Abstract
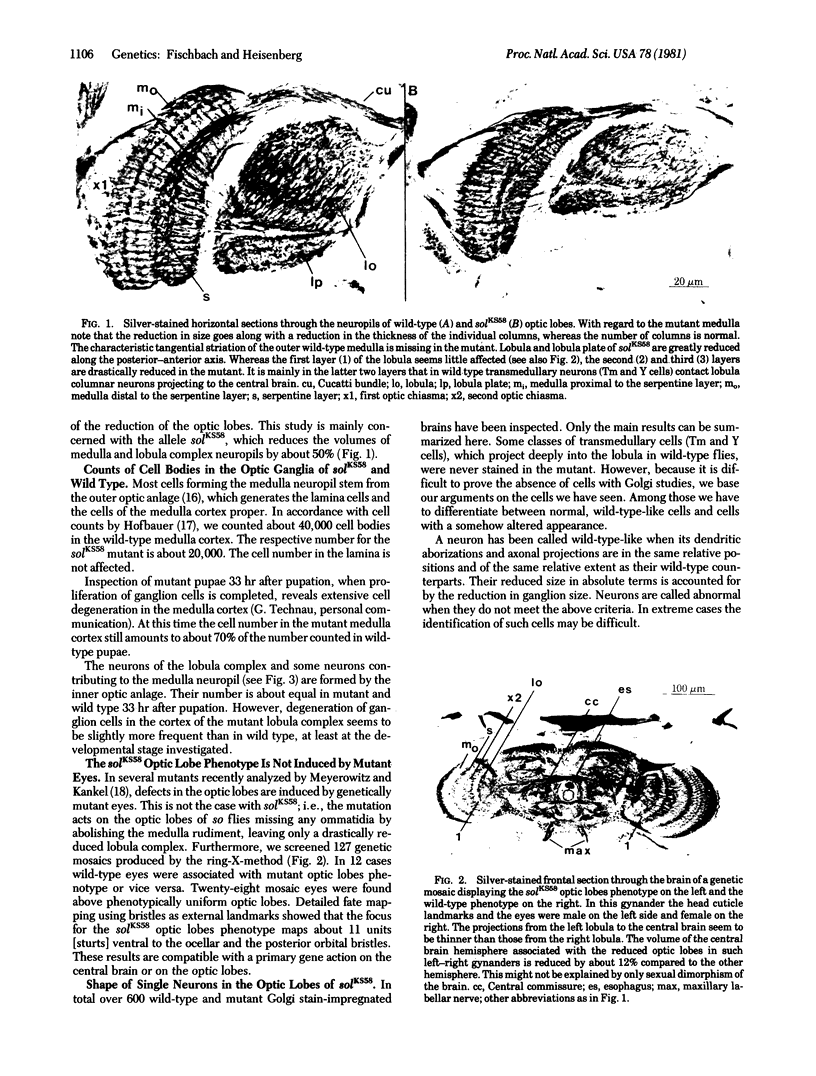
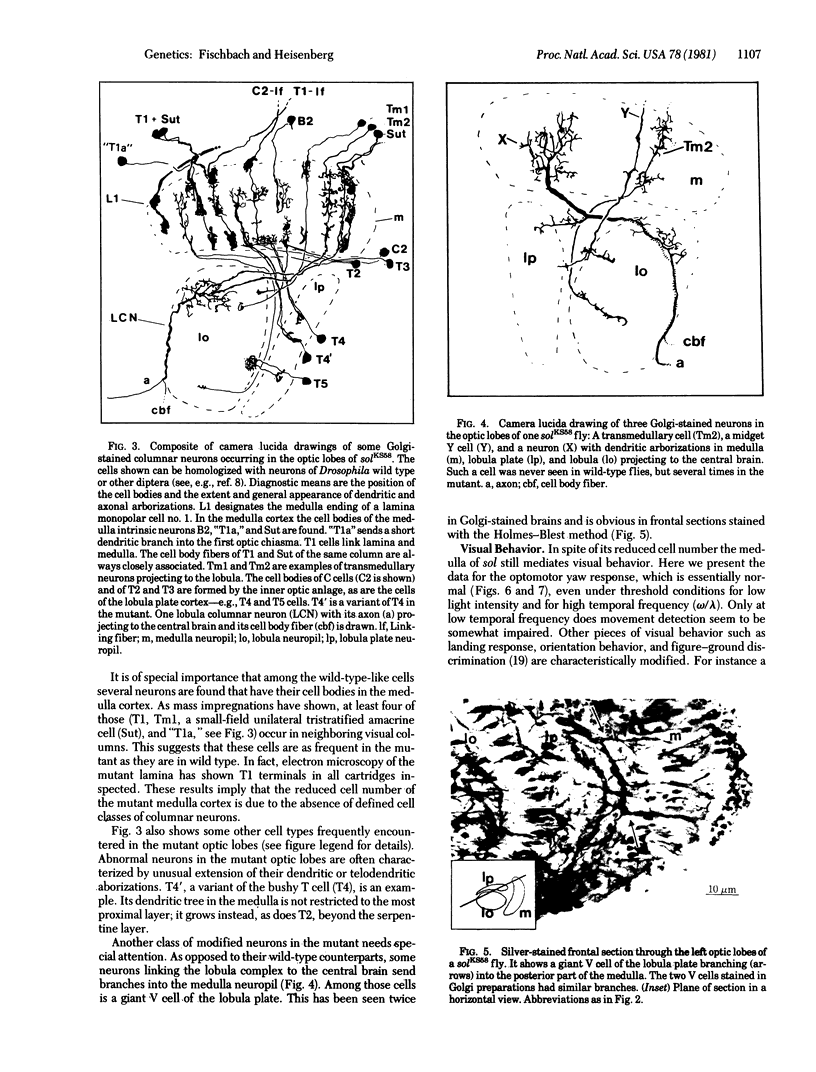
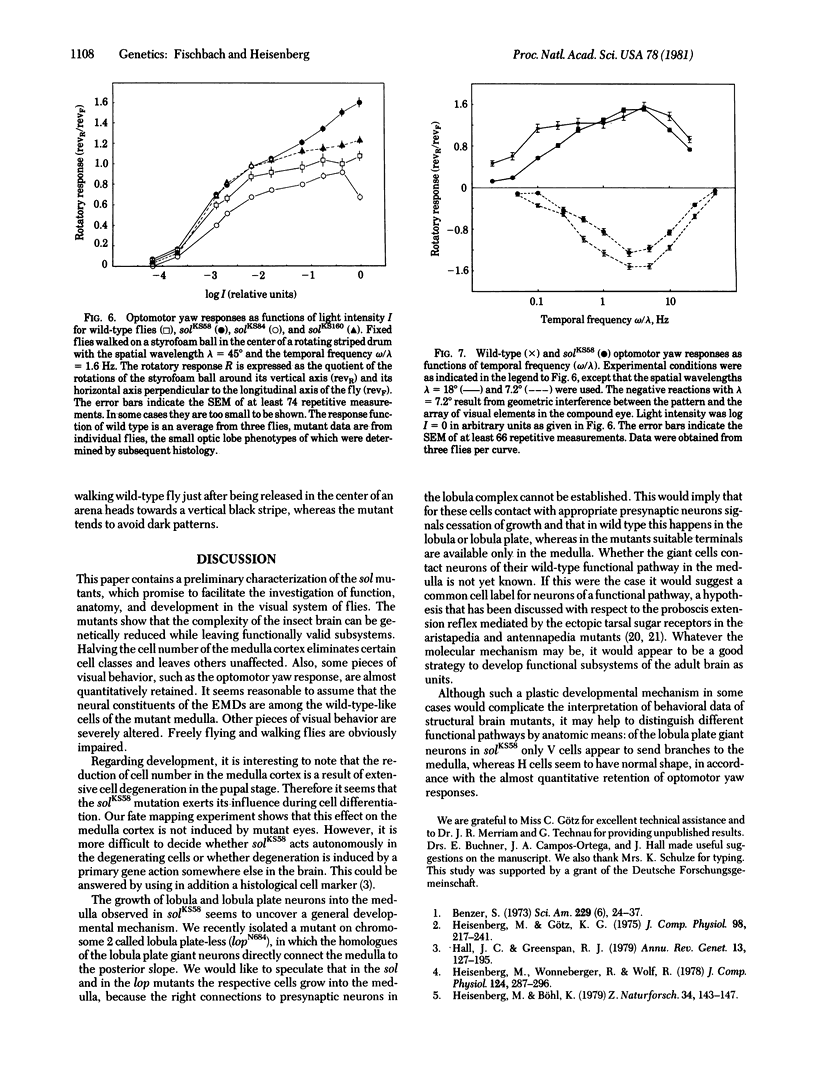
KS58, one out of six known alleles of the small optic lobes (sol) gene in Drosophila melanogaster, reduces the cell number in the medulla cortex by degeneration of ganglion cells in the pupae to about 50%. Also, about half the volume of the medulla and lobula complex neuropils is missing. Many Golgistained cells in the mutant optic lobes resemble their homologues in wild type. However, special classes of transmedullary columnar neurons projecting to the lobula or to both lobula and lobula plate are not seen in the mutant. Some neurons linking the lobula complex to the central brain send branches to the medulla (the branches do not exist in wild type); some other types seem to be missing. The fate mapping of the KS58 focus reveals a location ventral to the head bristles and in sine oculis (so) flies the mutation further reduces the rudiments of the optic lobes normally seen. Therefore