Background: FGF-23 and PTH inhibit phosphate transport in renal proximal tubules.
Results: FGF-23-mediated inhibition requires the PDZ-domain adaptor protein NHERF-1 and synergizes with PTH.
Conclusion: FGF-23 and PTH induce modifications of NHERF-1 by different but complementary pathways.
Significance: The interaction between FGF-23 and PTH on renal phosphate transport may provide a therapeutic rational for treatment of patients with FGF-23-associated hypophosphatemia.
Keywords: Fibroblast Growth Factor (FGF), Kidney, Parathyroid Hormone, Scaffold Proteins, Transporters, NHERF-1
Abstract
Fibroblast growth factor-23 (FGF-23) inhibits sodium-dependent phosphate transport in brush border membrane vesicles derived from hormone-treated kidney slices of the mouse and in mouse proximal tubule cells by processes involving mitogen-activated protein kinase (MAPK) but not protein kinase A (PKA) or protein kinase C (PKC). By contrast, phosphate transport in brush border membrane vesicles and proximal tubule cells from sodium-hydrogen exchanger regulatory factor-1 (NHERF-1)-null mice were resistant to the inhibitory effect of FGF-23 (10−9 m). Infection of NHERF-1-null proximal tubule cells with wild-type adenovirus-GFP-NHERF-1 increased basal phosphate transport and restored the inhibitory effect of FGF-23. Infection with adenovirus-GFP-NHERF-1 containing a S77A or T95D mutation also increased basal phosphate transport, but the cells remained resistant to FGF-23 (10−9 m). Low concentrations of FGF-23 (10−13 m) and PTH (10−11 m) individually did not inhibit phosphate transport or activate PKA, PKC, or MAPK. When combined, however, these hormones markedly inhibited phosphate transport associated with activation of PKC and PKA but not MAPK. These studies indicate that FGF-23 inhibits phosphate transport in the mouse kidney by processes that involve the scaffold protein NHERF-1. In addition, FGF-23 synergizes with PTH to inhibit phosphate transport by facilitating the activation of the PTH signal transduction pathway.
Introduction
Regulation of the urinary excretion of phosphate is a key component in maintaining the serum as well as the tissue content of inorganic phosphate. Following the molecular identification of the sodium-dependent phosphate transporters Npt2a, Npt2c, and Pit-2 located in the apical membrane of proximal tubule cells, two new major regulatory systems involving the trafficking of these transporters have been identified. The first related to the role played by scaffolding proteins such as NHERF-12 on basal phosphate transport in the kidney, on the ability of the kidney to adapt to changes in the phosphate content of the diet, and on the response to PTH (1–4). The second was the recognition that phosphatonins such as FGF-23 regulate the excretion of phosphate in the urine. FGF-23 has been identified as an important factor in the regulation of phosphate and vitamin D homeostasis in man and in animals, and the biologic effects of FGF-23 are unique to this member of the FGF family of proteins (for reviewed, see Refs. 5–7). FGF-23 inhibits the renal transport of phosphate in the proximal convoluted portion of the kidney, and FGF-23 levels are increased in subjects ingesting a diet high in phosphate. FGF-23 levels are also increased in patients with renal insufficiency in response to elevations in the serum concentration of phosphate. In patients with renal insufficiency, the plasma concentration of FGF-23 has been suggested to serve as a biomarker predicting the progression of renal injury and, in a more general context, as a predictor of cardiovascular disease. Finally, elevations in the serum concentration of FGF-23 are pathogenic in a number of clinical disorders such as oncogenic hypophosphatemic osteomalacia, autosomal dominant hypophosphatemic rickets, and vitamin D-resistant rickets, clinical conditions characterized by a decrease in the serum concentration of phosphate and vitamin D deficiency-related bone diseases.
