Abstract
AIM: To explore the dynamic changes of prion protein (PrPc) in the process of gastric cancer drug resistance and the role of PrPc expression in the prognosis of gastric cancer patients receiving chemotherapy.
METHODS: A series of gastric cancer cell lines resistant to different concentrations of adriamycin was established, and the expression of PrPc, Bcl-2 and Bax was detected in these cells. Apoptosis was determined using Annexin V staining. Western blotting and immunohistochemistry were performed to detect the expression of PrPc in patients receiving chemotherapy and to explore the role of PrPc expression in predicting the chemosensitivity and the outcome of gastric cancer patients receiving chemotherapy. Follow-up was performed for 2 years.
RESULTS: PrPc expression was increased with the increase in drug resistance. Bcl-2, together with PrPc, increased the level of anti-apoptosis of cancer cells. Increased PrPc expression predicted the enhanced level of anti-apoptosis and resistance to anticancer drugs. PrPc expression could be used as a marker for predicting the efficacy of chemotherapy and the prognosis of gastric cancer. Increased PrPc expression predicted both poor chemosensitivity and a low 2-year survival rate. Contrarily, low PrPc expression predicted favorable chemosensitivity and a relatively high 2-year survival rate.
CONCLUSION: PrPc expression is associated with histological types and differentiation of gastric cancer cells; The PrPc expression level might be a valuable marker in predicting the efficacy of chemotherapy and the prognosis of gastric cancer patients receiving chemotherapy.
Keywords: Prion protein, Gastric cancer, Drug resistance, Chemotherapy, Apoptosis
INTRODUCTION
Prion protein (PrPc or PrP) is a pathogen that causes bovine spongiform encephalopathy (also known as “mad cow disease”). In 1982, Prusiner[1] isolated the protease- and heat-resistant protein from the brains of scrapie-infected hamsters. PrPc is encoded by the host prion gene but not by the prion gene. Thereafter, a new field of biomedicine, prion biology, was founded, and a new concept of pathology, protein conformation diseases, was also established. In 2003, our study showed that PrPc was associated with the multidrug resistance of gastric cancer[2,3], and thereafter, a series of studies have been conducted to explore the role of PrPc in the differentiation, proliferation and metastasis of cancer cells and to deeply investigate the structural domains of PrPc. These studies have achieved great progress in prion biology and pathogenesis[4-13]. The present study aimed to investigate PrPc expression in gastric cancer cell lines and its role in predicting the outcome of gastric cancer patients undergoing chemotherapy. Our study may elucidate the clinical significance of PrPc over-expression in cancers and provide a potential indicator for predicting the outcome of cancer patients.
MATERIALS AND METHODS
Materials
Gastric cancer cell lines (KATO III, BGC-823, GES, HGC-27, AGS, SGC7901 and MGC803) were purchased from the Academy of Military Medical Sciences and were maintained in our department. PrPc and β-actin monoclonal antibodies (Sigma, United States), enhanced chemiluminescence (ECL) kit (Amersham Pharmacia) and immunohistochemistry kit (Beijing biodev-tech Co., Ltd) were used in this study.
A total of 185 patients with gastric cancers at stages
I to IV (6th edition of the UICC TNM classification and Lauren classification) were recruited. The characteristics of these patients are summarized in Table 1. There were 134 men and 51 women included in this study. The age of these patients ranged from 33 to 76 years, with a mean age of 54.2 years. The cancers of these patients were pathologically proven to be moderately or well-differentiated (n = 43), poorly differentiated (n = 33), undifferentiated (n = 46), mucinous (n = 16) and mixed cancers (n = 47). In addition, normal gastric tissues without cancer infiltration were used as controls.
Table 1.
