Abstract
The Ca2+ signaling pathway appears to regulate the processes of the early development through its antagonism of canonical Wnt/β-catenin signaling pathway. However, the underlying mechanism is still poorly understood. Here, we show that nuclear factor of activated T cells (NFAT), a component of Ca2+ signaling, interacts directly with Dishevelled (Dvl) in a Ca2+-dependent manner. A dominant negative form of NFAT rescued the inhibition of the Wnt/β-catenin pathway triggered by the Ca2+ signal. NFAT functioned downstream of β-catenin without interfering with its stability, but influencing the interaction of β-catenin with Dvl by its competitively binding to Dvl. Furthermore, we demonstrate that NFAT is a regulator in the proliferation and differentiation of neural progenitor cells by modulating canonical Wnt/β-catenin signaling pathway in the neural tube of chick embryo. Our findings suggest that NFAT negatively regulates canonical Wnt/β-catenin signaling by binding to Dvl, thereby participating in vertebrate neurogenesis.
Keywords: β-Catenin, Calcineurin, Neurodifferentiation, Neuroprogenitor Cell, NFAT Transcription Factor, Wnt Pathway, Dishevelled
Introduction
Wnt signaling plays important roles in various physiological and pathological processes (1–4). The Wnt pathway has been divided into two branches, β-catenin-dependent (5) and β-catenin-independent (6–8). The β-catenin-dependent pathway, also called canonical Wnt pathway, triggers its signaling by disrupting the β-catenin destruction complex to block β-catenin phosphorylation, subsequently leading to the stabilization of cytoplasmic β-catenin. The accumulated β-catenin then translocates to the nucleus where it regulates target gene expression through partnerships with the lymphoid enhancer-binding factor 1 (LEF1)2/T cell-specific transcription factors (TCF) family (5).
Dishevelled (Dvl) is an essential effector in this pathway. Despite its function on β-catenin accumulation, it also plays a role at β-catenin transcriptional activity. Previous studies have demonstrated that Dvl is also localized in the nucleus (9–11), and its nuclear localization is required for canonical Wnt signaling (10, 11). Nuclear Dvl associates with the β-catenin-LEF1/TCF transcriptional complex by interacting with β-catenin and c-Jun to stabilize β-catenin-LEF1/TCF transcriptional complex formation (11).
Proteins of the nuclear factor of activated T cells (NFAT) family are Ca2+-regulated transcription factors, which were initially identified in the immune system but now are known to have integral function in multiple signaling pathways (12–16). Five members of this family, including NFATc1/2/c, NFATc2/1/p, NFATc3/4/x, NFATc4/3, and NFAT5/TonEBP, have been identified in mammals. All members of the NFAT family have highly conserved DNA-binding domain, the Rel homology region (RHR), which confers common DNA-binding specificity (17). Except for NFAT5, NFATc1–c4 are activated upon a rise in intracellular Ca2+, which stimulates the serine/threonine phosphatase activity of calcineurin (18, 19). Calcineurin directly dephosphorylates NFAT resulting in the nuclear import of NFAT. In the nucleus, NFAT transcription complexes reassemble on DNA to initiate gene transcription (19). It has been revealed that NFAT can act as both a positive and negative regulator of transcription. A study of cyclin-dependent kinase 4 (CDK4) suggested that NFATc2 represses CDK4 expression (20). NFATc1 also induces transcriptional repression by interacting with histone deacetylases after sumoylation modification (21, 22).
Previously, it has been reported that Ca2+ signaling or Wnt/Ca2+ signaling can block the canonical Wnt signaling transduction through an activation of NFAT in Xenopus embryos (23). However, the underlying mechanism is not clear. Here, we report that NFAT participates in the inhibition of Wnt signaling through direct interaction with Dvl in the nucleus. The binding of NFAT to Dvl prevents Dvl recruiting to the β-catenin transcription complex, thus reducing the transcriptional activity of β-catenin. Furthermore, our data also reveal that the cross-talk between NFAT and Wnt signaling is involved in the proliferation and differentiation of neural progenitor cells in the neural tube of chick embryo.
EXPERIMENTAL PROCEDURES
Constructs, Antibodies, Ligand, and Chemicals
Mouse NFATc4 cDNA was cloned into CMV promoter-based mammalian cell expression vector and subcloned into pET-28c plasmid. The expression plasmid of Dvl has been described previously (11). Constitutively active calcineurin (ΔCnA) was a gift from Dr. C. W. Chow at Albert Einstein College of Medicine, New York. Mouse anti-β-catenin (BD Bioscience), anti-TCF-4 (Millipore), anti-FLAG (Sigma), anti-HA (Covance), and rabbit anti-phosphor-β-catenin (Ser-33/Ser-37/Thr-41) (Cell Signaling Technology) were acquired commercially. Wnt-3a conditioned medium (CM) and its control were described previously (24). Ionomycin and cyclosporin A (CsA) were from Sigma.