Using proximal tubule-like OK cells Yamashita et al. and using isolated perfused rabbit proximal tubules Baum et al. have defined elements of the inhibitory effect of FGF-23 on renal phosphate transport (8, 9). The present experiments were initially designed to explore the potential role of NHERF-1 in the renal response to FGF-23. The rationale for these experiments derived from recent studies from our laboratory indicating that PTH failed to inhibit renal phosphate transport in the absence of NHERF-1 and in the absence of PTH-mediated phosphorylation of Ser77 and Thr95 of NHERF-1 (4). Our current results indicate that although NHERF-1 is required for FGF-23 to inhibit phosphate transport, the involvement of Ser77 and Thr95 differed between the hormones. Given both the differences in the signal transduction pathways and the involvement of the modifiable residues Ser77 and Thr95, we extended the experiments to determine whether PTH and FGF-23 interact with one another in the regulation of phosphate transport in the renal proximal tubule. The results indicate that combining low doses of PTH and FGF-23, doses that individually do not affect phosphate transport, results in marked inhibition of phosphate transport.
EXPERIMENTAL PROCEDURES
Animals, Kidney Slices, and Cell Cultures
Animals were housed in standard cages in compliance with Association for Assessment and Accreditation of Laboratory Animal Care international guidelines in the Baltimore Veterans Affairs Animal Care Facility. All animal experiments were approved by the University of Maryland School of Medicine Animal Protocol Review Board. Inbred C57BL/6 wild-type mice and NHERF-1−/− mice bred into a C57BL/6 background were used in the present experiments (1).
The protocols for preparation of kidney slices and the isolation of brush border membrane vesicles, as well as the preparation of primary proximal tubule cells from wild-type and NHERF-1 knock-out mice, have previously been detailed from this laboratory (4, 10–12). The proximal tubule cells were plated on Matrigel-coated (BD Biosciences) plastic culture dishes and maintained in an incubator at 37 °C in 5% CO2. The cultures were left undisturbed for 48 h, after which the medium was replaced every 2 days until the cells achieved confluence. The cultured proximal tubule cells were grown in DMEM/F12 medium containing 0.9 mm phosphate. Where indicated, the cultured proximal tubule cells were infected with recombinant adenoviruses which were produced using AdEasy (Stratagene) as described (4). Proximal tubule cells were incubated with 107 plaque-forming units of adenovirus-GFP, adenovirus-GFP-NHERF-1 (wild type), adenovirus-GFP-NHERF-1 containing a S77A mutation, adenovirus-GFP-NHERF-1 containing a phosphomimetic T95D mutation, or adenovirus-GFP-NHERF-1 containing a T95A mutation for 24 h. The virus was removed and functional studies initiated 24 h later.
Measurements of Sodium-dependent Phosphate Transport in Isolated Renal Brush Border Membrane Vesicles and Primary Cultured Proximal Tubule Cells
For the study of any given concentration of hormone, one kidney of an animal was used as the control while the other kidney was incubated in recombinant rat PTH(1–34), recombinant human FGF-23, or both hormones for 30 min. The sodium-dependent uptake of phosphate was then measured in BBMs prepared from the kidney slices. This protocol permitted each experimental value to be expressed as percentage change from non-hormone-treated control tissue.
To approximate an initial rate, the 30-s uptake of phosphate (100 μm) in BBM vesicles harvested from control and hormone-treated kidney slices was determined by the rapid filtration technique using a transport medium containing 137 mm NaCl. 5.4 mm KCl, 2.8 mm CaCl2, 1.2 mm MgSO4, 0.1 mm KH2PO4, and 32Pi (17). The uptake reaction was stopped by washing the BBM vesicles three times with ice-cold fresh medium in which sodium chloride was substituted with tetramethylammonium chloride and 0.5 mm sodium arsenate was added.
Sodium-phosphate transport was also measured in the primary cultures of renal proximal tubule cells (11). Cells were grown in serum-free medium for 24 h. The uptake of phosphate was determined under control conditions or after hormone treatment for 30 min by incubation in transport medium consisting of 137 mm NaCl or 137 mm tetramethylammonium chloride, 5.4 mm KCl, 2.8 mm CaCl2, 1.2 mm MgSO4, and 0.1 mm KH2PO4. Phosphate uptake was initiated by addition of 32Pi to the transport medium. After 10 min at room temperature, the cells were washed three times with ice-cold fresh medium in which sodium chloride was substituted with tetramethylammonium chloride and 0.5 mm sodium arsenate was added. After the uptake measurements were completed, the cells were solubilized in 0.5% Triton X-100 for 90 min at room temperature and analyzed by liquid scintillation spectroscopy.
