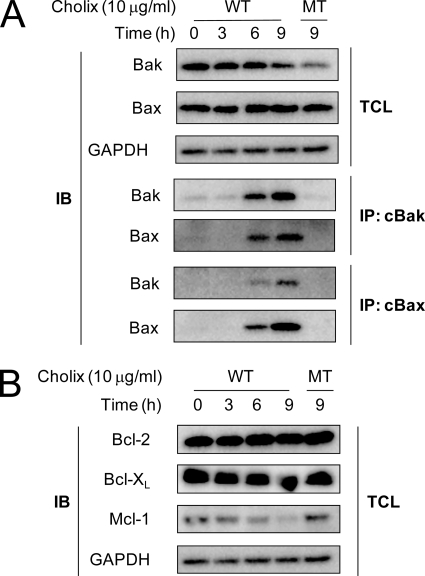
MOMP is a major event leading to mitochondrial pores; it is induced by pro-apoptotic Bcl-2 family proteins Bak or Bax, which undergo specific conformational changes in response to pro-apoptotic stimuli and assemble on the mitochondrial outer membrane by oligomerization (15). We examined whether Cholix induces Bak or Bax conformational changes in HeLa cells. Protein expression levels of Bak and Bax did not change after Cholix treatment for 9 h (Fig. 3A). By immunoprecipitation analysis using conformation-specific monoclonal primary antibodies against Bak or Bax, in Cholix-treated HeLa cells, Cholix induced conformational changes of Bak (cBak) and Bax (cBax) in a time-dependent manner. These changes were not seen in Cholix(E581A)-treated cells. In addition, Bax and Bak were co-immunoprecipitated by cBak, and Bak was also precipitated by cBax, suggesting that Cholix-induced oligomerization of Bak/Bax proceeded through the conformational changes.
FIGURE 3.
Effects of Cholix on Bcl-2 family proteins. A, Cholix-induced conformational changes and association of Bak and Bax in HeLa cells. Cells (2 × 105 cells/well) in a 6-well dish were grown for 24 h and then incubated with wild-type (WT) or mutant (MT) Cholix (10 μg/ml) for the indicated duration times. Cells were lysed and immunoprecipitated with anti-conformation-specific Bak (cBak) or Bax (cBax) antibodies as described in the previous report (19). The top panel shows the total cell lysate (TCL), and the lower panel shows Bak and Bax, which were immunoprecipitated with anti-cBak or anti-cBax antibodies, respectively. Data are representative of three separate experiments. B, effects of protein synthesis inhibition induced by Cholix on anti-apoptotic Bcl-2 family proteins. Cells (1 × 105 cells/well) in a 12-well dish were grown for 24 h and then incubated with 10 μg/ml Cholix WT for 0, 3, 6, and 9 h or with MT for 9 h. The expression levels of Bcl-2, Bcl-XL, and Mcl-1 in the total cell lysate were detected by Western blotting. Data are representative of three separate experiments.
Anti-apoptotic Bcl-2 family proteins such as Bcl-2, Bcl-XL, and Mcl-1 are known to prevent the conformational changes of Bak by binding to Bak and thus promoting mitochondrial membrane integrity in surviving cells (14, 20, 21). We investigated effects of Cholix on endogenous protein level of anti-apoptotic Bcl-2 family proteins. Our results demonstrated that the amount of Mcl-1 was significantly decreased, whereas amounts of Bcl-2 and Bcl-XL were not changed (Fig. 3B). In the presence of proteasome inhibitor MG132, loss of Mcl-1 induced by Cholix was inhibited (data not shown). These data indicate that Cholix induces inhibition of protein synthesis and then certain anti-apoptotic proteins, e.g. Mcl-1, are ubiquitinated, degraded by proteasome, and rapidly turned over, followed by Bak/Bax conformational changes.