Cell Transfection and Luciferase Assay
HEK293T were transfected with DNA using Lipofectamine Plus (Invitrogen). SW480, Caco-2, and LS174T cells were transfected with DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The LacZ plasmid was added to make the total amount of DNA equal (0.5 μg/well in a 24-well plate for SW480 cells and 0.25 μg/well for HEK293T cells). Luciferase assays were performed as described previously (25).
Immunoprecipitation, Protein Purification, and in Vitro Pulldown Assay
HEK293T cells were transiently transfected with the indicated constructs for 24 h. A co-immunoprecipitation experiment was then performed as described previously (25). His6-tagged and GST-fused proteins were expressed in Escherichia coli and were partially purified with nickel-nitrilotriacetic acid and GSH-Sepharose 4B beads, respectively. The in vitro pulldown assay was performed as described previously (11).
RT-PCR and Quantitative Real-time PCR
The extraction of Total RNAs and the reverse transcription of the purified RNA was performed as described previously (11). The quantification of all gene transcripts was done by quantitative PCR using the SYBR Premix Ex Taq (TaKaRa) and an ABI 7500 apparatus (ABI). The primer pair used for human Axin2 was 5′-CTGGCTTTGGTGAACTGTTG-3′ and 5′-AGTTGCTCACAGCCAAGACA-3′, for DKK1 was 5′-CTGCAAAAATGGAATATGTGT-3′ and 5′-CTTCTTGTCCTTTGGTGTGA-3′, and for GAPDH was 5′-GCACCACCAACTGCTTA-3′ and 5′-AGTAGAGGCAGGGATGAT-3′.
Cell Fractionations and Endogenous Interaction
HEK293T cells were scraped into PBS and were fractionationed as described previously (26). For the endogenous interaction assay, the nucleus of 3–5 × 107 HEK293T cells were lysed, and the nuclear extracts were sonicated four times for 5 s in 1 ml of buffer containing 20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Triton X-100, 2 mm EDTA, and CompleteTM protease inhibitors (Roche Applied Science).
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed as described previously (27); however, the immunoprecipitated DNA was quantified using quantitative PCR. The primer pair for human Axin2 TBE site was 5′-CTGGAGCCGGCTGCGCTTTGATAA-3′ and 5′-CGGCCCCGAAATCCATCGCTCTGA-3′. For human c-myc TBE site was 5′-CTCTCCACTTGCCCCTTTTAG-3′ and 5′-GAAGAGACAAATCCCCTTTGC-3′.
In Ovo Chick Embryo Electroporation
Fertilized eggs were obtained from the Shanghai Academy of Agricultural Sciences. The chick embryo in ovo electroporation was performed as described previously (28).
In Situ Hybridization
Section in situ hybridization was performed as described previously (29).
RESULTS AND DISCUSSION
Identification of NFAT as a Dvl-binding Protein
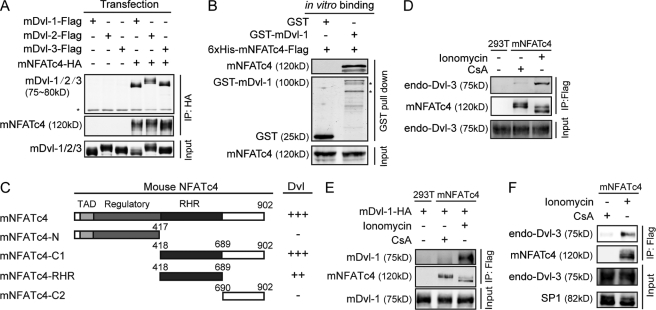
The Dvl protein family is an essential yet also perhaps the most enigmatic component of the Wnt signaling pathway. To address its function further, we sought to identify proteins acting directly with Dvl. A yeast two-hybrid screen of a mouse embryonic cDNA library was performed using mouse Dvl-1-C1 (residues 377–695) as the bait. A positive clone that encodes the C-terminal half of mouse NFATc4 (mNFATc4-C1) interacted strongly with the bait. To confirm this interaction, we carried out co-immunoprecipitation after transfecting HEK293T cells with HA-tagged mNFATc4 and FLAG-tagged Dvl-1, 2, and 3. All three Dvl homologs were co-immunoprecipitated with HA-tagged mNFATc4 (Fig. 1A), which suggests that NFAT binds to Dvl and has a universal binding activity to Dvl family members. We further purified His6-tagged full-length mNFATc4 and GST-mDvl-1 from E. coli. GST-mDvl-1, not GST alone, pulled down recombinant His6-tagged mNFATc4 in vitro (Fig. 1B), suggesting that NFAT can physically interact with Dvl. Moreover, we delineated the binding region within mNFATc4. As described in Fig. 1C, the N terminus of NFAT composed of the transactivation domain and regulatory domain is dispensable for interacting with Dvl, whereas the RHR has binding activity slightly less than that of full-length NFAT. Thus, we consider the RHR as the region critical for binding to Dvl.
FIGURE 1.
