Abstract
Bone is a frequent target of lung cancer metastasis and is associated with significant morbidity and a dismal prognosis. Interaction between cancer cells and the bone microenvironment causes a vicious cycle of tumor progression and bone destruction. This study analyzed the soluble factors secreted by lung tumor-associated osteoblast (TAOB), which are responsible for increasing cancer progression. The addition of bone morphogenetic protein-2 (BMP-2), present in large amounts in TAOB conditioned medium (TAOB-CM) and lung cancer patient sera, mimicked the inductive effect of TAOB-CM on lung cancer migration, invasion, and epithelial-to-mesenchymal transition. In contrast, inhibition of BMP by noggin decreases the inductive properties of TAOB-CM and lung cancer patient sera on cancer progression. Induction of lung cancer migration by BMP-2 is associated with increased ERK and p38 activation and the up-regulation of Runx2 and Snail. Blocking ERK and p38 by a specific inhibitor significantly decreases cancer cell migration by inhibiting Runx2 up-regulation and subsequently attenuating the expression of Snail. Enhancement of Runx2 facilitates Rux2 to recruit p300, which in turn enhances histone acetylation, increases Snail expression, and decreases E-cadherin. Furthermore, inhibiting Runx2 by siRNA also suppresses BMP-2-induced Snail up-regulation and cell migration. Our findings provide novel evidence that inhibition of BMP-2 or BMP-2-mediated MAPK/Runx2/Snail signaling is an attractive therapeutic target for osteolytic bone metastases in lung cancer patients.
Keywords: Bone Morphogenetic Protein (BMP), Cell Migration, Cell-Cell Interaction, Lung, MAP Kinases (MAPKs), Epithelial-to-Mesenchymal Transition, Lung Tumor, Osteoblast, Runx2, Snail
Introduction
The skeleton is a frequent target of lung cancer metastasis. Approximately 30–40% of patients with advanced lung cancer will develop bone metastasis, resulting in a dramatic increase of mortality rates and severely diminishing quality of life (1, 2). Bone metastasis are usually symptomatic, the most frequent pain complained by cancer patients is pain from bone metastases. Approximately 80% of lung cancer patients with bone metastases may suffer from localized bone pain, followed by extremity weakness (14.9%) (3, 4). Skeletal-related events, including pathologic fracture, spinal cord compression, hypercalcemia, or pain requiring surgery, radiotherapy, or opioid analgesics. Most lung cancer patients with bone metastases experience impaired quality of life because of pathologic bone fracture, especially the long bones, significantly impairing their functional status (4). The median survival time of lung cancer with synchronous bone metastasis ranges from 5 to 6 months, and the 2-year survival rate is only 3% (3). Interaction between cancer cells and the bone microenvironment causes a vicious cycle of tumor progression and bone destruction (5, 6). Cancer cells produce some soluble factors that alter osteoblast and osteoclast proliferation, maturation, and activation, resulting in an increase of bone resorption. In addition, resorption of the bone matrix causes the release of soluble factors that enhance cancer cell growth and promote metastasis from the primary site to the bones (5–7).
Bone morphogenetic proteins (BMPs),2 belonging to TGF-β superfamily members, were originally investigated because of their ability to regulate the formation of new bone (8). Current studies have indicated that BMP-2 is also involved in various biological processes such as cell proliferation, differentiation, and migration (9, 10). BMP-2 has been shown to play a role in the dysregulation of many types of cancers and to play a crucial role in the occurrence and development of breast and prostate cancers (11–13). Signaling by BMP proteins is mediated through heterodimerization of types I and II serine/theronine kinase receptors. After stimulation and activation of BMP type I receptor, SMAD1, SMAD5, and SMAD8 accumulate in the nuclei and control the transcription of a large number of target genes (14). Alternatively, binding of BMP-2 to its receptor can also activate MAPK family members ERK1/2 and p38, which participate in the regulation of the biological processes of cancer, including cell proliferation, migration, and metastasis (15, 16).
Although the regulation of bone metastasis has been the focus of intensive investigation, relatively little is known about the molecular mechanisms that control the process. Understanding these mechanisms contributing to bone metastasis is essential for developing biomarkers of the disease's progression, as well as for the development of more effective therapies. In this study, we tested the hypothesis that BMP-2 contributes to lung cancer-related bone metastasis by examining the effects of BMP-2 on the migration and epithelial-to-mesenchymal transition (EMT) of lung cancer. Our observations have enhanced our understanding of signaling pathways modulated by BMP-2 in the metastasis of lung cancer cells to the bones, providing a molecular basis explaining the correlation of lung cancer with bone metastasis.
EXPERIMENTAL PROCEDURES
Cell Cultures and Conditioned Medium
Human primary osteoblasts were obtained from Lonza (Walkersville, MD) and cultured in osteoblast growth medium. The low invasive human lung adenocarcinoma cell line (CL1–0) was kindly provided by Dr. Pan-Chyr Yang (Department of Internal Medicine, National Taiwan University Hospital) and cultured in RPMI1640 supplemented with 10% FBS and 1% penicillin-streptomycin (Invitrogen) (17, 18).
To obtain the A549- and CL1–0-conditioned medium (CM), the cells were seeded at 2 × 106 cells/100-mm dish and cultivated for 24 h. A549 tumor associated osteoblast (A549-OB) and CL1–0-OB were generated by culturing human osteoblasts presenting in A549-CM and CL1–0-CM for 24 h. After washing, the supernatants were collected and defined as A549-OB-conditioned medium (A549-OB-CM) and CL1–0-OB-CM (supplemental Fig. S1A).
Cell Proliferation, Migration, and Invasion
The cells were treated with 0, 10, and 20 ng/ml BMP-2 (R & D Systems Europe, Abingdon, UK) for 48 h. At the end of the assay period, cell proliferation was measured by WST-1 assay. The amount of color produced is directly proportionate to the number of metabolically active cells.
Cell migration was assessed by scratch wound healing assay. The cells were allowed to grow into full confluence in 24-well plates. The following day, a uniform scratch was made down the center of the well using a micropipette tip, followed by washing once with PBS. Vehicle control and BMP-2 were added to the respective wells for the indicated times. Photographic imaging was performed using the Olympus 1 × 50 inverted microscope. Cell migration and invasion were also assessed by QCMTM 24-well cell migration assay and invasion system (Millipore), according to the manufacturer's instructions.