Other Biochemical Assays, Immunoblots, Proteins, and Statistics
PKA and PKC activity was determined as described previously using the cAMP-dependent Protein Kinase (PKA) Radioactive Assay Kit (SignaTECT®, Promega) and Promega SignaTECT PKC Assay System containing a specific PKC substrate and capture membrane (10). MAPK activation was determined using a rabbit polyclonal antibody specific for phosphorylated MAPK. The phosphorylated MAPK bands were quantitated using laser densitometry. Western immunoblotting was performed on BBMs derived from lysates of kidney slices using a commercially available antibody to Npt2a and an antibody to Npt2c generously provided by Dr. Rochelle Cunningham (University of Maryland School of Medicine). Equality of loading was determined using Ponceau S staining or ezrin antibodies as described previously (13). Corrections were applied for minor differences in loading. Protein concentrations were determined using the method of Lowry et al. (14). Statistical analyses were performed using Peritz ANOVA. p values < 0.05 were considered statistically significant.
RESULTS
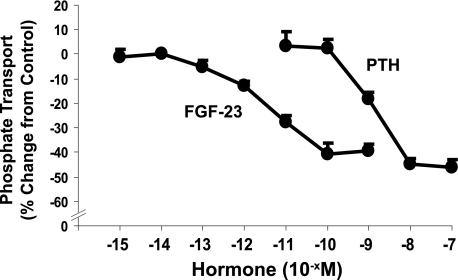
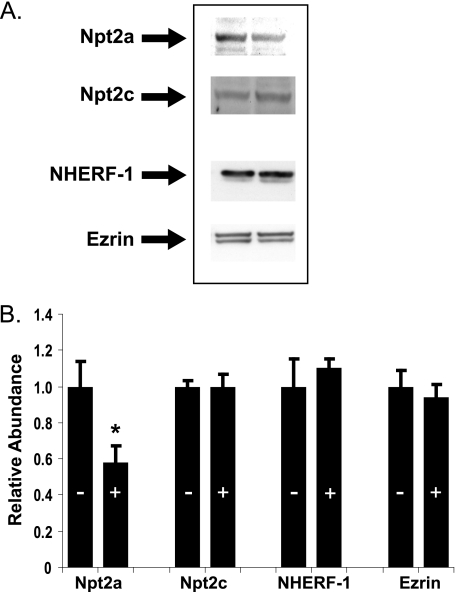
In initial studies, we established the dose-response relations among FGF-23, PTH, and phosphate transport. Kidney slices were incubated in control diluent or hormone for 30 min. BBM vesicles were then harvested, and the initial rate of sodium-dependent phosphate uptake was determined. Fig. 1 demonstrates the dose-response relations among FGF-23, PTH, and phosphate transport. No exogenous heparin was added in any of the tissue slice studies. Maximal inhibition of ∼40% was attained with each hormone individually at the highest concentrations tested. To determine the effect of FGF-23 (10−9 m) on the abundance of Npt2a and Npt2c, the BBM vesicles were subjected to SDS-PAGE, transferred, and immunoblotted using antibodies to the two transporters. As shown in Fig. 2, there was a 58 ± 7% (n = 4, p < 0.05) decrease in the abundance of Npt2a but not Npt2c in response to FGF-23 (10−9 m). There was also no change in the apical membrane abundance of NHERF-1 or ezrin.
FIGURE 1.
The dose-response relationship between FGF-23 and PTH, and phosphate transport was determined in BBM vesicles derived from wild-type mouse kidney slices incubated in the indicated concentration of hormone for 30 min. Non-hormone-treated controls were obtained for each concentration tested, and the results are expressed as percentage change from control ± S.E. (error bars). Three or more experiments were obtained with each concentration of hormone.
FIGURE 2.
The relative abundance of Npt2a, Npt2c, NHERF-1, and ezrin was determined by quantitative immunoblotting in BBM vesicles from control (−) or FGF-23 (10−9m) treated wild-type kidney slices. A, representative immunoblots. B, summary of BBM vesicle abundance after hormone treatment (+) expressed relative to non-hormone-treated control (−) BBM vesicles ± S.E. (error bars). n = 4, *, p < 0.05.