NFAT interacts directly with Dvl under the stimulation of calcium signaling. A, NFAT interacts with the Dvl family in HEK293T cells. The cells were transfected with the indicated plasmids. 24 h after transfection, a co-immunoprecipitation(IP) assay was performed, and immunoprecipitates were resolved with Western blotting. *, IgG. B, NFAT physically interacts with Dvl in vitro. His6-mNFATc4-FLAG was mixed with GST-mDvl-1 or GST-bound GSH-Sepharose 4B for 3 h. Samples were subjected to SDS-PAGE and Western blotting. *, nonspecific band. C, schematic represents mapping of the Dvl-interacting region in mNFATc4. The numbers indicate amino acids. The binding of full-length NFAT and various NFAT fragment to Dvl was examined by co-immunoprecipitation. TAD, transactivation domain. −, no interaction; +, strength of interaction. D, endogenous interaction occurred between NFAT and Dvl-3 upon stimulation with CsA or ionomycin. mNFATc4-FLAG stable cells were stimulated with 1 μg/ml CsA or 1 μg/ml ionomycin for 8 h. A co-immunoprecipitation assay was carried out with an anti-Dvl-3 antibody. E, difference in the interaction with Dvl between two different forms of NFAT is shown. NFAT stable cells were stimulated as indicated, and then the immunoprecipitation was carried out with anti-FLAG antibody. 2 h later, the Dvl expressed in 293T cells was added for another 1 h. F, endogenous interaction between NFAT and Dvl-3 in the nucleus. mNFATc4-Flag stable cells were stimulated with 1 μg/ml CsA or 1 μg/ml ionomycin for 8 h. Nuclear extracts were lysed for the co-IP experiment with anti-FLAG antibody.
Proteins of the NFAT family play important roles in the Ca2+ signaling which induces dephosphorylation of NFAT leading to its nuclear transport. Thus, we examined whether calcium affected the interaction of NFAT and Dvl. We used the calcium ionophore ionomycin to mimic the Ca2+ signaling (30–33). Because the available NFAT-specific antibody does not detect endogenous NFAT well, we stably expressed FLAG-tagged mNFATc4 in HEK293T cells. NFAT was highly phosphorylated upon the stimulation with CsA, a calcineurin inhibitor, and was dephosphorylated after ionomycin was added (supplemental Fig. S1A), indicating that the cells responded to calcium signaling. We then carried out immunoprecipitation to detect the interaction of endogenous Dvl-3 with NFAT upon CsA or ionomycin treatment. As shown in Fig. 1D, Dvl-3 efficiently co-immunoprecipitated with NFAT in the presence of ionomycin, but did so weakly upon CsA treatment. To confirm further whether the two different forms of NFAT caused by CsA and ionomycin treatment have different affinity for Dvl, we stimulated the cells stably expressing NFAT with CsA or ionomycin and carried out immunoprecipitation with a FLAG antibody. Then, Dvl expressed in HEK293T cells was added. As shown in Fig. 1E, NFAT from Ca2+-stimulated samples appeared to have a higher binding affinity for Dvl. Given that the dephosphorylated form of NFAT is located predominantly in the nucleus. We further performed a co-IP experiment using an extract of the nucleus fraction to verify whether the interaction happens in the nucleus. Indeed, NFAT forms complex with Dvl in the nucleus upon the stimulation of Ca2+ signal (Fig. 1F). These data suggest that the interaction between NFAT and Dvl possibly takes place in the nucleus upon Ca2+ concentration elevation.
NFAT Negatively Regulates Wnt Signaling
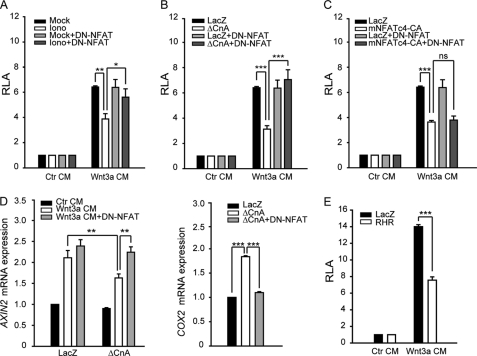
It has been reported that Ca2+ or Wnt/Ca2+ signaling plays a negative role in regulating Wnt/β-catenin signaling (23). Thus, we used the LEF1/TCF-dependent reporter TOP-Flash to validate its effect in our system. As shown in Fig. 2A, ionomycin or expression of ΔCnA inhibited reporter gene activity induced by Wnt3a CM. This inhibition was reversed by a dominant negative form of mNFATc4 (DN-NFAT, mNFATc4-N in Fig. 1C) which only contains the N-terminal region of mNFATc4 and blocks endogenous NFAT activation (34–36) (supplemental Fig. S1B). However, DN-NFAT could not reverse the inhibitory effect caused by a constitutively active form of mNFATc4 (mNFATc4-CA) (Fig. 2C). mNFATc4-CA lacks its phosphorylation sites (sp1, sRR1/2) and is constitutively localized in the nucleus (supplemental Fig. S1C). It also interacts with Dvl (supplemental Fig. S1D). We also examined the effects of calcineurin or DN-NFAT expression on the mRNA levels of Axin-2 and DKK1, specific Wnt target genes (Fig. 2D and supplemental Fig. S2A), which provides a coincident result as reporter gene assay. These data confirmed the previous findings that NFAT is not only a component of calcium signaling (Fig. 2D, right), but also a regulator that mediates the inhibition of the Wnt pathway by calcium signaling, especially via calcineurin.