Cholix-induced Cytochrome c Release Depends on Bak/Bax
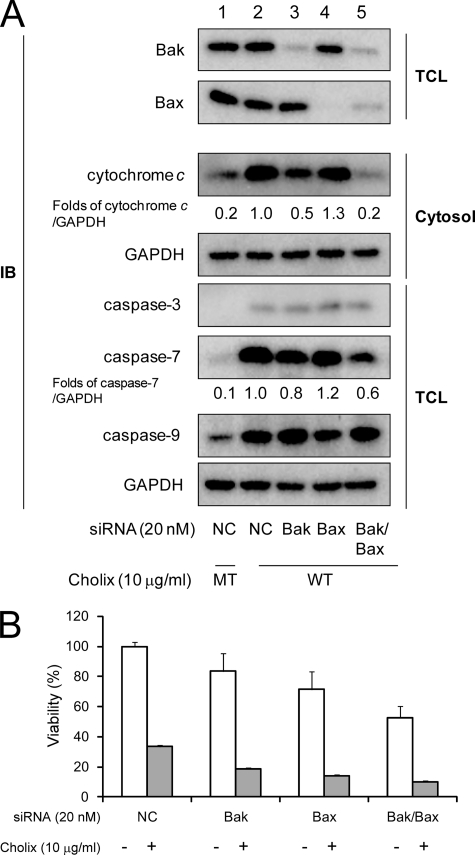
To clarify the roles of Bak or Bax on Cholix-induced cytochrome c release, we knocked down Bak and/or Bax with siRNA. Expression levels of Bak and Bax were effectively suppressed by the indicated siRNA transfection (Fig. 4A). Knockdown of Bak/Bax resulted in decreased viability in HeLa cells. In comparison with non-targeting control siRNA (Fig. 4A, lane 2), Bak knockdown significantly suppressed cytochrome c release (Fig. 4A, lane 3), indicating that Bak plays an important role in MOMP and cytochrome c release. Bax knockdown did not inhibit cytochrome c release (Fig. 4A, lane 4), however, in cells in which Bak and Bax were knocked down, cytochrome c release by Cholix was dramatically suppressed, suggesting that Cholix-induced cytochrome c release is regulated by Bak/Bax oligomerization. As we detected cytochrome c release from mitochondria associated with Bak/Bax conformational changes after Cholix treatment as shown above, mitochondria-mediated apoptotic signals could be involved in Cholix-induced cell death through formation of an apoptosome, containing Apaf-1, cytochrome c, and caspase-9 (22, 23). Next, we examined the effect of Bak and/or Bax knockdown on Cholix-induced caspase activation. Although Bak knockdown and Bak/Bax double knockdown suppressed caspase-7 activation, caspase-3 and -9 activation was not inhibited (Fig. 4A, lanes 3 and 5). Thus, these data suggested that Cholix-induced MOMP pathway is involved in caspase-7 activation, but caspase-3 and -9 activation was mediated by a pathway independent of cytochrome c release from mitochondria. Consistent with the results described above, cell viability was not increased in Cholix-treated Bak/Bax double-knockdown cells (Fig. 4B).
FIGURE 4.
Effect of Bak and/or Bax knockdown on Cholix-induced apoptotic signals. A, cells (1 × 105 cells/well) in a 12-well dish were grown for 24 h, and silencing of bak alone, bax alone, or bak/bax gene was performed with non-targeting control (NC), Bak, or Bax siRNA as described under “Experimental Procedures.” 48 h after transfection, cells were incubated with wild-type (WT) or mutant (MT) Cholix (10 μg/ml) for 18 h. Reduction of Bak or Bax protein level in total cell lysate (TCL) was confirmed by Western blotting with anti-Bak or anti-Bax antibody. Cytochrome c release was determined as described under “Experimental Procedures.” Cholix-induced caspases cleavage was determined by Western blotting with anti-cleaved caspase-3, -7, or -9 antibodies. Mean values were calculated on relative band intensity in three separate experiments. B, the cells transfected with the siRNA overnight (1 × 104 cells/well) were plated in a 96-well dish, grown overnight, and then treated with Cholix (10 μg/ml) for 24 h. Cell viability was determined by using the Cell Counting kit as described under “Experimental Procedures.” Data are the means ± S.D. value from three separate experiments. The Student's t test was used for comparisons with the non-targeting control siRNA-transfected samples incubated with Cholix.