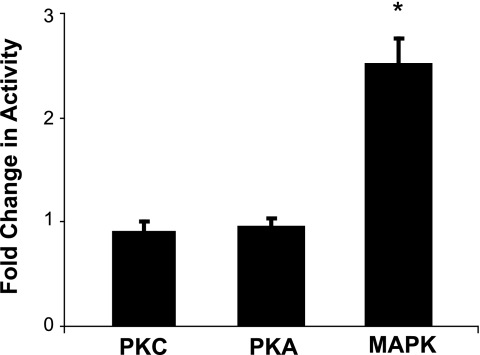
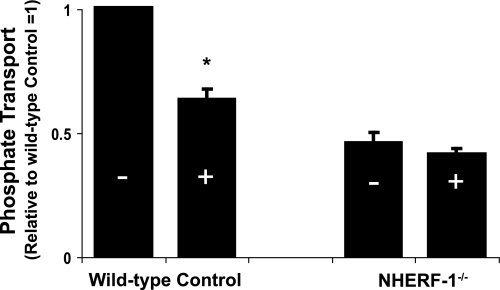
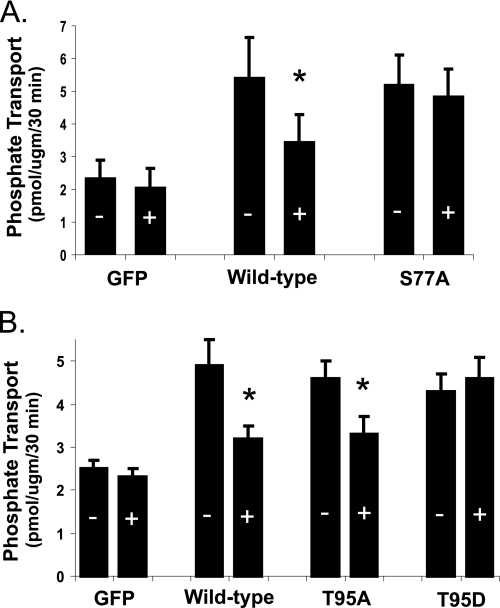
The signal transduction pathways for PTH in mouse and other renal tissues are well documented to involve activation of PKC and PKA (10). FGF-23 in a dose of 10−9 m, on the other hand, does not activate PKC or PKA but does activate MAPK in wild-type mice (Fig. 3). FGF-23 (10−9 m) also increased MAPK activity by 2.8 ± 0.5-fold (n = 3, p < 0.05) in tissue slices from NHERF-1-null mice. We next sought to determine the role of NHERF-1 in the inhibitory effect of FGF-23 on phosphate transport. BBM vesicles were harvested from wild-type and NHERF-1-null mice kidney slices incubated in control diluent or FGF-23 (10−9 m) for 30 min. Basal phosphate transport was ∼2-fold higher in BBM harvested from wild-type compared with NHERF-1-null mice (Fig. 4). Whereas FGF-23 (10−9 m) inhibited phosphate transport by 39.2 ± 2.6% (n = 6, p < 0.05) in BBM vesicles derived from wild-type mice, FGF-23 did not significantly inhibit phosphate transport in BBM vesicles from NHERF-1-null mice (percentage inhibition = 7.5 ± 2.7% (n = 4, p = not significant)). To study the role of NHERF-1 in greater detail, primary cultures of mouse NHERF-1-null proximal tubule cells were prepared and infected with control adenovirus-GFP, wild-type adenovirus-GFP-NHERF-1, or adenovirus-GFP-NHERF-1 containing a S77A mutation (Fig. 5A). A separate set of proximal tubule cells were infected with control adenovirus-GFP, wild-type adenovirus-GFP-NHERF-1, or adenovirus-GFP-NHERF-1 containing either a T95D or T95A mutation (Fig. 5B). All studies in cultured proximal tubule cells were performed in the presence of heparin (10 μg/ml) to stabilize the FGF-23/Klotho receptor (8). Sodium-dependent phosphate uptake was measured under control conditions or in the presence of FGF-23 (10−9 m) for 30 min. Basal phosphate transport was significantly higher in NHERF-1-null proximal tubule cells infected with wild-type NHERF-1 or NHERF-1 containing either a S77A, T95D, or T95A mutation as reported previously (11, 15). FGF-23 (10−9 m) did not inhibit phosphate transport in control adenovirus-GFP-infected cells but significantly inhibited phosphate transport by 38.4 ± 3.0% (n = 6, p < 0.05) (Fig. 5A) and 33.6 ± 1.2% (n = 6, p < 0.05) (Fig. 5B) in cells infected with wild-type adenovirus-GFP-NHERF-1. On the other hand, FGF-23 did not significantly inhibit phosphate transport in cells infected with adenovirus-GFP-NHERF-1 containing a S77A mutation (percentage inhibition = 6.4 ± 5.1 (n = 6, p = not significant)) (Fig. 5A). In cells infected with adenovirus-GFP-NHERF-1 containing a T95A mutation (Fig. 5B), FGF-23 inhibited phosphate transport by 27.0 ± 1.1% (n = 6, p < 0.05). In cells infected with adenovirus-GFP-NHERF-1 containing a T95D mutation, FGF-23 did not significantly inhibit phosphate transport (percentage inhibition = −6.4 ± 5.1% (n = 6, p = not significant)).