Demographics of gastric cancer patients at baseline (mean ± SD)
| Stage | n | Surgery + chemotherapy | Comprehensivetreatment | KPS beforetreatment | KPS(2 yr later) | ZPS before treatment | ZPS(2 yr later) | CR + PR(%) | Death |
| I | 52 | 43 | 9 | 87.12 ± 12.66 | 77.31 ± 19.72 | 1.019 ± 0.772 | 1.346 ± 1.254 | 74.6 | 2 |
| II | 66 | 41 | 25 | 77.73 ± 11.12 | 72.12 ± 16.56 | 1.803 ± 1.131 | 2.167 ± 1.344 | 52.1 | 1 |
| III | 47 | 32 | 15 | 66.81 ± 23.35 | 41.06 ± 35.20 | 2.383 ± 1.314 | 2.830 ± 1.872 | 36.8 | 17 |
| IV | 20 | 12 | 8 | 57.00 ± 16.46 | 33.00 ± 31.64 | 2.650 ± 1.014 | 3.650 ± 1.459 | 15.0 | 9 |
Male: 134; Female: 51; Mean age: 54.2 ± 21.5. KPS: Karnofsky Performance Scale; ZPS: Zubrod-ECOG-WHO Performance Status; CR: Complete remission; PR: Partial remission.
Methods
Establishment of adriamycin-resistant gastric cancer cell lines[10]: Adriamycin-resistant SGC7901 cells were selected by a stepwise increase in adriamycin concentration from 0.04 μg/mL to 0.6 μg/mL. In brief, the SGC7901 cells were maintained in medium containing 0.04 μg/mL adriamycin for 48 h. Then, the medium was refreshed with adriamycin-free medium. When normal growth was observed, the medium was replaced with that containing 0.04 μg/mL adriamycin followed by incubation for 48 h. The above mentioned procedures were repeated 3-5 times, and then the adriamycin concentration was increased once every 6-10 d. Finally, SGC7901 cells that were resistant to 0.04, 0.18, 0.32, 0.46 and 0.6 μg/mL adriamycin were obtained. Each adriamycin-resistant SGC7901 cell line was collected and stored in liquid nitrogen for the synchronization of experiments. The sensitivity of cells to adriamycin was determined by methyl thiazolyl tetrazolium assay followed by calculation of the half-maximal inhibitory concentration (IC50). The resistance index (RI) was calculated as follow: RI = IC50drug-resistant cells/IC50parental cells.
Detection of the expression of PrPc, Bcl-2 and Bax in each adriamycin-resistant SGC7901 cell line by Western blotting: After recovery from storage in liquid nitrogen, cells were maintained in fresh medium. Total proteins were extracted from cells in the logarithmic growth phase of growth, and the protein concentration was determined. Then, 40 μg total proteins were separated in odium dodecylsulphate (SDS) polyacrylamide gels and transferred onto nitrocellulose membranes. The primary antibodies used to detect the target proteins were anti-PrP, anti-Bcl-2 and anti-Bax monoclonal antibodies and anti-β-actin polyclonal antibody; horseradish peroxidase-conjugated goat anti-mouse IgG was used as the secondary antibody. The bands were visualized using the ECL system (Amersham Pharmacia Biotech, Piscataway, NJ, United States) according to the manufacturer’s instructions, and protein concentration was quantified by densitometry with TotalLab 2.0 software.
Flow cytometric analysis of apoptosis in each adriamycin-resistant SGC7901 cell line: Cells in the logarithmic phase of growth were transferred to a 6-well plate. After adherence, cells were maintained in medium with 0.7 μg/mL adriamycin at 37 °C for 48 h. The medium was then replaced with fresh medium containing 5 μL Annexin V-FITC and 1.5 mmol CaCl2, and the cells were incubated for 15 min at 37 °C. Subsequently, the cells were re-suspended in 490 μL binding buffer and 5 μL PI staining solution and incubated for 10 min at 4 °C. Apoptosis was then detected by flow cytometry, and the apoptosis index (AI) was calculated as follows: AI = (number of early and late apoptotic cells)/total number of cells × 100%.