Cholix-induced Apoptosis Depends on Caspase Activation
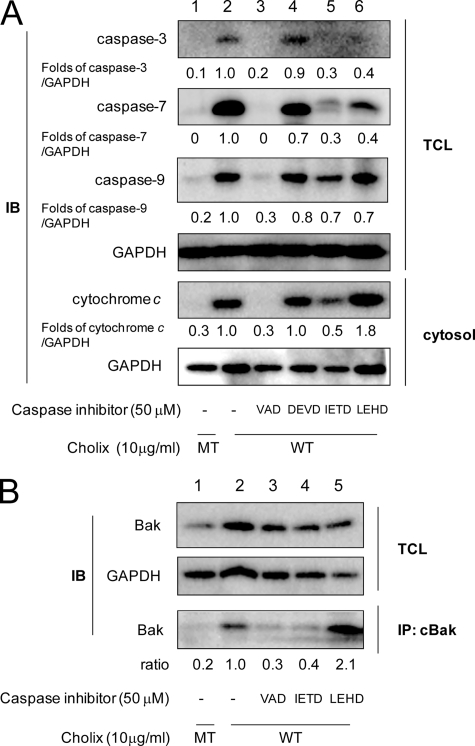
Cholix treatment of HeLa cells resulted in caspase-dependent apoptosis, in which several caspases are activated (Figs. 1B and 2A). As determined by Western blotting, pretreatment with the general caspase inhibitor significantly decreased Cholix-induced caspase activation and cytochrome c release (Fig. 5A, lane 3), indicating that Cholix induces activation of an initiator caspase followed by activation of other caspases and cytochrome c release in the apoptotic pathway.
FIGURE 5.
Effects of caspase inhibitors on Cholix-induced apoptotic signals. A, cells were grown overnight and then pretreated with the indicated caspase inhibitors (50 μm) as follows; VAD, general caspase inhibitor Z-VAD-FMK; DEVD, caspase-3 inhibitor Z-DEVD-FMK; IETD, caspase-8 inhibitor Z-IETD-FMK; and LEHD, caspase-9 inhibitor Z-LEHD-FMK. After 40 min, cells were incubated for 18 h with wild-type (WT) or mutant (MT) Cholix (10 μg/ml) in the presence or absence of the inhibitor. Lysates were collected from cells and resolved by immunoblotting to detect the indicated caspase cleavages. Cells were incubated with WT or MT Cholix (10 μg/ml) for 18 h, and then the cytosolic fraction was collected as described previously (18). Cytochrome c release into cytosol was detected using an anti-cytochrome c antibody. Mean values were calculated on relative band intensity in three separate experiments. B, cells were pretreated with the indicated caspase inhibitors (50 μm) for 40 min, and then incubated for 9 h with WT or MT Cholix (10 μg/ml) in the presence or absence of the inhibitor. Cells were lysed and immunoprecipitation with anti-conformation-specific Bak (cBak) antibody as described in Fig. 4. The top and middle panels show the total cell lysate (TCL), and the bottom panel shows Bak, which was immunoprecipitated (IP) with anti-cBak antibody. Mean values were calculated on relative band intensity in three separate experiments.
Caspase-2, -8, and -9 are initiator caspases; the type of cellular stress determines which caspase acts as the initiator in the apoptotic pathway (24). Although caspase-2 has been reported to be involved in MOMP through truncation of pro-apoptotic Bcl-2 family protein Bid (25–27), neither capase-2 knockdown by siRNA nor pretreatment with a caspase-2 inhibitor (Z-VDVAD-FMK) suppressed cytochrome c release or caspase activation in Cholix-treated HeLa cells (data not shown).
We further examined the effects of caspase-3, -8, and -9 inhibitors in Cholix-treated HeLa cells. Pretreatment with a caspase-3 inhibitor (Z-DEVD-FMK) slightly suppressed caspase-7 activation (Fig. 5A, lane 4). Notably, pretreatment with a caspase-8 inhibitor (Z-IETD-FMK) dramatically inhibited cleavage of caspases-3, -7, and -9 and cytochrome c release (Fig. 5A, lane 5). Pretreatment with a caspase-9 inhibitor (Z-LEHD-FMK) suppressed toxin-induced caspase-3 and -7 activation similar to the effect of caspase-8 inhibitor, but activation of caspase-9 and cytochrome c release were not inhibited (Fig. 5A, lane 6). These results suggest that caspase-8 plays an important role in Cholix-induced MOMP, followed by caspase activation. To verify whether Cholix-induced caspase activation regulates cytochrome c release, conformationally changed Bak was immunoprecipitated using anti-cBak antibody in the presence or absence of the indicated caspase inhibitors. Cholix-induced Bak conformational changes were clearly suppressed by a caspase-8 inhibitor (Z-IETD-FMK) (Fig. 5B, lane 4) similar to the effect of pretreatment with a general caspase inhibitor (Z-VAD-FMK) (Fig. 5B, lane 3), whereas pretreatment with a caspase-9 inhibitor (Z-LEHD-FMK) did not have any effect on Bak conformation (Fig. 5B, lane 5). These results proved that caspase-8 activity is involved in Cholix-induced Bak conformational changes, leading to the initiation of MOMP.