FIGURE 3.
The activation of PKC, PKA, and MAPK was measured in control and FGF-23-treated (10−9m) wild-type kidney slices. The results are expressed as -fold change from controls. *, p < 0.05; error bars, S.E.
FIGURE 4.
The effect of FGF-23 on the transport of phosphate was examined in wild-type and NHERF-1-null kidneys. Phosphate transport was determined in BBM vesicles harvested from control (−) or FGF-23 (+) (10−9 m)-treated kidney slices (30 min). The results are expressed relative to wild-type controls ± S.E. (error bars). *, p < 0.05.
FIGURE 5.
Phosphate transport was determined in cultured proximal tubule cells harvested from NHERF-1-null mice. A, cells were infected with adenovirus-GFP (GFP), adenovirus-GFP-NHERF-1 (wild-type), or adenovirus-GFP-NHERF-1 containing a S77A mutation (S77A). B, a separate set of cells was infected with adenovirus-GFP, adenovirus-GFP-NHERF-1, adenovirus-GFP-NHERF-1 containing a T95A mutation (T95A), or adenovirus-GFP-NHERF-1 containing a T95D mutation (T95D). Results are expressed as pmol/μg protein per 30 min in the absence (−) or presence (+) of FGF-23 (10−9 m) ± S.E. (error bars). *, p < 0.05 (non-treated versus treated cells). n = 5 or more for all groups.
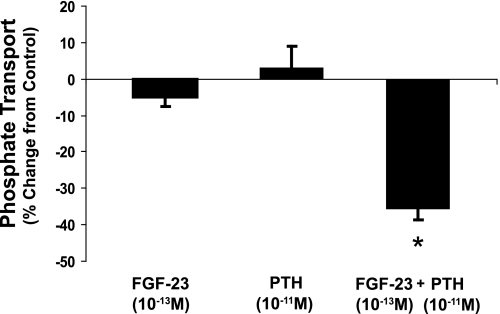
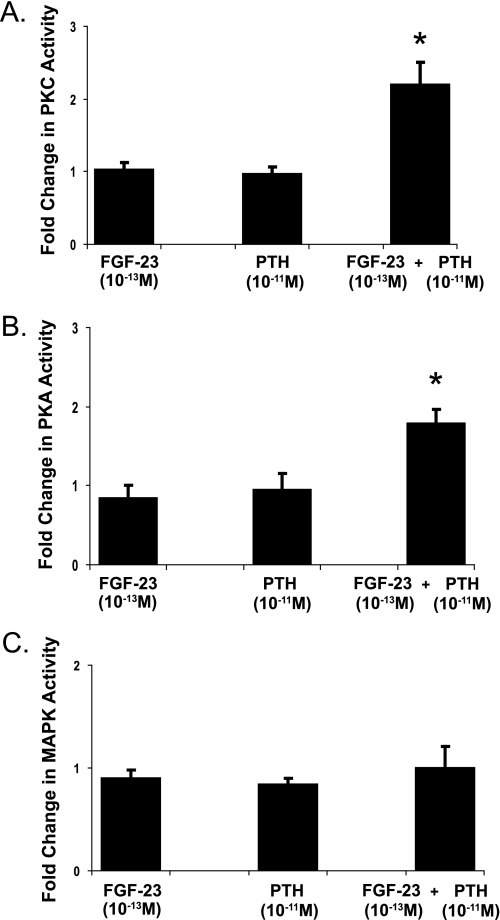
We next sought to determine whether there was synergy between the inhibitory effects of PTH and FGF-23 on phosphate transport. From the dose-response relations (Fig. 1), we studied the combined effects of PTH in a concentration of 10−11 m and FGF-23 in a concentration of 10−13 m. Fig. 6 compares the percent inhibition of phosphate uptake in the BBM vesicles derived from wild-type mice incubated for 30 min in either PTH (10−11 m), FGF-23 (10−13 m), or both hormones simultaneously. Although the individual hormones at these low concentrations were without effect, combined treatment with both hormones together resulted in a significant decrease in phosphate transport of 35.7 ± 2.9% (n = 7, p < 0.05). To determine the signal transduction pathway, PKC, PKA, and