Abstract
Murine embryonal carcinoma cells from line PCC4.aza1R differentiate readily in response to retinoic acid. By treating PCC4.aza1R cells with the mutagen N-methyl-N' -nitro-N-nitrosoguanidine, we derived two embryonal carcinoma lines in which the cells failed to differentiate during exposure to retinoic acid. Although these dif(RA)- cells maintained the tumorigenic potential of the parental cells, they differentiated poorly in tumor form. Similarly, the tendency of dif(RA)- cells to differentiate when aggregated in vitro was diminished relative to that of PCC4.zaz1R cells. The rate of retinoic acid uptake in cells from the two mutant lines did not appear to be reduced compared with the rate in cells from the parental line; however, specific cytoplasmic retinoic acid-binding protein activity was virtually absent in both mutants. These results strengthen the view that differentiation of embryonal carcinoma cells in response to retinoic acid requires formation of retinoic acid-cytoplasmic retinoic acid-binding protein complexes.
Full text
PDF


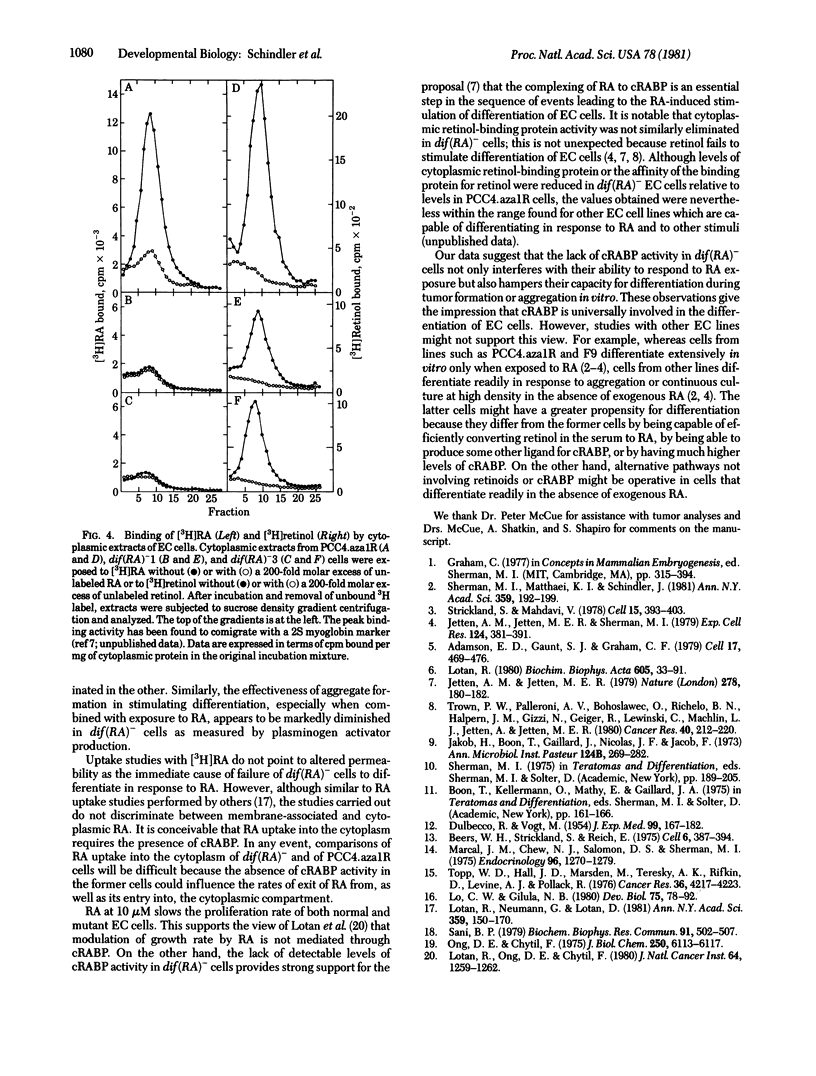
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Gaunt S. J., Graham C. F. The differentiation of teratocarcinoma stem cells is marked by the types of collagen which are synthesized. Cell. 1979 Jul;17(3):469–476. doi: 10.1016/0092-8674(79)90254-x. [DOI] [PubMed] [Google Scholar]
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Possible role of retinoic acid binding protein in retinoid stimulation of embryonal carcinoma cell differentiation. Nature. 1979 Mar 8;278(5700):180–182. doi: 10.1038/278180a0. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Stimulation of differentiation of several murine embryonal carcinoma cell lines by retinoic acid. Exp Cell Res. 1979 Dec;124(2):381–391. doi: 10.1016/0014-4827(79)90213-1. [DOI] [PubMed] [Google Scholar]
- Lo C. W., Gilula N. B. PCC4azal teratocarcinoma stem cell differentiation in culture. I. Biochemical studies. Dev Biol. 1980 Mar;75(1):78–92. doi: 10.1016/0012-1606(80)90145-1. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Lotan R., Neumann G., Lotan D. Characterization of retinoic acid-induced alterations in the proliferation and differentiation of a murine and a human melanoma cell line in culture. Ann N Y Acad Sci. 1981 Feb 27;359:150–170. doi: 10.1111/j.1749-6632.1981.tb12744.x. [DOI] [PubMed] [Google Scholar]
- Lotan R., Ong D. E., Chytil F. Comparison of the level of cellular retinoid-binding proteins and susceptibility to retinoid-induced growth inhibition of various neoplastic cell lines. J Natl Cancer Inst. 1980 May;64(5):1259–1262. [PubMed] [Google Scholar]
- Marcal J. M., Chew N. J., Salomon D. S., Sherman M. I. Delta5,3beta-hydroxysteroid dehydrogenase activities in rat trophoblast and ovary during pregnancy. Endocrinology. 1975 May;96(5):1270–1279. doi: 10.1210/endo-96-5-1270. [DOI] [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Retinoic acid-binding protein in rat tissue. Partial purification and comparison to rat tissue retinol-binding protein. J Biol Chem. 1975 Aug 10;250(15):6113–6117. [PubMed] [Google Scholar]
- Sani B. P. Retinoic acid-binding protein: a plasma membrane component. Biochem Biophys Res Commun. 1979 Nov 28;91(2):502–507. doi: 10.1016/0006-291x(79)91550-x. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., Matthaei K. I., Schindler J. Studies on the mechanism of induction of embryonal carcinoma cell differentiation by retinoic acid. Ann N Y Acad Sci. 1981 Feb 27;359:192–199. doi: 10.1111/j.1749-6632.1981.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Topp W., Hall J. D., Marsden M., Teresky A. K., Rifkin D., Levine A. J., Pollack R. In vitro differentiation of teratomas and the distribution of creatine phosphokinase and plasminogen activator in teratocarcinoma-derived cells. Cancer Res. 1976 Nov;36(11 Pt 2):4217–4223. [PubMed] [Google Scholar]
- Trown P. W., Palleroni A. V., Bohoslawec O., Richelo B. N., Halpern J. M., Gizzi N., Geiger R., Lewinski C., Machlin L. J., Jetten A. Relationship between binding affinities to cellular retinoic acid-binding protein and in vivo and in vitro properties for 18 retinoids. Cancer Res. 1980 Feb;40(2):212–220. [PubMed] [Google Scholar]