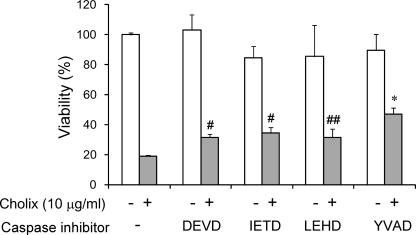
Next we examined the effects of the caspase inhibitors on cytotoxicity after Cholix treatment (Fig. 6). Although pretreatment with a caspase-8 inhibitor suppressed Cholix-induced caspase-3, -7, and -9 cleavage and cytochrome c release, Cholix-induced cytotoxicity remained. In addition, neither pretreatment with caspase-3 nor -9 inhibitors suppressed Cholix-induced cytotoxicity. These results raised the possibility that other types of caspases are involved in the early stage of Cholix-induced apoptotic signals. We found that pretreatment with cytotoxicity was partially repressed by Z-YVAD-FMK, which inhibits activity of caspase-1, -4, and -5, indicating that these caspases might play an important role as an initiator of Cholix-induced apoptotic signaling pathway.
FIGURE 6.
Effects of the caspase inhibitors on Cholix-induced cytotoxicity. Cells (1 × 104 cells/well) in a 96-well dish were grown for 24 h, treated with or without the indicated concentrations of caspase inhibitors for 40 min followed by incubation for 24 h with Cholix (10 μg/ml) in the presence or absence of the inhibitor. The utilized inhibitors were represented as follows; DEVD, caspase-3 inhibitor Z-DEVD-FMK; IETD, caspase-8 inhibitor Z-IETD-FMK; and LEHD, caspase-9 inhibitor Z-LEHD-FMK; YVAD, inflammatory caspase family (caspase-1, -4, and -5) inhibitor Z-YVAD-FMK. Cell viability was determined by using the Cell Counting kit as described under “Experimental Procedures.” Data are representative of three separate experiments. The Student's t test was used for comparisons with samples, which were incubated with Cholix for 24 h without any inhibitor. ##, #, and * represent 0.1 < p, p < 0.1, and p < 0.01, respectively.
Inflammatory Caspase Regulates Caspase-8 Activation in Cholix-induced Apoptosis
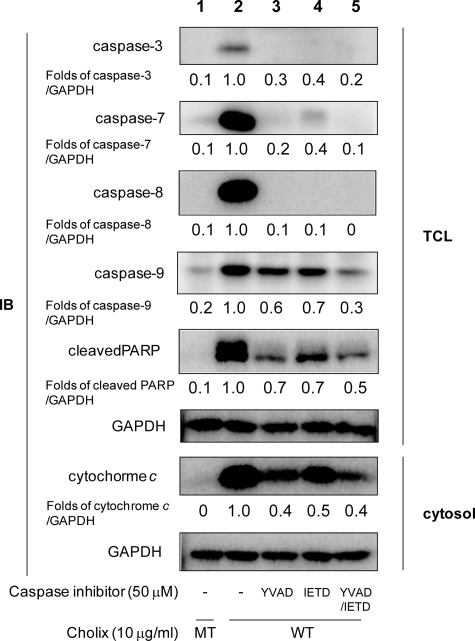
To clarify the initial caspase in Cholix-induced apoptotic pathways, we tested the effects of several caspase inhibitors on toxin-induced apoptosis. Pretreatment with a caspase-8 inhibitor (Z-IETD-FMK) suppressed truncation of procaspase-8 into caspase-8 (Fig. 7, lane 4). This result agreed with a previous report showing that caspase-8 activation is initiated by self-digestion of procaspase-8 (28). Interestingly, we found that pretreatment with Z-YVAD-FMK, an inhibitor for caspase-1, -4, and -5, also suppressed truncation of procaspase-8 (Fig. 7, lane 3). These data suggest that caspase-1, -4, and -5 regulate caspase-8 activation in Cholix-induced apoptosis. In addition, pretreatment with both Z-YVAD-FMK and Z-IETD-FMK more strongly suppressed caspase-7 and -9 activation and cytochrome c release than caspase-8 inhibitor treatment alone (Fig. 7, lane 5). Our findings indicate that toxin-activated caspase-1, -4, and -5 regulate not only caspase-3, -7, and -9 activation and MOMP through caspase-8 activation but also might directly activate apoptotic signals independent of caspase-8.
FIGURE 7.