MAPK activities were measured in kidney slices incubated with each hormone individually or with both hormones together in the same concentrations as in the transport studies above. Low concentrations of either hormone individually did not affect PKC, PKA, or MAPK activity. Combined treatment, on the other hand, significantly stimulated PKC and PKA, but not MAPK (Fig. 7).
FIGURE 6.
The percentage inhibition of phosphate transport compared with untreated controls was measured in BBM vesicles derived from kidney slices from wild-type mice incubated in FGF-23 (10−13m), PTH (10−11m), or both hormones for 30 min. Results are expressed as percentage change from control ± S.E. (error bars). *, p < 0.05. n = 7 for all groups.
FIGURE 7.
The activation of PKC (A), PKA (B), and MAPK (C) was measured in wild-type control kidney slices and kidney slices incubated in FGF-23 (10−13m), PTH (10−11m), or both hormones for 30 min. The results are expressed as -fold change from controls. *, p < 0.05. n = 4 or more in each group. Error bars, S.E.
DISCUSSION
Recent experiments have defined a critical role for FGF-23 in the regulation of not only phosphate excretion but also the metabolism of vitamin D (1–4, 16–18). In studies focused on the mechanism by which FGF-23 inhibits renal phosphate transport, Yamashita et al. in proximal tubule-like OK cells and Baum et al. in isolated perfused rabbit proximal tubules demonstrated that high concentrations of FGF-23, concentrations that approximate serum levels in patients with FGF-23 producing tumors, inhibit phosphate transport (8, 9). Our results in the mouse confirm that FGF-23 inhibits phosphate transport by mechanisms independent of the activation of PKC or PKA, the pathways used by PTH. Rather, treatment of renal tissue with FGF-23 is associated with activation of MAPK. The current experiments also indicate that the acute inhibition of phosphate transport by FGF-23 was associated with a decrease in the abundance of Npt2a but not Npt2c in the apical membrane of proximal tubule cells.