Detection of PrPc expression in different gastric cancer cell lines by Western blotting: Gastric cancer cells in the logarithmic growth phase of growth were harvested and the total protein was extracted, after which the proteins were separated on SDS polyacrylamide gels and transferred onto nitrocellulose membranes. The primary antibodies used to detect the target proteins were anti-PrP, anti-Bcl-2 and anti-Bax monoclonal antibodies and anti-β-actin polyclonal antibody; horseradish peroxidase-conjugated goat anti-mouse IgG was used as the secondary antibody. The bands were visualized using the ECL system (Amersham Pharmacia Biotech, Piscataway, NJ, United States) according to manufacturer’s instructions.
Detection of PrPc expression in different gastric cancers by tissue microarray: Anti-PrP monoclonal antibody (Sigma, Swampscott, MA, United States) and horseradish peroxidase-conjugated goat anti-mouse IgG antibody were used for immunohistochemical detection of PrPc with the StreptAvidin Biotin Complex staining kit according to the manufacturer’s instructions. The bands were visualized using diaminobenzidine substrate. The primary antibody was replaced with phosphate-buffered saline as the negative control.
Analysis was performed with Image-Pro Plus 5.0 software. Five high-power fields were randomly selected, and 100 cells were counted. The proportion of PrP-positive cells per sample was calculated, and tissue grading was performed as follows: < 5% as negative (score 0); 5%-25% as “+” (score 1); 26%-50% as “++” (score 2); 51%-75% as “+++” (score 3), and > 75% as “++++” (score 4). Scores of greater than 3 were regarded as strongly positive.
Detection of PrPc expression in gastric cancer tissues by Western blotting: Gastric cancer tissues, including 52 adenocarcinomas, 26 undifferentiated cancers, 33 mixed cancers, 17 poorly differentiated cancers and 11 precancerous lesions (severe atrophic gastritis), were obtained from 139 patients with gastric cancers who underwent biopsy or gastrectomy. Total protein was extracted with Trizol, the protein concentration was determined, and the proteins were separated by SDS-PAGE. Anti-PrP monoclonal antibody (Sigma, Swampscott, MA, United States) and anti-β-actin polyclonal antibody were used as primary antibodies and horseradish peroxidase-conjugated goat anti-mouse IgG as the secondary antibody.
Relationship between PrPc expression and the efficacy of chemotherapy in gastric cancer patients
The treatment strategies included surgery + chemotherapy and surgery + chemotherapy + interventional therapy. The treatment efficacy was recorded as complete remission (CR), partial remission (PR), stable disease, and progressive disease according to the criteria developed by the World Health Organization (WHO). The overall efficacy was calculated as CR + PR. According to the Karnofsky Performance Scale (KPS), the patients were stratified into one of the following groups: the improvement group (the increase in KPS score was greater than 10 after treatment), the deterioration group (the decrease in KPS score was greater than 10 after treatment), and the stability group (the changes in KPS score were less than 10 after treatment). The performance status was evaluated according to Zubrod-ECOG-WHO, and survival was followed up for 2 years. The demographics of these patients at baseline and after 2 years of follow-up were summarized in Table 1, respectively. PrPc expression in the patient samples was detected by immunohistochemistry and Western blotting, and the patients were divided into PrPc-positive and PrPc-negative groups. The whole protocol was approved by the Institutional Review Board of the Ethics Committee of our hospital. Informed consent was obtained before the study.
Statistical analysis
Statistical analysis was performed using SPSS software. Quantitative data were analyzed using the t test, and qualitative data were analyzed using the χ2 test. A value of P < 0.05 was considered statistically significant.
RESULTS
PrPc, Bcl-2 and Bax expression in gastric cancer cells resistant to different concentrations of adriamycin
The RI of gastric cancer cells resistant to adriamycin were increased accompany with the deepened concentrations which the value of RI 0.92 ± 0.072 vs the adriamycin concentration 0 μg/mL, 1.478 ± 0.098a vs 0.04 μg/mL, 3.096 ± 0.201a vs 0.18 μg/mL, 4.5 ± 0.334a vs 0.32 μg/mL, 5.086 ± 0.302a vs 0.46 µg/mL, 6.65 ± 0.354a vs 0.6 μg/mL (aP ≤ 0.01 vs 0 μg/mL group).