Inhibitory effects of Z-YVAD-FMK and Z-IETD-FMK on Cholix-induced apoptotic signals. Cells were grown overnight and then pretreated with the indicated inhibitors (50 μm). After 40 min, cells were incubated for 18 h with wild-type (WT) or mutant (MT) Cholix (10 μg/ml) in the presence or absence of the inhibitor. Total cell lysate (TCL) was collected from cells and resolved by immunoblotting to detect the indicated caspases cleavages or PARP cleavage. The cytosolic fraction was collected as described in the previous report (18). Cytochrome c release into cytosol was detected using an anti-cytochrome c antibody. Mean values were calculated on relative band intensity in three separate experiments. The utilized inhibitors were represented as follows; YVAD, caspase-1, -4, and -5 inhibitor Z-YVAD-FMK; and IETD, caspase-8 inhibitor Z-IETD-FMK.
DISCUSSION
First, we examined cytotoxicity of Cholix for Caco-2, HCT116, RKO, and HeLa cells. Although Cholix-induced cell death was observed in these cells in a dose-dependent manner, cell viability after incubation with Cholix in Caco-2, HCT116, and RKO cells was not increased by general caspase inhibitor (Z-VAD-FMK), suggesting that Cholix induces caspase-independent apoptosis or necrosis in these cells. On the other hand, Cholix-induced HeLa cell death was repressed by Z-VAD-FMK, revealing that Cholix induces caspase-dependent apoptosis in HeLa cells. These findings indicate that Cholix-induced cell death pathway differs in some cell types. In this study, we analyzed the mechanism of Cholix-induced cytotoxicity in HeLa cells. Cholix exhibits high structural homology to ETA, which was shown to cause caspase-3- and -8-dependent apoptosis in human mast cells (29). In ETA-treated MEF cells, MOMP-dependent apoptosis is induced (17). Unlike these reports, we found that Cholix-induced HeLa cell death involved multiple apoptotic pathways, including mitochondria-dependent and -independent apoptotic signals and that caspase-1, -4, and -5 seemed to be responsible at an early stage for caspase-dependent apoptosis.
The mitochondria-dependent pathway was initiated by assembly of Bcl-2 family proteins Bak and Bax through specific conformational changes (Fig. 3A). Bcl-2 family proteins, which have either pro- or anti-apoptotic activities, have classically been grouped into three classes (13). One class inhibits apoptosis (Bcl-2, Bcl-XL, BCL-w, Mcl-1, Bcl-B, and Bcl-2A1) (30), whereas a second class promotes apoptosis (Bak, Bax, and Bok) (31). A third divergent class (Bad, Bik, Bid, Hrk, Bim, Bmf, Noxa, and Puma) regulates the anti-apoptotic Bcl-2 proteins to promote apoptosis (32).
We showed that Cholix decreased Mcl-1, which is an anti-apoptotic Bcl-2 family protein that represses Bak conformational changes and maintains integrity of mitochondrial outer membrane in surviving cells (Fig. 3B). A previous report showed that Mcl-1 is a short-lived protein and rapidly lost when cells were treated with cycloheximide, which inhibits protein synthesis (33). Probably the loss of Mcl-1 resulted from inhibition of synthesis.
Du et al. reported that ETA-induced cytotoxicity in MEF cells was suppressed by overexpression of Mcl-1, suggesting that ETA induces intrinsic pathway-dependent apoptosis initiated by MOMP in MEF cells (17). Conformational change in Bak was induced concurrent with loss of Mcl-1 in Cholix-treated HeLa cells (Fig. 3, A and B); however, Cholix-induced cytotoxicity was not suppressed by overexpression of V5-tagged Mcl-1 (data not shown) using the plasmid constructed previously (34). This result agreed with our data showing that gene silencing of bak did not suppress Cholix-induced cytotoxicity (Fig. 4).
In this study, we found that Bak/Bax knockdown in HeLa cells significantly suppressed Cholix-induced cytochrome c release and caspase-7 activation (Fig. 4A). These results suggest that Bak/Bax-initiated MOMP triggers the intrinsic apoptotic pathway in Cholix-treated HeLa cells. However, caspase-3 and -9 activation was observed in Bak/Bax knockdown HeLa cells as well as in control cells. These findings indicate that the Cholix-induced apoptotic pathway can utilize not only the mitochondria-dependent pathway but also the a mitochondria-independent pathway.