Prior studies from our laboratory have indicated that ∼35–50% of the Npt2a transporter, the major phosphate transporter in renal proximal tubule cells of rodents, is bound to the PDZ domain containing scaffold protein NHERF-1 and that the Npt2a-NHERF-1 complex is the unique target of the downstream protein kinase cascades of the hormone-activated PTH1 receptor (4, 19). Npt2a not bound to NHERF-1, however, is resistant to the inhibitory effect of PTH (11, 13). In the current studies, FGF-23 did not significantly inhibit phosphate transport in brush border membrane vesicles harvested from kidney slices from NHERF-1−/− animals or in primary cultures of proximal tubule cells from these mice. FGF-23 did, however, stimulate MAPK in renal tissue from NHERF-1-null mice, indicating that the FGF-23 receptor complex was functional in the absence of NHERF-1. In cultured NHERF-1−/− proximal tubule cells, infection with wild-type adenovirus-GFP-NHERF-1 increased basal sodium-dependent phosphate transport as we have demonstrated previously (4, 10, 15). The increased basal rates of phosphate transport following infection with wild-type NHERF-1 likely reflect the property of NHERF-1 to function as a membrane retention signal for Npt2a and to extend the lifetime of this transporter in the apical membrane of proximal tubule cells. When infected with wild-type NHERF-1, treatment of the NHERF-1-null cells with FGF-23 strongly inhibits phosphate transport. PTH and dopamine inhibit phosphate transport by phosphorylating Ser77 in the first PDZ domain of NHERF-1 thereby disrupting its binding to Npt2a (4, 15). Our prior studies also indicated an absolute requirement for the phosphorylation of Thr95 to condition the phosphorylation of Ser77 and thereby effect PTH-mediated inhibition of phosphate transport (15). To determine whether these residues are involved in FGF-23-mediated inhibition of phosphate transport, we studied NHERF-1-null proximal tubule cells infected with adenovirus-GFP-NHERF-1 containing a S77A mutation, a T95A mutation, or a phosphomimetic T95D mutation. NHERF-1-null cells infected with adenovirus-GFP-NHERF-1 containing a S77A mutation had a higher basal rate of phosphate transport but were resistant to the inhibitory effect of FGF-23. This suggests that like PTH and dopamine, modification of Ser77 is a determinant of the inhibitory effect of FGF-23. Cells infected with adenovirus-GFP-NHERF-1 containing a T95A mutation had also higher basal rates of phosphate transport and were responsive to the inhibitory effect of FGF-23, although the absolute magnitude of inhibition was somewhat less than wild-type NHERF-1. On the other hand, cells infected with NHERF-1 containing a T95D mutation were resistant to FGF-23. These findings indicate that the role of modifications of Thr95 in FGF-23-mediated inhibition of phosphate transport is clearly different from that of PTH which requires phosphorylation of this residue to inhibit phosphate transport. The most conservative interpretation of these findings is that FGF-23 inhibits phosphate transport in renal proximal tubule cells by a NHERF-1-dependent mechanism involving the modification of Ser77 in the first PDZ domain of NHERF-1 but by a pathway that differs from that of PTH and dopamine.
The difference in the signal transduction pathways between PTH and FGF-23 raises the question of whether these two powerful regulatory hormones act independently or synergistically to modulate the renal transport of phosphate. This question is of potential clinical significance given some clinical reports that indicated that the hypophosphatemia associated with elevated levels of FGF-23 could be abrogated by treatment with drugs that inhibit the release of PTH (20). The serum concentrations of phosphate as well as 1,25-dihydroxy vitamin D are determinants of both PTH and FGF-23 levels, and perturbations in the levels of PTH might be predicted to affect the concentration and/or metabolic actions of FGF-23. In addition, evidence has been advanced that FGF-23 directly inhibits PTH synthesis and release from parathyroid glands (21). Our results, however, demonstrate a heretofore unrecognized direct interaction between these hormones at the level of the renal proximal convoluted tubule. FGF-23 and PTH in doses that individually had no effect on phosphate transport in the kidney, dramatically inhibited phosphate transport when cells were treated with both hormones simultaneously. Based on the findings that the combined low doses of PTH and FGF-23 stimulated PKC and PKA activity but did not stimulate MAPK, we would suggest that FGF-23, in some manner, sensitizes renal proximal tubule cells to PTH. The biochemical nature of the interaction between the two hormones is not known at the present time, but given the protocol of our experiments and the time frame of the response, it would appear that the interaction between these hormones does not necessarily engage long term adaptive pathways involving tissues other than the kidney.
In summary, the present experiments indicate that FGF-23 is a potent inhibitor of sodium-dependent phosphate transport in the kidney of the mouse and establish for the first time that the inhibitory effect of FGF-23 involves the multi-PDZ domain scaffold protein NHERF-1. These studies also demonstrate a direct synergism between PTH and FGF-23 to inhibit phosphate transport in proximal tubule cells thereby providing a physiologic explanation for clinical observation that the low serum phosphate concentrations in patients with elevations in FGF-23 can be corrected, at least in part, by drugs that inhibit the release of PTH from the parathyroid glands.
This work was supported, in whole or in part, by National Institutes of Health Grant DK55881 (to E. J. W.). This work was also supported by the Research Service, Department of Veterans Affairs (to E. J. W.).
- NHERF-1
- sodium-hydrogen exchanger regulatory factor-1
- BBM
- brush border membrane
- PTH
- parathyroid hormone.