FIGURE 2.
NFAT participates in the inhibition of Wnt pathway triggered by calcium signal. A and B, DN-NFAT rescues the inhibition of Wnt3a CM-induced TOP-Flash activity by ionomycin and ΔCnA. 18 h after transfection, HEK293T cells were stimulated with Wnt3a CM for 6 h. Then, cells were lysed, and luciferase activities were determined as described previously (44). C, DN-NFAT has no effect on the inhibition of Wnt3a CM-induced TOP-Flash activity by NFAT-CA. D, DN-NFAT can rescue the Wnt-induced the Axin-2 expression. 18 h after transfection, cells were stimulated with Wnt3a CM for 6 h. The Axin-2 mRNA level was detected by quantitative PCR using GAPDH as an inner control. E, the RHR domain also negatively regulates Wnt3a CM-induced TOP-Flash activity. The data are expressed as the mean ± S.E. (error bars) of at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
NFAT proteins can also act as transcriptional activator. We investigated whether the transcriptional activity of NFAT was required for its inhibitory effect on Wnt signaling. The RHR domain of mNFATc4 contains the DNA-binding activity but not transactivation activity (supplemental Fig. S2B). The fact that RHR alone could suppress Wnt signaling (Fig. 2E) suggests that the transcriptional activity of NFAT is dispensable for its ability to suppress Wnt signaling.
NFAT Functions Downstream of β-Catenin
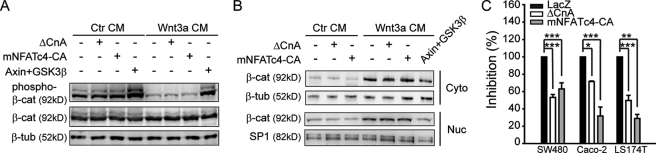
It has been reported previously that NFAT participates in the regulation of Wnt signaling by acting upstream of β-catenin (23). However, in our system, neither calcineurin expression nor NFAT-CA expression interfered with the contents of phosphorylated β-catenin (Ser-33, Ser-37, and Thr-41) or β-catenin nuclear accumulation whereas expression of GSK3β or Axin did (Fig. 3, A and B). These results indicate that NFAT may not influence the stability of β-catenin. Therefore, we postulated that NFAT may repress Wnt signaling in the nucleus by blocking the transactivation function of β-catenin. This hypothesis is consistent with the aforementioned finding that the interaction of NFAT with Dvl is induced by Ca2+ signals in the nucleus. To test this hypothesis, LS174T cells that contain oncogenic mutation of β-catenin gene (37, 38) and Caco-2 or SW480 cells which contain mutated adenomatous polyposis coli (APC) (39) were used to examine the effect of Ca2+ signaling/NFAT on Wnt reporter activity. As shown in Fig. 3C, overexpression of calcineurin or NFAT-CA led to a repression of Wnt reporter gene activity in these cells. Taken together, these data suggest that calcineurin and NFAT regulate Wnt signaling downstream of β-catenin. Saneyoshi et al. reported that NFAT functions upstream of β-catenin when co-expressed with β-catenin in Xenopus (23). We believed that this difference may be caused by using different system or different detection approach.
FIGURE 3.
NFAT functions downstream of β-catenin. A, ΔCnA and NFAT-CA do not influence the phosphorylation level of endogenous β-catenin. After transfection, HEK293T cells were stimulated with 20 μm MG132 and Wnt3a CM for 3 h. Then, cells were lysed, and the phosphorylation level of β-catenin was detected by Western blotting. B, ΔCnA and NFAT-CA do not affect the accumulation and subcellular translocation of β-catenin. After transfection, cells were stimulated with Wnt3a CM for 3 h. The levels of cytoplasmic and nuclear β-catenin were resolved with Western blotting. C, ΔCnA and NFAT-CA inhibit Wnt signaling in three colon cancer cell lines. The cells were transfected with FOP-Flash or TOP-Flash along with ΔCnA or NFAT-CA. 24 h after transfection, cells were lysed, and luciferase activity was detected. The inhibition represents the ratio of TOP-Flash to FOP-Flash, and the activity of LacZ was set as 100%. The data are expressed as the mean ± S.E. (error bars) of at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
NFAT Physically Influences the Interaction between Dvl and β-Catenin
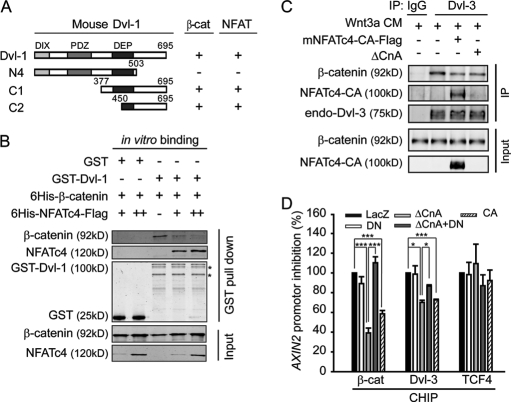