Serum Samples from Lung Cancer Patients
Preoperative blood samples were obtained from 25 lung cancer patients and 15 healthy donors admitted to the Division of Pulmonary and Critical Care Medicine, Kaohsiung Medical University Hospital (Kaohsiung, Taiwan). Serum was separated by centrifugation and frozen at −80 °C. Approval for these studies was obtained from the institutional review board of Kaohsiung Medical University Hospital. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Immunoblot/Immunoprecipitation
Cells were treated with A549-OB-CM (20%), CL1–0-OB-CM or 20 ng/ml BMP-2 for the indicated times then lysed on ice for 15 min in a solution containing 50 mm Tris, 1% Triton X-100, 0.1% SDS, 150 mm NaCl, 2 mm Na3VO4, 2 mm EGTA, 12 mm β-glycerolphosphate, 10 mm NaF, 16 μg/ml benzamidine hydrochloride, 10 μg/ml phenanthroline, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 1 mm phenylmethylsulfonyl fluoride. Cell lysate was centrifuged at 14,000 × g for 15 min, and the supernatant fraction was collected for immunoblot. Equivalent amounts of protein were resolved by SDS-PAGE (10–12%) and transferred to PVDF membranes. After blocking for 1 h in 5% nonfat dry milk in Tris-buffered saline, the membranes were incubated with the desired primary antibody for 1–16 h. The membranes were then treated with appropriate peroxidase-conjugated secondary antibody, and the immunoreactive proteins were detected using an enhanced chemiluminescence kit (Amersham Biosciences) according to the manufacturer's instructions. The primary antibodies used in this study targeted unphosphorylated and phospho-MAPK family proteins, SMAD1/5/8, vimentin, N-cadherin, and E-cadherin, which were obtained from Cell Signaling Technology; p300, Runx2, and Snail antibody were obtained from Abcam, Ltd. (Cambridge, UK). β-Actin was obtained from Upstate Biotechnology (Lake Placid, NY). For immunoprecipitation, cell lysates (200 μg of total protein) were incubated with 2 μg of anti-p300 overnight and then with 20 μl of protein A-agarose beads (Millipore, Bedford, MA) for 2 h at 4 °C. Association of Runx2 with p300 was detected by incubating the blots with anti-Runx2 antibodies (Abcam).
Gene Knockdown and Overexpression
Knockdown of Runx2, p300, and Snail in CL1–0 and A549 cells was performed by using a lentiviral expression system provided by the National RNAi Core Facility (Taipei, Taiwan). The lentiviruses were produced by co-transfecting HEK293T with pLKO-AS2, pLKO-AS2-RUNX2, pLKO-AS2-p300, or pLKO-AS2-SNAI1 shRNA and two packaging plasmids (pCMVVDR8.91 and pMD.G). The efficacy of Runx2, p300, and Snail shRNA plasmid was assessed by real time PCR. Runx2-transfected A549 and CL1–0 cell were transected with pCMV or pSnail plasmid (Origene, Rockville, MD), and stable clones were established by G418 and puromycin.
Real Time RT-PCR
RNA isolation was performed using the TRIzol reagent (Invitrogen). cDNA was prepared using an oligo(dT) primer and reverse transcriptase (Takara, Shiga, Japan) following standard protocols. Real time PCR was performed using SYBR Green on the ABI 7500 real time PCR system (Applied Biosystems, Foster City, CA). Each PCR mixture contained 200 nm of each primer, 10 μl of 2×SYBR Green PCR Master Mix (Applied Biosystems), 5 μl of cDNA, and RNase-free water with a total volume of 20 μl. The PCR was carried out with a denaturation step at 95 °C for 10 min and then for 40 cycles at 95 °C for 15 s and 60 °C for 1 min. All of the PCRs were performed in triplicate and normalized to internal control GAPDH mRNA. Relative expression was presented using the 2−ΔΔCT method.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed by agarose ChIP kit (Pierce). Immunoprecipitated DNA was analyzed by quantitative PCR (Light Cycler, as described above) using SYBR Green dye. The primers used were TACTCTGAGACAGGCGCATG and GGGACAGAGGCAGTAAGCAG for Snail, yielding a 147-bp fragment.
Animal Model
LLC cells were transplanted into C57BL/6 mice by tail vein injection. BMP-2 (0.5 mg/kg) was administered by intraperitoneal injection three times every 3 days for after transplantation. The animals were sacrificed on day 10 after LLC transplantation, and the number of tumor nodules was recorded for analysis of lung cancer incidence.
Statistical Analysis
The data were expressed as the means ± standard errors. Statistical comparisons of the results were made using analysis of variance. Significant differences (p < 0.05) between the means of test groups were analyzed by Dunnett's test.
RESULTS
Lung Tumor-associated Osteoblast-derived BMP-2 Increases the Migration and EMT of Lung Cancer Cells
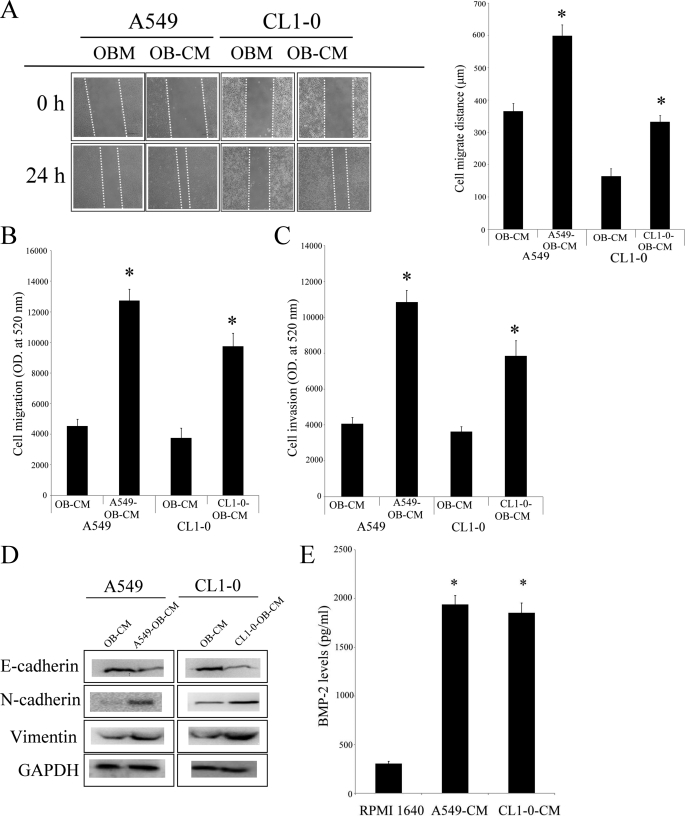
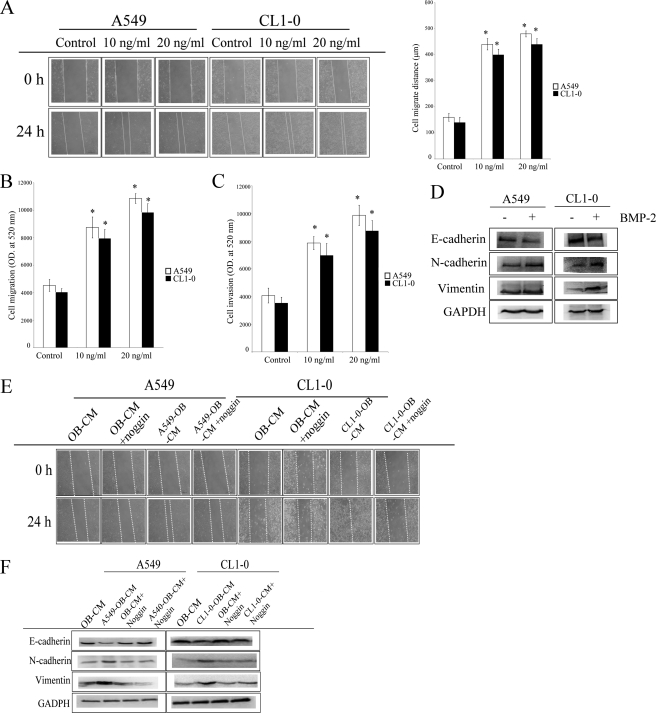