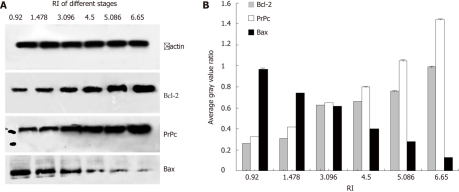
PrPc, Bcl-2 and Bax expression in gastric cancer cells resistant to different concentrations of adriamycin are shown in Figure 1. Analysis indicated that the expression of PrPc and Bcl-2 were markedly increased (P < 0.01) in an adriamycin concentration-dependent manner, while that of Bax was significantly reduced (P < 0.01). The results indicated that the resistance index was 3.096 when the adriamycin concentration was 0.18 μg/mL.
Figure 1.
Expressions of PrPc, Bcl-2 and Bax in gastric cancer cells resistant to different concentrations of adriamycin. Analysis indicated that the expressions of PrPc and Bcl-2 were markedly increased (P < 0.01), but that of Bax was significantly reduced (P < 0.01), which were in an adriamycin concentration dependent manner. Resistance index was 3.096 when the adriamycin concentration was 0.18 μg/mL. PrPc: Prion protein; RI: Resistence index.
Apoptosis of gastric cancer cells resistant to different concentrations of adriamycin
The AI is displayed in Figure 2. A significant difference in the AI was not noted between the 0.04 and 0 μg/mL adriamycin groups (P > 0.05). In SGC7901 cells resistant to 0.18 μg/mL adriamycin, the anti-apoptotic capability was significantly increased and drug sensitivity markedly decreased with an RI of 3.096 (P < 0.001) when compared with SGC7901 cells without adriamycin treatment.
Figure 2.
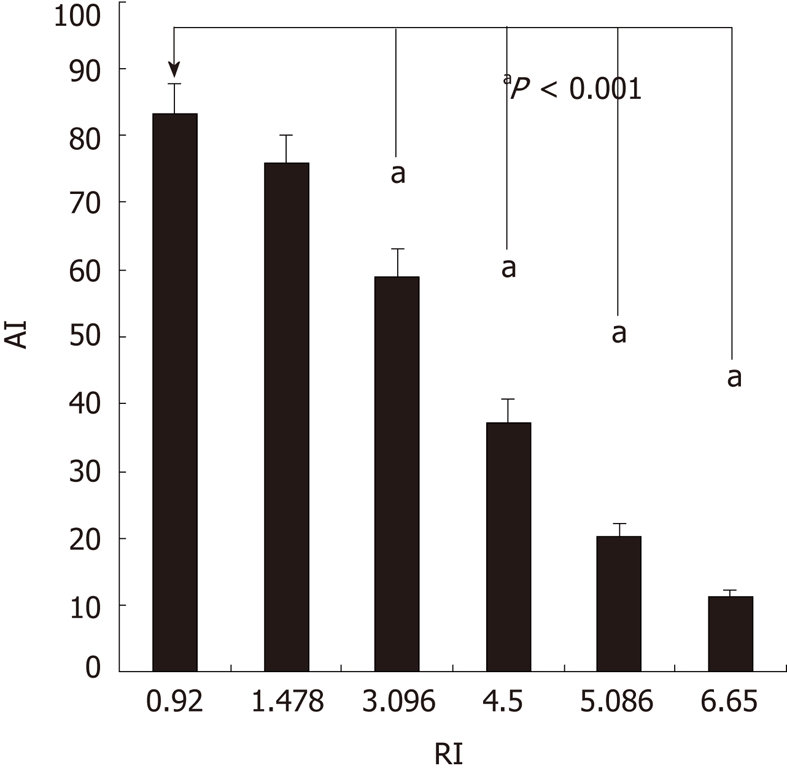
Apoptosis index of gastric cancer cells resistant to adriamycin of different concentrations. In the SGC7901 cells resistant to 0.18 μg/mL adriamycin, the anti-apoptotic capability was significantly increased with the resistence index of 3.096 (aP < 0.001) when compared with SGC7901 cells without adriamycin treatment. AI: Apoptosis index; RI: Resistence index.