We further delineated the regions within Dvl responsible for its interaction with NFAT to residues 450–695, which is the same region that binds to β-catenin (Fig. 4A). The interaction of Dvl with β-catenin was shown to enhance the stability of β-catenin-TCF complex (11). Thus, we suspected that NFAT might disrupt the interaction between Dvl and β-catenin to inhibit Wnt/β-catenin signaling. We purified His6-tagged β-catenin, His6-tagged NFAT-FLAG, and GST-mDvl-1 from E. coli. In the competition binding assay, NFAT disrupted GST-mDvl-1 and His6-β-catenin interaction in a dose-dependent manner (Fig. 4B). Furthermore, the endogenous interaction of Dvl and β-catenin in the nucleus under Wnt stimulation could also be disrupted by overexpression of NFAT-CA and ΔCnA (Fig. 4C). These data together suggest that NFAT inhibits Wnt signaling by interfering with the formation of stable Dvl-β-catenin complex. Given that the interaction of Dvl and β-catenin is important for the transcription complex formation on the Wnt target gene promoter (11), we used ChIP to determine the effect of NFAT on the binding of β-catenin and Dvl to the Axin-2 (Fig. 4D) and the c-myc promoters (supplemental Fig. S2C). Overexpression of ΔCnA or NFAT-CA decreased the amounts of Dvl and β-catenin on the Axin-2 promoters, whereas TCF4 bound to the promoters remained unaffected (Fig. 4D). Moreover, DN-NFAT could rescue the inhibition effect of ΔCnA (Fig. 4D). These results support a conclusion that NFAT functions downstream of β-catenin probably by disrupting the interaction of β-catenin with Dvl.
FIGURE 4.
NFAT disrupts the interaction between Dvl and β-catenin. A, schematic represents mDvl-1 fragments. The interaction of Dvl-1 with β-catenin or NFAT was detected by co-immunoprecipitation. B, NFAT competes with β-catenin in binding to Dvl-1 in an in vitro binding assay. His6-β-catenin was mixed with GST-mDvl-1 or GST-bound GSH-Sepharose 4B with or without His6-mNFATc4-FLAG for 3 h. Samples were subjected to Western blotting. C, NFAT-CA and ΔCnA reduce the endogenous interaction of Dvl-3 and β-catenin in the nucleus. HEK293T cells were treated with Wnt3a CM for 3 h. Nuclear extracts were lysed for the co-immunoprecipitation experiment with anti-Dvl-3 antibody. D, ΔCnA and NFAT-CA affect the accumulation of β-catenin and Dvl-3 on the promoter of the Axin2 gene. HEK293T cells were treated with Wnt3a CM for 2 h, and ChIP-quantitative PCR analyses were performed with anti-β-catenin, anti-Dvl-3, and anti-TCF4 antibodies and normalized by input. The activity of LacZ was set as 100%. The data are expressed as the mean ± S.E. (error bars) of three independent experiments. *, p < 0.05; ***, p < 0.001.
NFAT Antagonizes Wnt Signal in Regulating the Proliferation and Differentiation of the Neural Progenitor Cells in the Neural Tube of Chick Embryo
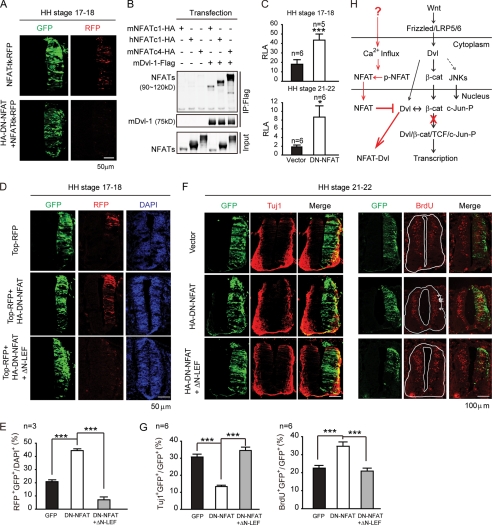
To investigate the physiological role of NFAT in modulating canonical Wnt signaling, we extended our analysis to the central nerve system development in chick embryos. Wnt proteins are important morphogens during the development of the CNS. Wnt signaling through activation of the β-catenin/TCF pathway promotes the proliferation of neural precursor cells and prevents differentiation (40). We sought to determine whether NFAT plays a role in this process via its regulation of the Wnt/β-catenin signaling. First, we performed in situ hybridization analysis to detect the expression of three predicted chick NFAT genes, NFATc1, c2, and c3 in the developing spinal cord. As shown in supplemental Fig. S3, NFATc1 is expressed predominantly in the neural tube among three NFATs in chick spinal cord. Interestingly, the expression of NFATc1 is restricted in the ventricular zone during development, and it is highly expressed in the late stages, where Wnt activity must be limited for progenitor cell differentiation (40). Second, we electroporated a NFAT reporter pNFAT-RE-TK-RFP into the developing neural tube of chicken embryos at Hamburger and Hamilton (HH) stages 10 and 11 (41) to detect the active form of endogenous NFAT. The expression of RFP was controlled by four repeats of the NFAT responsible element (NFAT-RE) from NFAT-luciferase reporter and was analyzed at HH stages 17 and 18 to visualize the endogenous NFAT activity. As shown in Fig. 5A, RFP was expressed across the neural tube from the dorsal to the ventral side, which indicates that endogenous NFAT is activated during the course of development. Moreover, we tested whether NFATc1 could also interact with Dvl. We cloned both human and mouse NFATc1, respectively, and the immunoprecipitation was carried out. As shown in Fig. 5B, hNFATc1 and mNFATc1 were co-immunoprecipitated with Dvl as well as mNFATc4. Given that the RHR domain in NFAT is responsible for binding to Dvl and it is conserved among species, we assume that chick NFAT protein could also interact with Dvl.