Increase in bone remodeling and soluble factors released from the bone matrix may be involved in the progression of cancer (19). To investigate this hypothesis, we collected lung tumor-associated osteoblast conditioned medium (TAOB-CM), then assessed the effects of the TAOB-CMsA549-OB-CM and CL1–0-OB-CM on the proliferation and migration of two lung cancer cell lines, A549 and CL1–0. As shown in Fig. 1 (A–D), both A549-OB-CM and CL1–0-OB-CM increased migration, invasion, and EMT of both A549 and CL1–0 cells but did not affect cell proliferation in either cell line (supplemental Fig. S1B). In addition, we found that lung tumor-associated osteoblasts express a high amount of BMP-2 protein after A549-OB-CM and CL1–0-OB-CM treatment (Fig. 1E). Next, we determined the effect of BMP-2 on the proliferation, migration, and EMT in A549 and CL1–0 cells. As shown in supplemental Fig. S2A, after treatment of the two cancer cell lines for 48 h, BMP-2 did not increase cell proliferation in either A549 or CL1–0 cells but did enhance lung cancer cell migration and invasion abilities (Fig. 2, A–C). In addition, BMP-2 also caused A549 and CL1–0 cells to undergo EMT, including the down-regulation of epithelial marker (E-cadherin) and up-regulation of fibroblast markers (vimentin and N-cadherin) (Fig. 2D). Furthermore, noggin, a BMP-2 antagonist, decreased TAOB-CM-mediated cell migration and EMT in both cancer cell lines (Fig. 2, E and F). The enhancement effect of BMP-2 on A549 and CL1–0 migration was also confirmed by BMP-2 depletion (supplemental Fig. S2B). These data clearly show that BMP-2 in TAOB-CMs mediates the changes in lung cancer progression.
FIGURE 1.
TAOB-CMs increases lung cancer migration and EMT. TAOB-CMs enhanced the migratory ability of A549 and CL1–0 lung cancer cells. A and B, TAOB-CMs enhanced cell migratory ability, as determined by scratch wound healing assay (A) and Transwell system (B). C, TAOB-CMs enhanced cell invasion ability. D, TAOB-CMs caused EMT in cancer cells. E, the expression of BMP-2 in OB-CM, For A, the migration ability of lung cancer cells was assessed by wound healing assay. OB-CM (control group) and TAOB-CMs (20%) act as a chemoattractant of cancer migration. Quantification of cell migration was carried out by measuring the distance between the migratory fronts of cells in four random selected microscopic fields for each condition and time point. The degree of cell movement is expressed as the percentage of wound closure as compared with the zero time point. For B and C, the invasiveness and migration ability of A549 and CL1–0 cells were quantified by QCMTM 24-well cell migration and invasion assay. The cells were seeded in the upper inset, and the OB-CM (control group) and TAOB-CMs (20%) acted as the chemoattractant for cancer migration and invasion. For D, A549 and CL1–0 cells were treated with TAOB-CMs (20%) for 24 h, and then the expression of various proteins was assessed by immunoblot assay. For E, primary osteoblasts were treated RPMI 1640 (20%), A549-CM (20%), and CL1–0-CM for 24 h. The BMP-2 levels were assessed by BMP-2 ELISA kits. Each value is the mean ± S.D. of three independent experiments. The asterisk indicates a significant difference between control and test groups, as analyzed by Dunnett's test (p < 0.05).
FIGURE 2.
BMP-2 is involved in TAOB-CM-mediated enhancement of migration and EMT in lung cancer. A and B, BMP-2 increased migratory ability, as determined by scratch wound healing assay (A) and Transwell system (B). C and D, BMP-2 increased the invasion ability (C) and EMT (D) of A549 and CL1–0 cells. E and F, noggin decreased TAOB-CM-mediated cell migration (E) and EMT (F). The migration ability of lung cancer cells was assessed by wound healing assay, in accord with the description under “Experimental Procedures.” BMP-2 (20 ng/ml for EMT assay) acts as the chemoattractant for cancer migration. For E and F, A549 and CL1–0 cells were pretreated with or without noggin for 1 h, and then OB-CM and TAOB-CMs were added for another 24 h. Cell migration was assessed by wound healing assay, and the expression of various proteins was determined by immunoblot assay. Each value is the mean ± S.D. of three independent experiments. The asterisk indicates a significant difference between control and test groups, as analyzed by Dunnett's test (p < 0.05).
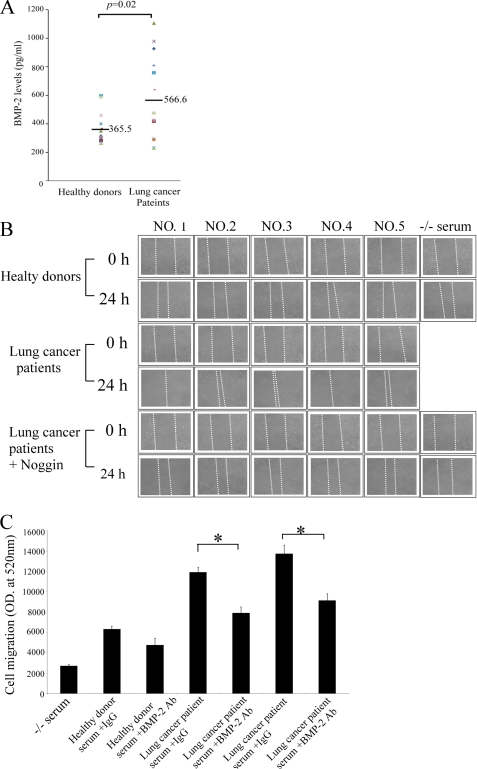
To further elucidate the nature of the soluble factors present in the sera of stage IV lung cancer patients, we examined the levels of BMP-2 in healthy donors and patients. The average BMP-2 serum level in 25 patients' sera was 566.6 pg/ml. The levels of BMP-2 in healthy donor ranges were lower than those in patient sera (Fig. 3A). CL1–0 cells were used to assess cell migration in the presence of 15% serum from five stage IV lung cancer patients. As shown in Fig. 3B, the migration of CL1–0 cells was dramatically enhanced in the presence of patient sera. Furthermore, noggin prevented the induction of cell migration by lung cancer patient sera. Depletion of BMP-2 also decreased lung cancer patient serum-mediated cell migration (Fig. 3C). Our results convincingly prove that patients with invasive lung cancer have soluble factors released from bone into their sera, which effectively increases cancer cell migration.