the sol phenotype is not induced by mutant eyes and the primary gene action seems to be on nervous tissue. The structural alterations of the small optic lobes are reflected in visual orientation behavior. The optomotor yaw response, however, is almost quantitatively preserved. The respective neural network should still be present in the mutant optic lobes.
Keywords: cell degeneration, developmental plasticity, orientation behavior, Golgi staining, genetic mosaics
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. Genetic dissection of behavior. Sci Am. 1973 Dec;229(6):24–37. doi: 10.1038/scientificamerican1273-24. [DOI] [PubMed] [Google Scholar]
- Buchner E., Buchner S., Hengstenberg R. 2-Deoxy-D-glucose maps movement-specific nervous activity in the second visual ganglion of Drosophila. Science. 1979 Aug 17;205(4407):687–688. doi: 10.1126/science.111349. [DOI] [PubMed] [Google Scholar]
- COLONNIER M. THE TANGENTIAL ORGANIZATION OF THE VISUAL CORTEX. J Anat. 1964 Jul;98:327–344. [PMC free article] [PubMed] [Google Scholar]
- Deak I. I. Demonstration of sensory neurones in the ectopic cuticle of spineless-aristapedia, a homoeotic mutant of Drosophila. Nature. 1976 Mar 18;260(5548):252–254. doi: 10.1038/260252a0. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Greenspan R. J. Genetic analysis of Drosophila neurobiology. Annu Rev Genet. 1979;13:127–195. doi: 10.1146/annurev.ge.13.120179.001015. [DOI] [PubMed] [Google Scholar]
- Harris W. A., Stark W. S., Walker J. A. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol. 1976 Apr;256(2):415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Kankel D. R. A genetic analysis of visual system development in Drosophilia melanogaster. Dev Biol. 1978 Jan;62(1):112–142. doi: 10.1016/0012-1606(78)90096-9. [DOI] [PubMed] [Google Scholar]
- Reichardt W., Poggio T. Visual control of orientation behaviour in the fly. Part I. A quantitative analysis. Q Rev Biophys. 1976 Aug;9(3):311-75, 428-38. doi: 10.1017/s0033583500002523. [DOI] [PubMed] [Google Scholar]