FIGURE 5.
NFAT negatively regulates Wnt signal in the proliferation and differentiation of neural progenitor cell in the neural tube of chick embryo. A, endogenous NFAT activity in the chick neural tube is shown. At HH stages 10–11, pNFAT-RE-TK-RFP and pCIG2-IRES-GFP were co-electroporated with the vector or DN-NFAT into the chick neural tubes. The transverse sections of HH stages 17–18 were analyzed for the expression of GFP and RFP. B, mouse and human NFATc1 also interact with Dvl as well as NFATc4. The cells were transfected with the indicated plasmids. 24 h after transfection, a co-immunoprecipitation assay was performed with an anti-FLAG antibody. C, DN-NFAT up-regulates endogenous Wnt-induced TOP-Flash activity in the chick neural tube. The experiment was performed as in A with the expression plasmids as indicated. The electroporated portions of HH stages 17–18 and HH stages 21–22 were dissected using luciferase assay normalized by Renilla. D, DN-NFAT up-regulates endogenous Wnt-induced TOP-TK-RFP expression throughout the whole neural tube. The experiment was performed as in A with the expression plasmids as indicated. E, the ratio of RFP+GFP+ (yellow) in all of the DAPI+ (blue) cells is shown. F, DN-NFAT regulates the proliferation and differentiation of neural progenitor cells through modulation of the Wnt signaling. The right side of the neural tube was electroporated with the indicated plasmids. The transverse sections of HH stages 22–23 were immunostained with an anti-Tuj1 antibody. For BrdUrd labeling, electroporated embryos were injected with BrdUrd and incubated for 1 h. They were harvested at HH stages 22 and 23. Transverse sections were immunostained with anti-BrdUrd antibody. G, the ratio of Tuj1+GFP+ (yellow) and BrdUrd+GFP+ (yellow) in all of the GFP+-transfected cells (green) in D is shown. H, model for NFAT in modulating canonical Wnt signaling is shown. The error bars indicate S.E. *, p < 0.05; ***, p < 0.001.
We further co-electroporated the pTOP-Flash reporter with DN-NFAT to test whether endogenous NFAT would modulate Wnt signaling in the neural tube. Overexpression of DN-NFAT which inhibits endogenous NFAT activity (Fig. 5A) increased the luciferase activity of pTOP-Flash (Fig. 5C), which suggests an inhibitory effect of NFAT in Wnt signaling. Because chick NFATc1 is expressed predominantly in the neural tube (supplemental Fig. S3), we performed RNAi experiment to knock down endogenous cNFATc1 (supplemental Fig. S4A), which led to increase in the activity of TOP-Flash (supplemental Fig. S4B). We also used pTOP-TK-RFP to mark the range of activity of the Wnt signaling. RFP expression was also up-regulated by DN-NFAT and spread throughout the whole neural tube, with a disturbed Wnt gradient along the dorsal-ventral axes (Fig. 5, D and E). Taken together, these results indicate that endogenous NFAT activity also negatively regulates Wnt signaling in chick neural tube.