FIGURE 3.
Sera from lung cancer patients increase lung cancer migration. A, the levels of BMP-2 in lung cancer patient sera. B, lung cancer sera enhance the migratory ability of lung cancer cells. C, depletion of BMP-2 decreased lung cancer patient serum-mediated cell migration. The levels of BMP-2 were assessed by ELISA. Horizontal bars represent means. The cells were treated with or without noggin for 1 h, and then culture medium containing healthy donor sera (15%) or lung cancer patient sera (15%) was added for another 24 h. Cell migration was assessed by wound healing assay. For C, BMP-2 depleted from lung cancer patient serum was performed using anti-BMP-2 and antibodies (4 μg/ml) and Sepharose A/G beads, following regular immunoprecipitation techniques. The migration ability of A549 and CL1–0 cells were quantified by QCMTM 24-well cell migration assay kit. Each value is the mean ± S.D. of three independent experiments. The asterisk indicates a significant difference between control and test groups, as analyzed by Dunnett's test (p < 0.05).
TAOB-CMs and BMP-2 Activate ERK1/2 and p38 and Increase the Expression of Runx2 and Snail
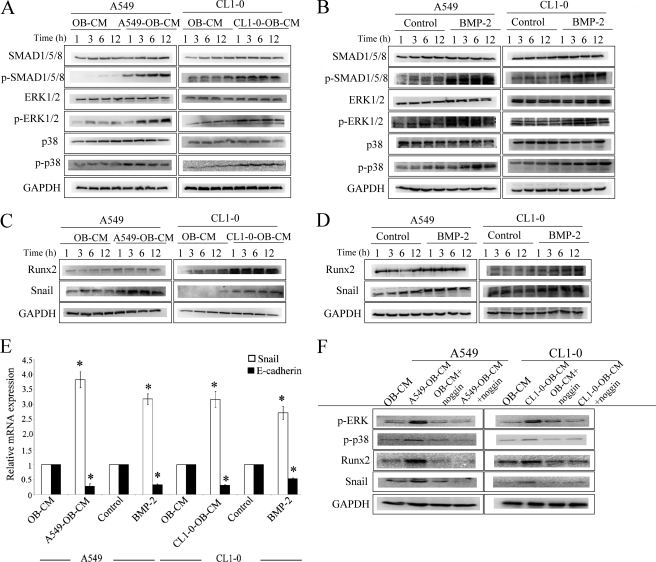
Previous studies showed that binding of BMP-2 to BMP-2 receptor increases the phosphorylation of SMAD1/5/8 and MAPK, which in turn enhances the expression of Runx2 (9, 10, 20). Therefore, we assessed the effect of TAOB-CMs and BMP-2 on MAPK cascade and Runx2 expression. The results showed that exposure of A549 and CL1–0 cells to TAOB-CMs and BMP-2 results in the phosphorylation of SMAD1/5/8, p38 and ERK1/2 (Fig. 4, A and B). On the other hand, the expression of SMAD1/5/8, p38, and ERK1/2 (unphosphorylated form) was unaltered by TAOB-CMs and BMP-2 treatment in either A549 or CL1–0 cells. In addition, TAOB-CMs and BMP-2 also cause the up-regulation of Runx2 in both A549 and CL1–0 cells, as determined by immunoblot (Fig. 4, C and D). Snail has been reported to decrease E-cadherin expression by means of transcription repression, inducing epithelial-mesenchymal transition and cell migration (21, 22). TAOB-CMs and BMP-2 increase Snail levels while decreasing the expression of E-cadherin in both A549 and CL1–0 cells at mRNA and protein levels (Figs. 1D, 2D, and 4, C–E).
FIGURE 4.
TAOB-CMs and BMP-2 increase the activation of MAPK and elevate the expression of Runx2 and Snail. A and B, TAOB-CMs (A) and BMP-2 (B) increase the phosphorylation of SMAD, ERK, and p38. C and D, TAOB-CMs (C) and BMP-2 (D) enhance the expression of Runx2 and Snail protein. Cells were treated with OB-CM (20%), TAOB-CMs (20%), or BMP-2 (20 ng/ml) for the indicated times. The expressions of various proteins were determined by immunoblot assay. E, TAOB-CMs and BMP-2 enhance the expression of Runx2 and Snail mRNA. The cells were treated with OB-CM (20%), TAOB-CMs (20%), or BMP-2 (20 ng/ml) for a specific time (3 h for snail and 12 h for E-cadherin). The expressions of mRNA were determined by quantitative PCR. F, noggin decreases TAOB-CM-mediated MAPK activation and Runx2 and Snail up-regulation. The cells were treated with OB-CM (20%), TAOB-CMs (20%), or BMP-2 (20 ng/ml) for the indicated times. The expressions of mRNA and various proteins were determined by quantitative PCR and immunoblot assay. For F, A549 and CL1–0 cells were pretreated with or without noggin for 1 h and then treated with BMP-2 (20 ng/ml) for 6 h. The expression of various proteins was then assessed by immunoblot assay. The data shown are representative of three independent experiments. Each value is the mean ± S.D. of three independent experiments. The asterisk indicates a significant difference between control and test groups, as analyzed by Dunnett's test (p < 0.05).
To further investigate the role of BMP-2, we assessed the effect of noggin on TAOB-CM-mediated p38 and ERK1/2 phosphorylation, Runx2 and Snail up-regulation, and E-cadherin down-regulation. As shown in Fig. 4F, noggin prevents TAOB-CM-mediated phosphorylation of ERK1/2 and p38. Similarly, noggin also decreases TAOB-CM-mediated Runx2 and Snail up-regulation and E-cadherin down-regulation (Figs. 2F and 4F), suggesting that BMP-2 plays an important role in TAOB-CM-mediated EMT.