Pretreatment with a general caspase inhibitor in HeLa cells completely suppressed Cholix-induced caspase activation and cytochrome c release (Fig. 5), suggesting that both mitochondria-dependent and -independent apoptotic pathways are initiated by caspase activation. Pretreatment with caspase inhibitors Z-YVAD-FMK and Z-IETD-FMK significantly suppressed caspase-9 activation (Fig. 7), suggesting that caspases 1, 4, 5, and 8 are involved in both mitochondria-dependent and -independent apoptotic pathways in Cholix-treated HeLa cells.
Caspase-8 is known to initiate the extrinsic apoptotic pathway triggered by stimulation of a tumor necrosis factor (TNF) superfamily receptor, CD95 (Fas) or TNF-related apoptosis-inducing ligand receptor (35). TNF superfamily receptors are assembled with procaspase-8 and promote self-digestion of procaspase-8 in response to stimulation. There are many reports on involvement of caspase-8 in apoptosis. For example, caspase-8 truncates Bid into tBid, leading to Bax conformational changes, which can initiate MOMP (36). Caspase-3 and -9 are directly activated by caspase-8 without MOMP in the extrinsic apoptotic pathway in murine cells (37, 38). Jenkins et al. reported that a caspase-8 inhibitor (Z-IETD-FMK) suppresses ETA-induced apoptosis and that ETA treatment decreases FLIP, which is an endogenous caspase-8 inhibitor, in human mast cells, suggesting that caspase-8 plays an essential role for apoptosis in ETA-treated human mast cells (29). Based on this study, we suggest that caspase-8 regulates MOMP and activation of caspase-3, -7, and -9 (Figs. 5A and 7). Although caspase-3 and -9 were activated independent of MOMP, caspase-3 and -9 activation was significantly or partially suppressed by a caspase-8 inhibitor, indicating that caspase-3 and -9 are directly activated by caspase-8, independent of mitochondrial signals, as shown by previous reports on TNF-mediated caspase-8 activation(37, 38).
Caspases 1, 4, 5, and 12 are members of the inflammatory caspase family, and their genes are organized in a single locus (11). Human caspase-4 is the counterpart of murine caspase-12, because human caspase-12, containing several mutations, is functionally inactive (39). In this study, pretreatment with Z-YVAD-FMK, which inhibits caspase-1, -4, and -5, partially repressed Cholix-induced cell death (Fig. 6) and significantly suppressed caspase (caspase-3, -7, -8, and -9) activation and cytochrome c release (Fig. 7). These results indicate that caspases 1, 4, and 5 act upstream and regulate both mitochondria-dependent and -independent apoptotic pathways. Among the three caspases in human cells, caspase-4 has been reported to be involved in not only inflammation but also endoplasmic reticulum (ER) stress-induced apoptosis (40). Mitochondria-independent caspase-9 activation by caspase-4 was also reported recently by Yamamuro et al. in studies of ER stress-induced apoptosis in SH-SY5Y cells (41). It was suspected that Cholix induces some ER stress in HeLa cells. To examine whether Cholix induces ER stress in HeLa cells, we quantified the increased expression level of ER stress marker proteins, such as Bip and CHOP. However, the amounts of these proteins were not significantly changed in either Cholix- or Cholix(E581A)-treated HeLa cells (data not shown).
It remains unknown which factors are required between eEF2-ADP-ribosylation and activation of caspases in Cholix-induced apoptosis. Cholix-induced cell stress probably resulted from rapid loss of anti-apoptotic proteins. Pretreatment with MG132, a proteasome inhibitor, repressed Cholix-induced caspase activation (data not shown), indicating that loss of certain anti-apoptotic protein might be a trigger to induce caspase activation. In ETA-treated MEF cells, Mcl-1, one of the short-lived anti-apoptotic proteins, is believed to be the trigger (17). Although Mcl-1 was lost even in the absence of the general caspase inhibitor during Cholix treatment (supplemental Fig. S1, lane 6), pretreatment significantly repressed Cholix-induced cytotoxicity in HeLa cells even in the absence of Mcl-1, suggesting that not only Mcl-1 but also other short-lived proteins are involved in Cholix-induced caspase activation.
We examined the effects of Z-YVAD-FMK (caspase-1, -4, and -5 inhibitor) on ETA- or cycloheximide-treated HeLa cells (supplemental Fig. S2). In our studies, this inhibitor significantly repressed caspase-7 activation and cytochrome c release in ETA- and cycloheximide-treated HeLa cells, indicating that Cholix, ETA, and cycloheximide share a common pathway of caspase action in HeLa cells at an early stage.