During CNS development, the canonical Wnt pathway functions as a mitogenic signal of neural progenitor cells (40). Activation of the Wnt/β-catenin pathway blocks neural precursors differentiation (42). Thus, we hypothesized that NFAT may participate in regulating this process by interfering with Wnt signaling. Immunostaining of Tuj1, a neuron-specific class III β-tubulin, was used to measure the number of mature neurons at HH stages 20 and 21. Overexpression of DN-NFAT significantly decreased Tuj1-positive cells on the transfected side compared with the control side, whereas transfection of a GFP plasmid had no effect (Fig. 5, F and G, left). In contrast, BrdUrd staining, which monitors the proliferation of neural progenitor cells, was increased by DN-NFAT (Fig. 5, F and G, right). These data indicate that endogenous Ca2+/NFAT signaling negatively regulates neural progenitor cell proliferation and positively regulates differentiation. To test whether this function depends on its inhibition of Wnt signaling, we co-electroporated with ΔN-LEF1 to repress Wnt signaling through binding to the TBE site (Fig. 5, F and G). Consistent with our hypothesis, co-expression of ΔN-LEF1 rescued the decrease of Tuj1-positive cell number as well as the increase of BrdUrd cell numbers (Fig. 5, F and G). Taken together, the above data suggest that NFAT pathway regulates neural progenitor cell proliferation and differentiation in the neural tube of chick embryo by antagonizing Wnt signaling. We noticed that Graef and colleagues employed triple mutant mice to illustrate that the NFATc2/c3/c4 mutant did not interfere with βIII-tubulin or certain cell-specific markers in the CNS (43). In our system, chick NFATc1, among the three chick NFAT types, was expressed predominantly in the neural tube. Furthermore, we used a dominant negative form of NFAT to block the activation of all three NFAT types. Thus, as a result, we observed the function of NFAT in the regulation of neural differentiation. The negative regulation of Wnt signaling by NFAT allows the progenitor cell to exit the cell cycle and differentiate (Fig. 5, F and G). However, the upstream stimulation of NFAT is not clear yet. The Ca2+-calcineurin signal is the most important signal for regulating NFAT activation, but the signal that leads to Ca2+ influx during neural tube differentiation is still unclear.
In summary, we propose a framework in which NFAT modulates canonical Wnt signaling based on this work and our previous finding (11) (Fig. 5H). NFAT negatively regulates the Wnt signaling through interacting with Dvl, which disrupts the association of Dvl and β-catenin, leading to the destabilization of β-catenin-LEF1/TCF transcriptional complex. Furthermore, NFAT functions as a “modulator” of neural progenitor cell differentiation. In the neural tube of chick embryos, endogenous NFAT is progressively expressed in the ventricular zone of neural tube at the time when the Wnt signaling needs to be reduced. The suppression of the Wnt signaling induces precursor cells to exit the cell cycle and promotes their differentiation, which comprises the function of NFAT in the development of the CNS.
Acknowledgment
We thank D. Li for critical reading and commenting on this paper.
This work is supported by Ministry of Science and Technology of China Grants 2010CB912100 and 2007CB914500, National Natural Science Foundation of China Grants 30821065, 30930052, and 90813024, and the Science and Technology Commission of Shanghai Municipality.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- LEF1
- lymphoid enhancer-binding factor 1
- CA
- constitutively active
- CM
- conditioned medium
- ΔCnA
- constitutively active form of calcineurin
- CsA
- cyclosporin A
- DN
- dominant negative
- Dvl
- Dishevelled
- HH stages
- Hamburger and Hamilton stages
- NFAT
- nuclear factor of activated T cells
- RE
- responsible element
- RHR
- Rel homology region
- TCF
- T cell-specific transcription factor
- TK
- thymidine kinase
- RFP
- red fluorescent protein.
REFERENCES
- 1. Nusse R. (2005) Cell Res. 15, 28–32 [DOI] [PubMed] [Google Scholar]
- 2. Clevers H. (2006) Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 3. Bisson I., Prowse D. M. (2009) Cell Res. 19, 683–697 [DOI] [PubMed] [Google Scholar]
- 4. Lai S. L., Chien A. J., Moon R. T. (2009) Cell Res. 19, 532–545 [DOI] [PubMed] [Google Scholar]
- 5. Logan C. Y., Nusse R. (2004) Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 6. Kühl M., Sheldahl L. C., Park M., Miller J. R., Moon R. T. (2000) Trends Genet. 16, 279–283 [DOI] [PubMed] [Google Scholar]
- 7. Kohn A. D., Moon R. T. (2005) Cell Calcium 38, 439–446 [DOI] [PubMed] [Google Scholar]
- 8. Semenov M. V., Habas R., Macdonald B. T., He X. (2007) Cell 131, 1378. [DOI] [PubMed] [Google Scholar]