ERK1/2 and p38 Are the Upstream Event of Runx2 and Snail
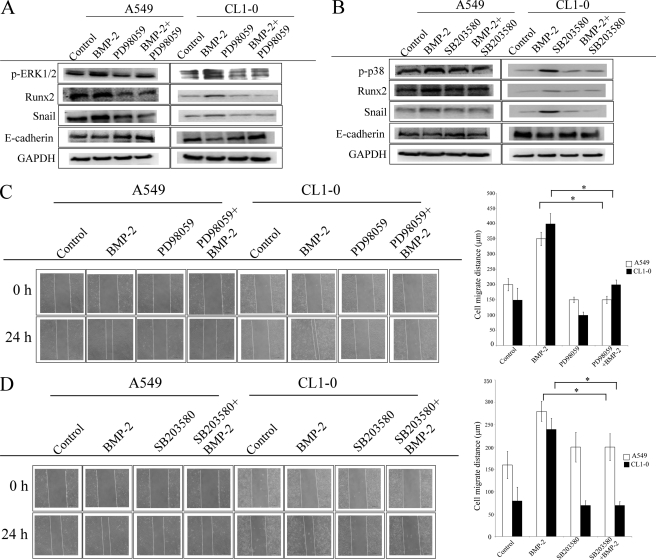
We next investigated the role of ERK1/2 and p38 on BMP-2-mediated up-regulation of Runx2 and Snail as well as on cell migration. As shown in Fig. 5A, ERK inhibitor PD98059 (MEK1/2 inhibitor) decreases up-regulation of Runx-2 and Snail induced by BMP-2 in both A549 and CL1–0 cells. Similarly, SB203580 (p38 inhibitor) pretreatment also inhibits the effect of BMP-2 on Runx2 and Snail enhancement in both A549 and CL1–0 cells (Fig. 5B). In addition, both PD98059 and SB203580 reverse E-cadherin down-regulation and cell migration in BMP-2-treated cells (Fig. 5 and supplemental Fig. S3). These data suggest that ERK1/2 and p38 are the upstream event of Runx2 and Snail.
FIGURE 5.
The role of ERK1/2 and p38 on Runx2 and Snail expression. A and B, ERK inhibitor (A) and p38 inhibitor (B) decrease BMP-2-mediated up-regulation of Runx2 and Snail as well as E-cadherin. C and D, ERK inhibitor (C) and p38 inhibitor (D) decrease BMP-2-mediated lung cancer cell migration. The cells were treated with PD98059 (ERK inhibitor, 10 μm) or SB203580 (p38 inhibitor, 10 μm) for 1 h, and then BMP-2 (20 ng/ml) was added for the specified times (cell migration, 24 h; Runx2 and Snail, 6 h; E-cadherin, 24 h). The expression of various proteins was then assessed by immunoblot assay. Cell migration was assessed by wound healing assay. The data shown are representative of three independent experiments. Each value is the mean ± S.D. of three independent experiments. The asterisk indicates a significant difference between control and test groups, as analyzed by Dunnett's test (p < 0.05).
Snail Is Involved in BMP-2-mediated E-cadherin Down-regulation and Cell Migration
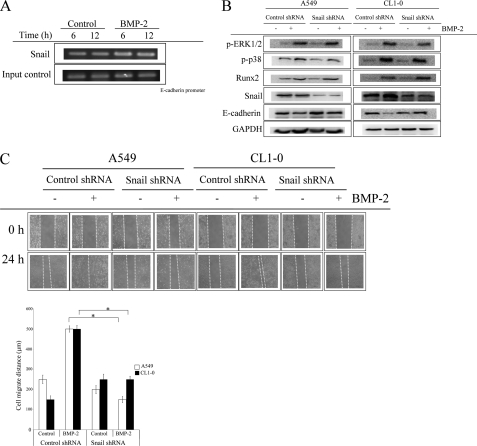
Snail plays a critical role in cancer EMT transition (21, 22). We therefore assessed whether the increase of A549 and CL1–0 migration and EMT were dependent on Snail. Chromatin immunoprecipitation assay verified that Snail bound to the promoter of E-cadherin at the E box region after BMP-2 treatment (Fig. 6A). Inhibition of Snail by shRNA transfection completely blocks the BMP-2-mediated down-regulation of E-cadherin in both A549 and CL1–0 cells (Fig. 6B). On the other hand, p38 and ERK1/2 phosphorylation and Runx2 up-regulation were unaltered by BMP-2 treatment in either Snail knockdown A549 or CL1–0 cells. These data suggest that the up-regulation of Snail by BMP-2 is in a Runx2-dependent manner. In addition, Snail knockdown also decreases BMP-2-mediated cell migration in both A549 and CL1–0 cells (Fig. 6C and supplemental Fig. S4).
FIGURE 6.
Snail is involved in BMP-2-mediated cell migration and EMT. A, the binding of Snail on E-cadherin promoter. B and C, knockdown of Snail decreases BMP-2 mediated E-cadherin down-regulation (B) and cell migration (C). The cells were treated with BMP-2 (20 ng/ml) for the indicated times. The Snail binding on E-cadherin was determined by chromatin immunoprecipitation. The cells were transfected with pLKO-AS2 or pLKO-AS2-SNAIL1 shRNA. Stable clones were created by puromycin selection, and the efficacy of shRNA was assessed by RT-PCR. The cells were treated with BMP-2 (20 ng/ml) for the specified times (cell migration, 24 h; Runx2 and Snail, 6 h; E-cadherin, 24 h). Then the expression of various proteins was then assessed by immunoblot assay. Cell migration was assessed by wound healing assay. The data shown are representative of three independent experiments. Each value is the mean ± S.D. of three independent experiments. The asterisk indicates a significant difference between control and test groups, as analyzed by Dunnett's test (p < 0.05). The data shown are representative of three independent experiments.
Runx2 Is Required for BMP-2-induced Snail Up-regulation and E-cadherin Down-regulation
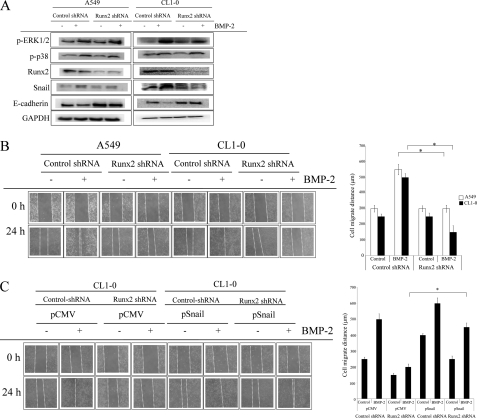
We next investigated the role of Runx2 on BMP-2-mediated EMT and migration. In comparison with control shRNA-transfected cells, Runx2 shRNA reduced Runx2 expression by ∼70% (data not shown). Selective genetic inhibition of Runx2 decreased the BMP-2-mediated Snail up-regulation and E-cadherin down-regulation (Fig. 7A). In contrast, Runx2 knockdown failed to affect the phosphorylation of ERK1/2 and p38, suggesting that Runx2 is the upstream regulator of Snail but not ERK1/2 and p38. In addition, Runx2 inhibition also decreases BMP-2-induced migration in both A549 and CL1–0 cells (Fig. 7B and supplemental Fig. S5A), whereas this effect of Runx2 shRNA is prevented by Snail overexpression (Fig. 7C and supplemental Fig. S5B).
FIGURE 7.
Runx2 is the upstream regulatory factor of Snail. A and B, inhibition of Runx2 decreases BMP-2-mediated Snail up-regulation and E-cadherin down-regulation (A), as well as cell migration (B). Cells were transfected with pLKO-AS2 or pLKO-AS2-RUNX2 shRNA. Stable clones were created by puromycin selection, and the efficacy of shRNA was assessed by RT-PCR. Cells were treated with BMP-2 (20 ng/ml) for the specified times (cell migration, 24 h; Runx2 and Snail, 6 h; E-cadherin, 24 h). Then the expression of various proteins was then assessed by immunoblot assay. C, overexpression of Snail reversed the inhibitory effect of Runx2 shRNA on BMP-2-mediated cell migration. Runx2-transfected A549 and CL1–0 cells were transected with pCMV or pSnail plasmid, and stable clones were established by G418 and puromycin. The asterisk indicates a significant difference between control and test groups, as analyzed by Dunnett's test (p < 0.05). The data shown are representative of three independent experiments.