REFERENCES
- 1. Shenolikar S., Voltz J. W., Minkoff C. M., Wade J. B., Weinman E. J. (2002) Proc. Nat. Acad. Sci. 99, 11470–11475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernando N., Déliot N., Gisler S. M., Lederer E., Weinman E. J., Biber J., Murer H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11957–11962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinman E. J., Boddeti A., Cunningham R., Akom M., Wang F., Wang Y., Liu J., Steplock D., Shenolikar S., Wade J. B. (2003) Am. J. Physiol. Renal Physiol. 285, F1225–1232 [DOI] [PubMed] [Google Scholar]
- 4. Weinman E. J., Biswas R. S., Peng G., Peng Q., Shen L., Turner C.L., E X., Steplock D., Shenolikar S., Cunningham R. (2007) J. Clin. Invest. 117, 3412–3420 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Seiler S., Heine G. H., Fliser D. (2009) Kidney Int. Suppl. 76, S34–S42 [DOI] [PubMed] [Google Scholar]
- 6. Yu X., White K. E. (2005) Cytokine Growth Factor Rev. 16, 221–232 [DOI] [PubMed] [Google Scholar]
- 7. Quarles L. D. (2008) J. Clin. Invest. 118, 3820–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamashita T., Konishi M., Miyake A., Inui K., Itoh N. (2002) J. Biol. Chem. 277, 28265–28270 [DOI] [PubMed] [Google Scholar]
- 9. Baum M., Schiavi S., Dwarakanath V., Quigley R. (2005) Kidney Int. 68, 1148–1153 [DOI] [PubMed] [Google Scholar]
- 10. Cunningham R., Biswas R., Brazie M., Steplock D., Shenolikar S., Weinman E. J. (2009) Am. J. Physiol. Renal Physiol. 296, F355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunningham R., Steplock D., E, X., Biswas R. S., Wang F., Shenolikar S., Weinman E. J. (2006) Am. J. Physiol. Renal Physiol. 291, F896–901 [DOI] [PubMed] [Google Scholar]
- 12. Weinman E. J., Biswas R., Steplock D., Douglass T. S., Cunningham R., Shenolikar S. (2010) J. Biol. Chem. 285, 13454–13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunningham R., E X., Steplock D., Shenolikar C., Weinman E. J. (2005) Am. J. Physiol. Renal Physiol. 289, F933–938 [DOI] [PubMed] [Google Scholar]
- 14. Lowry O. H., Rosenbrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 15. Weinman E. J., Steplock D., Zhang Y., Biswas R., Bloch R. J., Shenolikar S. (2010) J. Biol. Chem. 285, 25134–25138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White K. E., Jonsson K. B., Carn G., Hampson G., Spector T. D., Mannstadt M., Lorenz-Depiereux B., Miyauchi A., Yang I. M., Ljunggren O., Meitinger T., Strom T. M., Jüppner H., Econs M. J. (2001) J. Clin. Endocrinol. Metab. 86, 497–500 [DOI] [PubMed] [Google Scholar]
- 17. White K. E., Evans W. E., O'Riordan J. L. H., Speer M. C., Econs M. J., Lorenz-Depiereux B., Grabowski M., Meitinger T., Strom T. M. (2000) Nat. Genet. 26, 345–34811062477 [Google Scholar]
- 18. Shimada T., Mizutani S., Muto T., Yoneya T., Hino R., Takeda S., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Déliot N., Hernando N., Horst-Liu Z., Gisler S. M., Capuano P., Wagner C. A., Bacic D., O'Brien S., Biber J., Murer H. (2005) Am. J. Physiol. Cell Physiol. 289, C159–167 [DOI] [PubMed] [Google Scholar]
- 20. Geller J. L., Khosravi A., Kelly M. H., Riminucci M., Adams J. S., Collins M. T. (2007) J. Bone Miner. Res. 22, 931–937 [DOI] [PubMed] [Google Scholar]
- 21. Ben-Dov I. Z., Galitzer H., Lavi-Moshayoff V., Goetz R., Kuro-o M., Mohammadi M., Sirkis R., Naveh-Many T., Silver J. (2007) J. Clin. Invest. 117, 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]