- 9. Torres M. A., Nelson W. J. (2000) J. Cell Biol. 149, 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Itoh K., Brott B. K., Bae G. U., Ratcliffe M. J., Sokol S. Y. (2005) J. Biol. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gan X. Q., Wang J. Y., Xi Y., Wu Z. L., Li Y. P., Li L. (2008) J. Cell Biol. 180, 1087–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rao A., Luo C., Hogan P. G. (1997) Annu. Rev. Immunol. 15, 707–747 [DOI] [PubMed] [Google Scholar]
- 13. Crabtree G. R., Olson E. N. (2002) Cell 109, S67–79 [DOI] [PubMed] [Google Scholar]
- 14. Macian F. (2005) Nat. Rev. Immunol. 5, 472–484 [DOI] [PubMed] [Google Scholar]
- 15. Hogan P. G., Chen L., Nardone J., Rao A. (2003) Genes Dev. 17, 2205–2232 [DOI] [PubMed] [Google Scholar]
- 16. Wan Y. Y., Flavell R. A. (2009) J. Mol. Cell. Biol. 1, 20–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain J., Burgeon E., Badalian T. M., Hogan P. G., Rao A. (1995) J. Biol. Chem. 270, 4138–4145 [PubMed] [Google Scholar]
- 18. Clipstone N. A., Crabtree G. R. (1992) Nature 357, 695–697 [DOI] [PubMed] [Google Scholar]
- 19. Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., Curran T., Rao A. (1993) Nature 365, 352–355 [DOI] [PubMed] [Google Scholar]
- 20. Baksh S., Widlund H. R., Frazer-Abel A. A., Du J., Fosmire S., Fisher D. E., DeCaprio J. A., Modiano J. F., Burakoff S. J. (2002) Mol. Cell 10, 1071–1081 [DOI] [PubMed] [Google Scholar]
- 21. Terui Y., Saad N., Jia S., McKeon F., Yuan J. (2004) J. Biol. Chem. 279, 28257–28265 [DOI] [PubMed] [Google Scholar]
- 22. Nayak A., Glöckner-Pagel J., Vaeth M., Schumann J. E., Buttmann M., Bopp T., Schmitt E., Serfling E., Berberich-Siebelt F. (2009) J. Biol. Chem. 284, 10935–10946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saneyoshi T., Kume S., Amasaki Y., Mikoshiba K. (2002) Nature 417, 295–299 [DOI] [PubMed] [Google Scholar]
- 24. Mao J., Wang J., Liu B., Pan W., Farr G. H., 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., Wu D. (2001) Mol. Cell 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 25. Li L., Yuan H., Weaver C. D., Mao J., Farr G. H., 3rd, Sussman D. J., Jonkers J., Kimelman D., Wu D. (1999) EMBO J. 18, 4233–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li M., Wang H., Huang T., Wang J., Ding Y., Li Z., Zhang J., Li L. (2010) J. Biol. Chem. 285, 13397–13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang G., Balamotis M. A., Stevens J. L., Yamaguchi Y., Handa H., Berk A. J. (2005) Mol. Cell 17, 683–694 [DOI] [PubMed] [Google Scholar]
- 28. Yan Y., Bian W., Xie Z., Cao X., Le Roux I., Guillemot F., Jing N. (2004) Dev. Dyn. 231, 248–257 [DOI] [PubMed] [Google Scholar]
- 29. Birren S. J., Lo L., Anderson D. J. (1993) Development 119, 597–610 [DOI] [PubMed] [Google Scholar]
- 30. Macián F., García-Cózar F., Im S. H., Horton H. F., Byrne M. C., Rao A. (2002) Cell 109, 719–731 [DOI] [PubMed] [Google Scholar]
- 31. Okamura H., Aramburu J., García-Rodríguez C., Viola J. P., Raghavan A., Tahiliani M., Zhang X., Qin J., Hogan P. G., Rao A. (2000) Mol. Cell 6, 539–550 [DOI] [PubMed] [Google Scholar]
- 32. Chow C. W., Davis R. J. (2000) Mol. Cell. Biol. 20, 702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gregory M. A., Phang T. L., Neviani P., Alvarez-Calderon F., Eide C. A., O'Hare T., Zaberezhnyy V., Williams R. T., Druker B. J., Perrotti D., Degregori J. (2010) Cancer Cell 18, 74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow C. W., Rincón M., Davis R. J. (1999) Mol. Cell. Biol. 19, 2300–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo C., Burgeon E., Rao A. (1996) J. Exp. Med. 184, 141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Northrop J. P., Ho S. N., Chen L., Thomas D. J., Timmerman L. A., Nolan G. P., Admon A., Crabtree G. R. (1994) Nature 369, 497–502 [DOI] [PubMed] [Google Scholar]
- 37. Yang M., Zhong W. W., Srivastava N., Slavin A., Yang J., Hoey T., An S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 6027–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahmoudi T., Li V. S., Ng S. S., Taouatas N., Vries R. G., Mohammed S., Heck A. J., Clevers H. (2009) EMBO J. 28, 3329–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santoro I. M., Groden J. (1997) Cancer Res. 57, 488–494 [PubMed] [Google Scholar]
- 40. Megason S. G., McMahon A. P. (2002) Development 129, 2087–2098 [DOI] [PubMed] [Google Scholar]
- 41. Hamburger V., Hamilton H. L. (1992) Dev. Dyn. 195, 231–272 [DOI] [PubMed] [Google Scholar]
- 42. Ille F., Atanasoski S., Falk S., Ittner L. M., Märki D., Büchmann-Møller S., Wurdak H., Suter U., Taketo M. M., Sommer L. (2007) Dev. Biol. 304, 394–408 [DOI] [PubMed] [Google Scholar]
- 43. Graef I. A., Wang F., Charron F., Chen L., Neilson J., Tessier-Lavigne M., Crabtree G. R. (2003) Cell 113, 657–670 [DOI] [PubMed] [Google Scholar]
- 44. Li L., Yuan H., Xie W., Mao J., Caruso A. M., McMahon A., Sussman D. J., Wu D. (1999) J. Biol. Chem. 274, 129–134 [DOI] [PubMed] [Google Scholar]