Runx2 Increases Snail Expression through p300-mediated Histone Acetylation
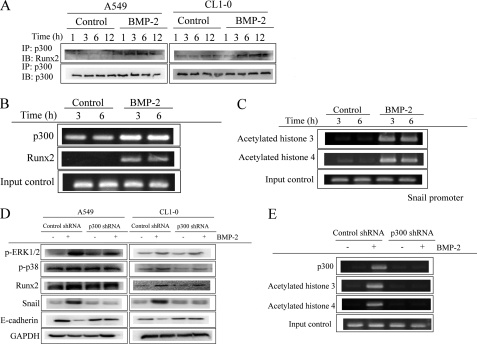
Runx2 has been reported to associate with histone acetyltransferase p300 and then to increase the expression of the cancer invasion-related gene (23). We used the TFSearch computational tool to search for p300 binding sites in Snail promoter. For this analysis, there are three p300-binding sites present at −2000 to +200 from the transcription start site in the Snail promoter, suggesting that Runx2 may cooperate with p300 to regulate the expression of Snail. To test whether Runx2 recruits p300, we first assessed the interaction of Runx2 and p300 by immunoprecipitation. As shown in Fig. 8A, BMP-2 increased the association of Runx2 with p300. Chromatin immunoprecipitation assay also verified that p300 and Runx2 bound to the promoter of Snail at the −1495 to −1642 region after BMP-2 treatment (Fig. 8B). The acetylation of histone H3 and H4 on the p300-binding region of Snail promoter was increased (Fig. 8C). In addition, inhibition of p300 by specific siRNA transfection decreases the up-regulation of BMP-2 on Snail and its downstream E-cadherin expression (Fig. 8D) but does not affect Runx2 expression, ERK and p38 phosphorylation. Furthermore, acetylation of histone H3 and H4 on the p300-binding region of Snail promoter was also abrogated by p300 siRNA (Fig. 8E). These data suggest that Runx2/p300 cooperation is involved in the regulation of Snail transcription.
FIGURE 8.
Runx2-p300 complex regulates the expression of Snail. A, the interaction of Runx2 with p300. B, the binding of p300 and Runx2 on Snail promoter. C, BMP-2 increased the acetylation of histone H3 and H4 on the p300-binding region of Snail promoter. D and E, inhibition of p300 prevents BMP-2-mediated Snail up-regulation (D) and histone acetylation on Snail promoter (E). The cells were treated with BMP-2 (20 ng/ml) for the indicated times. The interaction of Runx2 was assessed by immunoprecipitation (IP). The histone acetylation and p300/Runx2 binding on Snail were determined by chromatin immunoprecipitation. The cells were transfected with pLKO-AS2 or pLKO-AS2-p300. Stable clones were created by puromycin selection, and the efficacy of shRNA was assessed by RT-PCR. shRNA-transfected cells were treated with BMP-2 (20 ng/ml) for the specified times (Runx2 and Snail, 6 h; E-cadherin, 24 h). The expression of various proteins was then assessed by immunoblot (IB) assay. The data shown are representative of three independent experiments.
BMP-2 Increases Lung Cancer Metastasis in Vivo
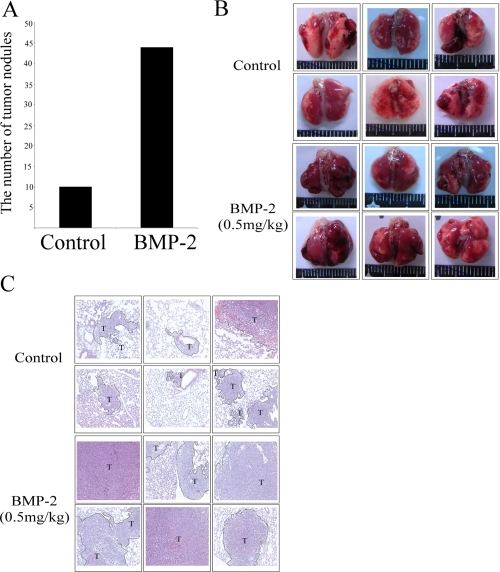
Next, we also used animal experiments to determine whether BMP-2 increases lung cancer metastasis in mice. We injected mouse lung cancer cell line LLC into mice and then allowed the cells to develop for 7 days. Treatment of mice by BMP-2 (0.5 mg/kg) increased the metastasis of LLC in 85% (six of seven) of the mice, in comparison with 75% (six of eight) of the control mice. In addition, the tumor nodules in the lungs of BMP-2-treated mice were greater than that of the control group mice (Fig. 9).
FIGURE 9.
BMP-2 increased the metastasis of lung cancer cell in vivo. A, BMP-2 increased tumor nodules in lungs. B and C, BMP-2 increased lung metastasis as revealed by photographs (B) and hematoxylin and eosin staining (C). LLC cells were injected into mice via the tail vein. The mice were dosed every 3 days with intraperitoneal injections PBS (n = 8) or BMP-2 (0.5 mg/kg) (n = 7). After 10 days, nontumorous and tumorous regions of the lungs were harvested, cut, hematoxylin- and eosin-stained, and analyzed by microscopy. The number of tumor nodules was recorded for analysis of lung cancer incidence. The asterisk indicates a significant difference with the control, as analyzed by analysis of variance with Student's t test post hoc. *, p < 0.05.
DISCUSSION
Bone is a common site of distant metastasis in cases of lung cancer (1, 24). Current studies show that BMP-2, found in the osteoblast, increases cell migration and EMT of pancreatic cancer cells (25). Invasive lung cancer patient sera containing high levels of BMP-2 also significantly increase the migration and invasion of A549 and CL1–0 cells. Pretreatment of A549 and CL1–0 cells with BMP-2 antagonist noggin decreases lung cancer patient sera-mediated cancer cell migration. Moreover, mice treated with BMP-2 showed increased number nodule of tumor in lung. These data suggest that BMP-2 may be a major factor enhancing the ability of lung cancer to metastasize from the primary site to bone.
Snail transcriptionally suppresses the adherent junction protein E-cadherin by binding to E2 box type elements within its promoter, resulting in epithelial-mesenchymal transition (26, 27). E-cadherin loss and EMT have been implicated in tumor progression and are closely correlated with poor prognosis (28). In our study, we found that A549 and CL1–0 cells treated with BMP-2 dramatically decrease the epithelial marker E-cadherin and increase mesenchymal markers vimentin and N-cadherin. Also, selective inhibition of Snail by siRNA decreased the effects of BMP-2 on cell migration and E-cadherin down-regulation, suggesting that the up-regulation of Snail plays a critical role in BMP-2-mediated cell migration and EMT.