PrPc expression in different gastric cancer cell lines
PrPc expression in different gastric cancer cell lines is presented in Figure 3. Results showed that the highest PrPc expression was found in undifferentiated gastric cancer cells (HGC-27) followed by poorly differentiated gastric cancer cells (KATO III), and the lowest PrPc expression was in GES-1 immortalized human gastric epithelial cells. These results were consistent with tissue microarray and Western blotting analyses of the cancer tissues. Of note, low PrPc expression was noted in immortalized GES-1 cells, which was different from the results from the tissue microarray analysis.
Figure 3.
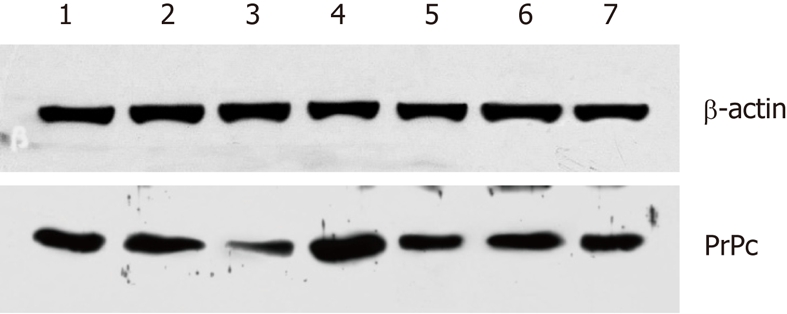
Prion protein expression in different gastric cancer cell lines. 1: KATOIII; 2: BGC-823; 3: GES; 4: HGC-27; 5: AGS; 6: SGC7901; 7: MGC80. Results showed that the highest prion protein (PrPc) expression was found in undifferentiated gastric cancer cells (HGC-27), followed by poorly differentiated gastric cancer cells (KATOIII), and the lowest PrPc expression in human gastric epithelial immortalized GES-1 cells. PrPc: Prion protein.
PrPc expression using tissue microarray assay
PrPc expression in the gastric cancers, precancerous lesions and normal tissues are presented in Figures 3, 4 and 5, respectively. χ2 tests revealed that the percentage of PrPc-positive cells in gastric cancers and precancerous lesions were dramatically increased when compared with normal tissues (P < 0.01). The percentage of PrP-positive cells in adenocarcinoma, mucinous carcinoma, undifferentiated cancer and poorly differentiated cancer are displayed in Figure 4. Analysis showed that PrPc-positive cells were related to the types of gastric cancer (P < 0.05). The proportion of PrPc-positive cells in undifferentiated cancer was significantly higher than that in adenocarcinoma (P < 0.01), but no significant differences in PrPc positive cells were found between mucinous carcinoma and poorly differentiated adenocarcinoma (Figure 5).
Figure 4.
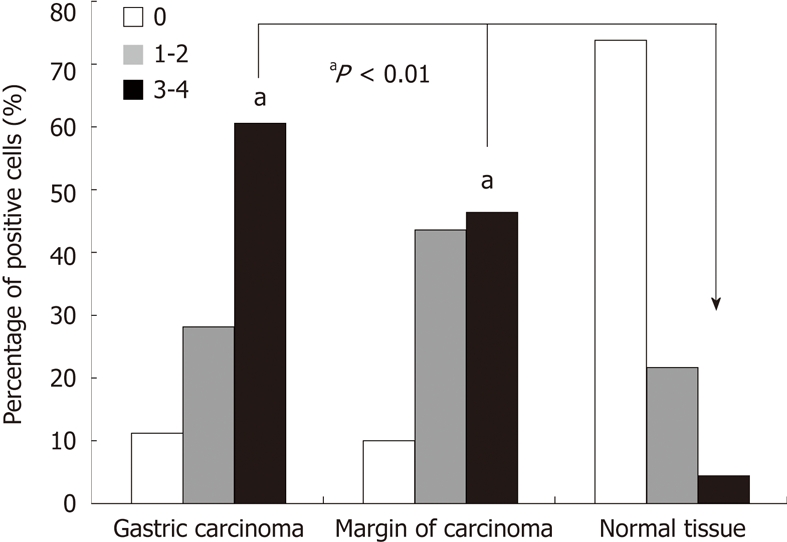
Prion protein expression in gastric cancers, precancerous lesions and normal tissues. χ2 test revealed that the number of prion protein positive cells in gastric cancers and precancerous lesions were dramatically increased when compared with normal tissues (aP < 0.01). PrPc: Prion protein.