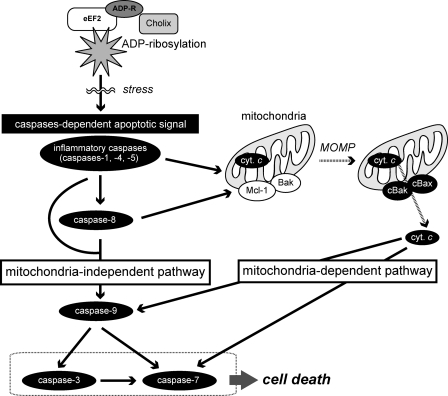
In conclusion, we propose Cholix-induced apoptotic pathways in HeLa cells occur as follows (Fig. 8). Inhibition of protein synthesis caused by Cholix-catalyzed eEF2-ADP-ribosylation results in a stress response, which initiates caspase-dependent apoptotic pathways by inflammatory caspase activation (caspase-1, -4, and -5) at an early stage of apoptosis. After caspase-8 activation by the inflammatory caspases, the caspases induce both mitochondria-dependent and -independent apoptotic pathways. The mitochondria-dependent pathway is initiated by Bak/Bax assembly, followed by cytochrome c release and caspase-7 activation. Although cytochrome c release has been known to activate caspase-9 through formation of an apoptosome, this caspase is also activated by the inflammatory caspases or caspase-8 independent of Bak/Bax assembly. After caspase-3 and -7 activation, these two apoptotic signaling pathways induce HeLa cell death.
FIGURE 8.
Proposed cascade of Cholix-induced apoptotic signals. This figure is described under “Discussion.”
Acknowledgments
We thank Dr. T. Hirayama (Institute of Tropical Medicine, Nagasaki University) and Dr. N. Morinaga (Chiba University) for helpful discussions and review of the manuscript. We acknowledge the expert technical assistance of C. Noritake and A. Kiuchi.
This work was supported by grants-in-aid for Scientific Research for Young Scientists (B) of Japan and Improvement of Research Environment for Young Researchers from Japan Science and Technology Agency.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- CT
- cholera toxin
- ETA
- Pseudomonas exotoxin A
- eEF2
- eukaryotic elongation factor 2
- ER
- endoplasmic reticulum
- MOMP
- mitochondrial outer membrane permeabilization
- EMEM
- Eagle's minimal essential medium
- MEF
- mouse embryo fibroblast
- PCSM
- 100 units/ml penicillin, and 100 μg/ml streptomycin
- Z
- benzyloxycarbonyl
- FMK
- fluoromethyl ketone
- PARP
- poly(ADP-ribose) polymerase.
REFERENCES
- 1. Peterson J. W., Ochoa L. G. (1989) Science 245, 857–859 [DOI] [PubMed] [Google Scholar]
- 2. Kaper J. B., Morris J. G., Jr., Levine M. M. (1995) Clin. Microbiol. Rev. 8, 48–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes J. M., Hollis D. G., Gangarosa E. J., Weaver R. E. (1978) Ann. Intern. Med. 88, 602–606 [DOI] [PubMed] [Google Scholar]
- 4. Morris J. G., Jr., Black R. E. (1985) N. Engl. J. Med. 312, 343–350 [DOI] [PubMed] [Google Scholar]
- 5. Petsaris O., Nousbaum J. B., Quilici M. L., Le Coadou G., Payan C., Abalain M. L. (2010) J. Med. Microbiol 59, 1260–1262 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y., Johnson J. A., Pusch G. D., Morris J. G., Jr., Stine O. C. (2007) Infect. Immun. 75, 2645–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Purdy A. E., Balch D., Lizárraga-Partida M. L., Islam M. S., Martinez-Urtaza J., Huq A., Colwell R. R., Bartlett D. H. (2010) Environ. Microbiol. Rep. 2, 198–207 [DOI] [PubMed] [Google Scholar]
- 8. Jørgensen R., Purdy A. E., Fieldhouse R. J., Kimber M. S., Bartlett D. H., Merrill A. R. (2008) J. Biol. Chem. 283, 10671–10678 [DOI] [PubMed] [Google Scholar]
- 9. Liu S., Milne G. T., Kuremsky J. G., Fink G. R., Leppla S. H. (2004) Mol. Cell. Biol. 24, 9487–9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roy V., Ghani K., Caruso M. (2010) PLoS One 5, e15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel R. M. (2006) Nat. Rev. Immunol. 6, 308–317 [DOI] [PubMed] [Google Scholar]