An increasing body of evidence supports the hypothesis that tumors have the ability to adapt to new environments when cancer cells metastasize (29, 30). Osteomimicry, an ability of cancer to acquire a bone cell phenotype, especially osteoblast-like, enhances the ability of cancer to survive and proliferate in bone tissue (31, 32). In addition, stimulating bone remodeling gives tumor cells more possibilities to successfully colonize this environment and support further tumor growth (29). Runx2, a pivotal factor in the progression of osteogenesis, has been found to be up-regulated in various types of malignancies that are metastatic to bone (33). Also, overexpression of Runx2 in cancer cells increases the expression of bone matrix and adhesion proteins, matrix metalloproteinases, and pro-angiogenic factors, which are strongly associated with cancer metastasis (34, 35). In contrast, inhibition of Runx2 in cancer cells decreases tumor growth in bone and the accompanying osteolytic disease. Runx2 complex, together with co-regulatory factors SMADs and other co-activator and co-repressor proteins, regulate target gene transcription (36). p300, a histone acetyltransferase, has been reported to interact with Runx2 and to subsequently regulate the expression of osteocalcin and MMP-13 (37, 38). In addition, p300 also acts as a stabilizer of Runx2 via acetylation (39). Our data are the first to demonstrate that BMP-2 increases the expression of Runx2, which interacts with p300 protein and in turn enhances the acetylation of histone H3 and H4, resulting in the up-regulation of Snail. Knockdown Runx2 by specific siRNA reversed the effects of BMP-2 on the elevation of Snail protein and the enhancement of the migratory ability of cancer. Overexpression of Snail significantly reversed the inhibitory effect of Runx2 siRNA on cancer cell migration. Indeed, genetic blockade of p300 also attenuated the acetylation of histone H3 and H4 and up-regulation of Snail induced by BMP-2, suggesting cooperation between Runx2 and p300 in the regulation of Snail.
ERK1/2 and p38, belonging to members of the MAPK family, are an important regulatory factors in the control of cell proliferation, survival, and migration (40). The ERK pathway has been shown to phosphorylate Runx2 on residues, which strongly correlates with enhanced Runx2 transactivation in osteoblastic differentiation and skeletal development (41). Similarly, it has been shown that p38 pathway, by interacting with SMADs signaling, is involved in BMP-2 induced bone matrix gene expression (42). Our study observed an increase in Runx2 expression following ERK1/2 and p38 activation, whereas suppression of ERK1/2 and p38 signaling by inhibitors abrogated Runx2 up-regulation. These data suggest that activation of ERK1/2 and p38 acts as an upstream activator of osteoblast-specific Runx2 transcription factor in BMP-2-mediated cancer progression.
MAPK has been reported to be relevant for BMP-2 biologic functions. Activation of p38 signaling pathway is required for the regulation of cell migration and actin cytoskeleton remodeling by BMP-2 (10). Trigger of ERK signaling by BMP-2 increases the stability and transcriptional activity of Runx2, which cooperatively regulates osteoblast differentiation (39). How BMP-2 activates ERK1/2 and p38 MAPK signaling is not clearly understood. Previous study has indicated that binding of BMP-2 to preformed homomeric BMP type II receptor complexes activate the SMAD pathway, whereas BMP-2-induced recruitment of hetero-oligomeric BMP type II and BMP type I receptors activates a different, SMAD-independent pathway, resulting in the induction of alkaline phosphatase activity via p38 MAPK (43). Indeed, binding of TGF-β to receptor has been elucidated to be involved in recruitment and ubiquitylation of TRAF6/TGF-β-associated kinase 1 and subsequently rapidly activates the p38 pathway (44). However, whether BMP-2-mediated BMP type II receptor/BMP type I receptor activation alters ubiquitylation system or receptor conformation to regulate p38 or ERK1/2 signaling requires further study.
Taken together, our findings indicate that BMP-2 in the bone matrix cooperates in an enhanced effect on lung cancer EMT and migration. ERK1/2/Runx2/Snail and p38/Runx2/Snail have been found responsible for induction of lung cancer cell migration and EMT. This study provides novel evidence that Runx2 regulates early metastatic events in lung cancer by increasing Snail expression, which in turn enhances the migratory ability of cancer. These findings provide important insights into the biology of tumors, with vicious interaction of osteoblasts and cancer, and establish new rationales for using anti-BMP-2 and anti-Runx2/Snail strategies to target bone metastasis in lung cancer.
This work is supported by National Science Council of Taiwan Grant NSC 98-2320-B-037-007-MY3) and Excellence for Cancer Research Center Grant DOH100-TD-C-111-002 from the Department of Health, Executive Yuan, Taipei, Taiwan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- BMP
- bone morphogenetic protein
- EMT
- epithelial-to-mesenchymal transition
- TAOB
- tumor-associated osteoblast
- CM
- conditioned medium.