Figure 5.
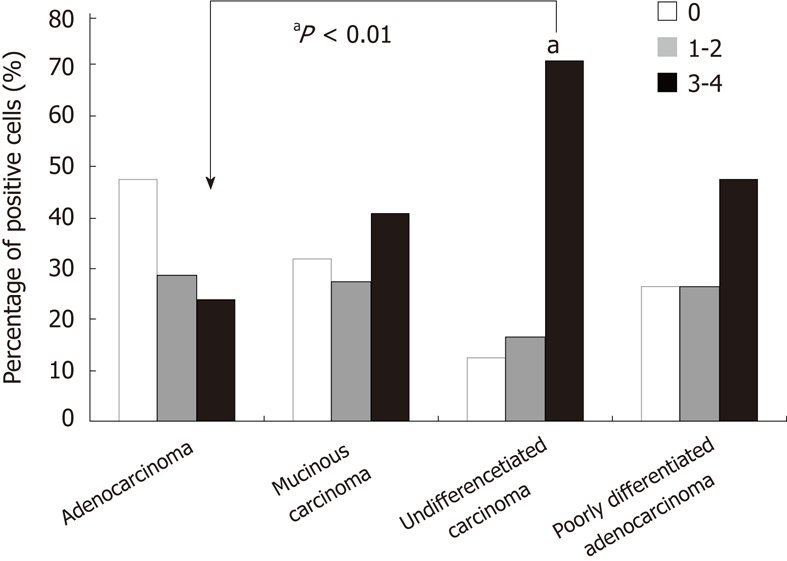
Prion protein expression in different type of gastric cancer. The proportion of prion protein positive cells in undifferentiated cancer was significantly higher than that in adenocarcinoma (aP < 0.01), but no significant difference in PrPc positive cells was found between mucinous carcinoma and poorly differentiated adenocarcinoma. PrPc: Prion protein.
PrPc protein expression in different types of gastric cancers
PrPc expression in the cancer and normal tissues is shown in Figure 6, and the immunohistochemistry for PrPc in gastric cancer is shown in Figure 7. Results showed that PrPc expression in gastric adenocarcinoma and mixed carcinoma was relatively low, and no significant difference in PrPc expression was observed between the remaining types of gastric cancer. Furthermore, PrPc expression in normal gastric tissues was much lower than that in gastric cancers.
Figure 6.
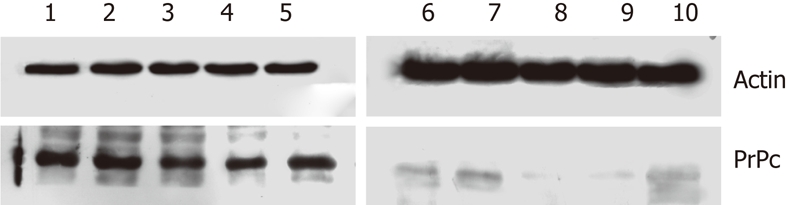
Prion protein expression in gastric cancers and normal tissues. The prion protein (PrPc) expression in gastric adenocarcinoma and mixed carcinoma was relatively low, and no significant difference in PrPc expression was noted between the remaining types of gastric cancer. Furthermore, PrPc expression in normal gastric tissues was much lower than that in gastric cancers. PrPc: Prion protein. 1: Tissue of atrophic gastritis; 2: Tissue of undifferentiated gastric cancer; 3: Tissue of gastric adenocarcinoma; 4: Tissue of mixed type carcinoma; 5: Tissue of poorly differentiated gastric cancer; 6-10: Corresponding normal gastric tissue.