- 12. Pop C., Salvesen G. S. (2009) J. Biol. Chem. 284, 21777–21781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 14. Vaux D. L., Cory S., Adams J. M. (1988) Nature 335, 440–442 [DOI] [PubMed] [Google Scholar]
- 15. Green D. R., Kroemer G. (2004) Science 305, 626–629 [DOI] [PubMed] [Google Scholar]
- 16. Chiu C. C., Chen H. H., Chuang H. L., Chung T. C., Chen S. D., Huang Y. T. (2009) J. Vet. Med. Sci. 71, 1–8 [DOI] [PubMed] [Google Scholar]
- 17. Du X., Youle R. J., FitzGerald D. J., Pastan I. (2010) Mol. Cell Biol. 30, 3444–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen M., Guerrero A. D., Huang L., Shabier Z., Pan M., Tan T. H., Wang J. (2007) J. Biol. Chem. 282, 33888–33895 [DOI] [PubMed] [Google Scholar]
- 19. Mikhailov V., Mikhailova M., Pulkrabek D. J., Dong Z., Venkatachalam M. A., Saikumar P. (2001) J. Biol. Chem. 276, 18361–18374 [DOI] [PubMed] [Google Scholar]
- 20. Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. (1993) Cell 74, 597–608 [DOI] [PubMed] [Google Scholar]
- 21. Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 3516–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. (1997) Cell 91, 479–489 [DOI] [PubMed] [Google Scholar]
- 23. Zou H., Henzel W. J., Liu X., Lutschg A., Wang X. (1997) Cell 90, 405–413 [DOI] [PubMed] [Google Scholar]
- 24. Boatright K. M., Salvesen G. S. (2003) Curr. Opin. Cell Biol. 15, 725–731 [DOI] [PubMed] [Google Scholar]
- 25. Bonzon C., Bouchier-Hayes L., Pagliari L. J., Green D. R., Newmeyer D. D. (2006) Mol. Biol. Cell 17, 2150–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo Y., Srinivasula S. M., Druilhe A., Fernandes-Alnemri T., Alnemri E. S. (2002) J. Biol. Chem. 277, 13430–13437 [DOI] [PubMed] [Google Scholar]
- 27. Krumschnabel G., Sohm B., Bock F., Manzl C., Villunger A. (2009) Cell Death Differ. 16, 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Y., Sui X., Ren H. (2010) J. Cell Physiol. 225, 316–320 [DOI] [PubMed] [Google Scholar]
- 29. Jenkins C. E., Swiatoniowski A., Issekutz A. C., Lin T. J. (2004) J. Biol. Chem. 279, 37201–37207 [DOI] [PubMed] [Google Scholar]
- 30. Vogler M., Dinsdale D., Dyer M. J., Cohen G. M. (2009) Cell Death Differ. 16, 360–367 [DOI] [PubMed] [Google Scholar]
- 31. Reed J. C. (2006) Cell Death Differ. 13, 1378–1386 [DOI] [PubMed] [Google Scholar]
- 32. Willis S. N., Adams J. M. (2005) Curr. Opin. Cell Biol. 17, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adams K. W., Cooper G. M. (2007) J. Biol. Chem. 282, 6192–6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yahiro K., Morinaga N., Moss J., Noda M. (2010) Microb. Pathog. 49, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Degterev A., Boyce M., Yuan J. (2003) Oncogene 22, 8543–8567 [DOI] [PubMed] [Google Scholar]
- 36. Li H., Zhu H., Xu C. J., Yuan J. (1998) Cell 94, 491–501 [DOI] [PubMed] [Google Scholar]
- 37. Ashkenazi A. (2008) Cytokine Growth Factor Rev. 19, 325–331 [DOI] [PubMed] [Google Scholar]
- 38. McDonnell M. A., Wang D., Khan S. M., Vander Heiden M. G., Kelekar A. (2003) Cell Death Differ. 10, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. (2000) Nature 403, 98–103 [DOI] [PubMed] [Google Scholar]
- 40. Bian Z. M., Elner S. G., Elner V. M. (2009) Invest. Ophthalmol. Vis. Sci. 50, 6006–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamuro A., Kishino T., Ohshima Y., Yoshioka Y., Kimura T., Kasai A., Maeda S. (2011) J. Pharmacol. Sci. 115, 239–243 [DOI] [PubMed] [Google Scholar]