REFERENCES
- 1. Roodman G. D. (2004) N. Engl. J. Med. 350, 1655–1664 [DOI] [PubMed] [Google Scholar]
- 2. Coleman R. E., Lipton A., Roodman G. D., Guise T. A., Boyce B. F., Brufsky A. M., Clézardin P., Croucher P. I., Gralow J. R., Hadji P., Holen I., Mundy G. R., Smith M. R., Suva L. J. (2010) Cancer Treat. Rev. 36, 615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosen L. S., Gordon D., Tchekmedyian S., Yanagihara R., Hirsh V., Krzakowski M., Pawlicki M., de Souza P., Zheng M., Urbanowitz G., Reitsma D., Seaman J. J. (2003) J. Clin. Oncol. 21, 3150–3157 [DOI] [PubMed] [Google Scholar]
- 4. Kvale P. A., Simoff M., Prakash U. B. (2003) Chest 123, (Suppl. 1) 284S–311S [DOI] [PubMed] [Google Scholar]
- 5. Onishi T., Hayashi N., Theriault R. L., Hortobagyi G. N., Ueno N. T. (2010) Nat. Rev. Clin. Oncol. 7, 641–651 [DOI] [PubMed] [Google Scholar]
- 6. Juárez P., Guise T. A. (2011) Bone 48, 23–29 [DOI] [PubMed] [Google Scholar]
- 7. Ooi L. L., Zheng Y., Stalgis-Bilinski K., Dunstan C. R. (2011) Bone 48, 66–70 [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi A., Katagiri T., Ikeda T., Wozney J. M., Rosen V., Wang E. A., Kahn A. J., Suda T., Yoshiki S. (1991) J. Cell Biol. 113, 681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuo P. L., Hsu Y. L., Chang C. H., Chang J. K. (2005) J. Pharmacol. Exp. Ther. 314, 1290–1299 [DOI] [PubMed] [Google Scholar]
- 10. Gamell C., Susperregui A. G., Bernard O., Rosa J. L., Ventura F. (2011) PLoS One 6, e16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bunyaratavej P., Hullinger T. G., Somerman M. J. (2000) Exp. Cell Res. 260, 324–333 [DOI] [PubMed] [Google Scholar]
- 12. Graham T. R., Odero-Marah V. A., Chung L. W., Agrawal K. C., Davis R., Abdel-Mageed A. B. (2009) Prostate 69, 168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park Y., Kang M. H., Seo H. Y., Park J. M., Choi C. W., Kim Y. H., Kim I. S., Kim J. S., Oh S. C. (2010) Med. Oncol. 27, 1192–1199 [DOI] [PubMed] [Google Scholar]
- 14. Liu F., Hata A., Baker J. C., Doody J., Cárcamo J., Harland R. M., Massagué J. A. (1996) Nature 381, 620–623 [DOI] [PubMed] [Google Scholar]
- 15. Scherberich A., Tucker R. P., Degen M., Brown-Luedi M., Andres A. C., Chiquet-Ehrismann R. (2005) Oncogene 24, 1525–1532 [DOI] [PubMed] [Google Scholar]
- 16. Lai T. H., Fong Y. C., Fu W. M., Yang R. S., Tang C. H. (2008) Prostate 68, 1341–1353 [DOI] [PubMed] [Google Scholar]
- 17. Chen H. W., Lee J. Y., Huang J. Y., Wang C. C., Chen W. J., Su S. F., Huang C. W., Ho C. C., Chen J. J., Tsai M. F., Yu S. L., Yang P. C. (2008) Cancer Res. 68, 7428–7438 [DOI] [PubMed] [Google Scholar]
- 18. Chu Y. W., Yang P. C., Yang S. C., Shyu Y. C., Hendrix M. J., Wu R., Wu C. W. (1997) Am. J. Respir. Cell Mol. Biol. 17, 353–360 [DOI] [PubMed] [Google Scholar]
- 19. Georges S., Ruiz Velasco C., Trichet V., Fortun Y., Heymann D., Padrines M. (2009) Cytokine Growth Factor Rev. 20, 29–41 [DOI] [PubMed] [Google Scholar]
- 20. Osyczka A. M., Leboy P. S. (2005) Endocrinology 146, 3428–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuo P. L., Chen Y. H., Chen T. C., Shen K. H., Hsu Y. L. (2011) J. Cell Physiol. 226, 1224–1231 [DOI] [PubMed] [Google Scholar]
- 22. Moreno-Bueno G., Portillo F., Cano A. (2008) Oncogene 27, 6958–6969 [DOI] [PubMed] [Google Scholar]
- 23. Boumah C. E., Lee M., Selvamurugan N., Shimizu E., Partridge N. C. (2009) Mol. Endocrinol. 23, 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nannuru K. C., Singh R. K. (2010) Curr. Osteoporos Rep. 8, 105–113 [DOI] [PubMed] [Google Scholar]
- 25. Gordon K. J., Kirkbride K. C., How T., Blobe G. C. (2009) Carcinogenesis 30, 238–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galván J. A., González M. V., Crespo G., Folgueras M. V., Astudillo A. (2010) Lung Cancer 69, 289–295 [DOI] [PubMed] [Google Scholar]
- 27. Yanagawa J., Walser T. C., Zhu L. X., Hong L., Fishbein M. C., Mah V., Chia D., Goodglick L., Elashoff D. A., Luo J., Magyar C. E., Dohadwala M., Lee J. M., St John M. A., Strieter R. M., Sharma S., Dubinett S. M. (2009) Clin. Cancer Res. 15, 6820–6829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin Q., Li M., Shen Z. Y., Xiong L. W., Pan X. F., Gen J. F., Bao G. L., Sha H. F., Feng J. X., Ji C. Y., Chen M. (2010) Jpn. J. Clin. Oncol. 40, 670–676 [DOI] [PubMed] [Google Scholar]
- 29. Langley R. R., Fidler I. J. (2011) Int. J. Cancer 128, 2527–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graves E. E., Maity A., Le Q. T. (2010) Semin. Radiat. Oncol. 20, 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knerr K., Ackermann K., Neidhart T., Pyerin W. (2004) Int. J. Cancer. 111, 152–159 [DOI] [PubMed] [Google Scholar]
- 32. Clezardin P., Teti A. (2007) Clin. Exp. Metastasis 24, 599–608 [DOI] [PubMed] [Google Scholar]
- 33. Baniwal S. K., Khalid O., Gabet Y., Shah R. R., Purcell D. J., Mav D., Kohn-Gabet A. E., Shi Y., Coetzee G. A., Frenkel B. (2010) Mol. Cancer 9, 258–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Onodera Y., Miki Y., Suzuki T., Takagi K., Akahira J., Sakyu T., Watanabe M., Inoue S., Ishida T., Ohuchi N., Sasano H. (2010) Cancer Sci. 101, 2670–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akech J., Wixted J. J., Bedard K., van der Deen M., Hussain S., Guise T. A., van Wijnen A. J., Stein J. L., Languino L. R., Altieri D. C., Pratap J., Keller E., Stein G. S., Lian J. B. (2010) Oncogene 29, 811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pratap J., Wixted J. J., Gaur T., Zaidi S. K., Dobson J., Gokul K. D., Hussain S., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2008) Cancer Res. 68, 7795–7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee M., Partridge N. C. (2010) J. Biol. Chem. 285, 38014–38022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paredes R., Arriagada G., Cruzat F., Olate J., Van Wijnen A., Lian J., Stein G., Stein J., Montecino M. (2004) J. Steroid Biochem. Mol. Biol. 89–90, 269–271 [DOI] [PubMed] [Google Scholar]
- 39. Jun J. H., Yoon W. J., Seo S. B., Woo K. M., Kim G. S., Ryoo H. M., Baek J. H. (2010) J. Biol. Chem. 285, 36410–36419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cuadrado A., Nebreda A. R. (2010) Biochem. J. 429, 403–417 [DOI] [PubMed] [Google Scholar]
- 41. Park O. J., Kim H. J., Woo K. M., Baek J. H., Ryoo H. M. (2010) J. Biol. Chem. 285, 3568–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greenblatt M. B., Shim J. H., Zou W., Sitara D., Schweitzer M., Hu D., Lotinun S., Sano Y., Baron R., Park J. M., Arthur S., Xie M., Schneider M. D., Zhai B., Gygi S., Davis R., Glimcher L. H. (2010) J. Clin. Invest. 120, 2457–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nohe A., Hassel S., Ehrlich M., Neubauer F., Sebald W., Henis Y. I., Knaus P. (2002) J. Biol. Chem. 277, 5330–5338 [DOI] [PubMed] [Google Scholar]
- 44. Sorrentino A., Thakur N., Grimsby S., Marcusson A., von Bulow V., Schuster N., Zhang S., Heldin C. H., Landström M. (2008) Nat. Cell Biol. 10, 1199–1207 [DOI] [PubMed] [Google Scholar]