Figure 7.
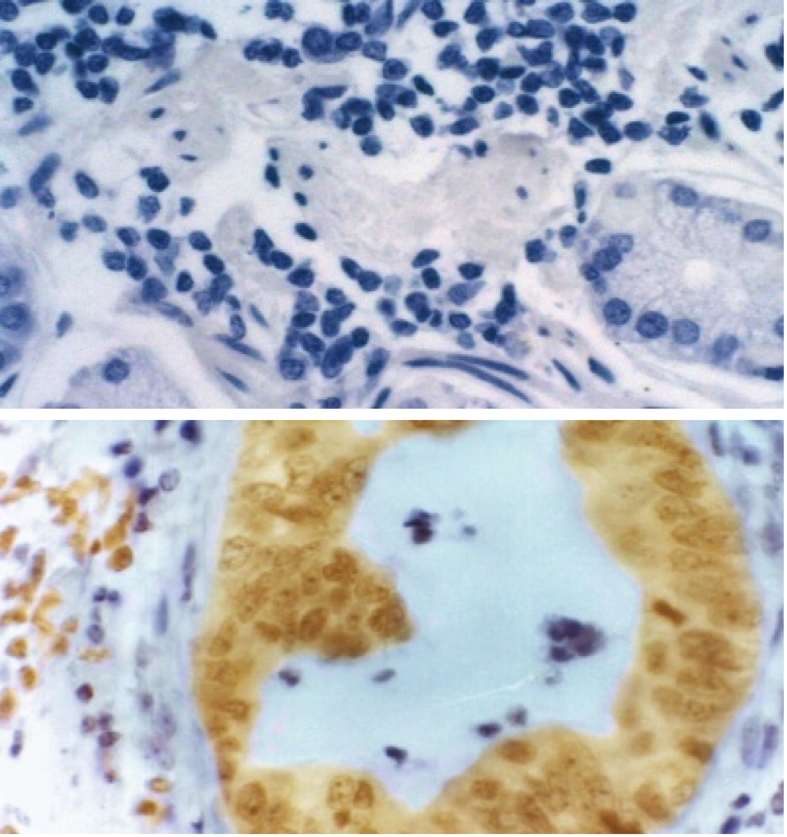
Prion protein expression in human gastric cancer (× 200). A: Negative control; B: Positive results.
Association of PrPc expression and chemotherapeutic efficacy in gastric cancer patients
The demographics of 185 patients at baseline are presented in Table 1, and those after 2 years of follow-up are presented in Table 2. Analysis showed that PrPc expression was significantly correlated with the pathological type of gastric cancer (P < 0.05). PrPc expression in undifferentiated cancers was significantly higher than that in moderately or well-differentiated cancer and mixed cancers (P < 0.01). However, no significant difference in PrPc expression was observed between poorly differentiated cancers and mucinous cancer (P > 0.05). The relationship between PrPc expression and the efficacy of chemotherapy are shown in Tables 2 and 3. The curative effects, KPS scores, and performance statuses were also evaluated. As shown in Table 2, PrPc expression was associated with the efficacy of chemotherapy. Patients with higher PrPc expression had a poorer response to chemotherapy (P < 0.05). As shown in Table 3, PrPc expression was associated with survival (P < 0.05). High PrPc expression predicted poorer prognosis, where a lower 2-year survival rate and higher mortality rate were observed when compared with low PrPc expression group. Table 4 showed KPS scores at diagnosis and after 2 years of follow-up in patients with different levels of PrPc expression. Results showed the overall survival status was deteriorated (KPS score was decreased more than 10), and the decrease in KPS score was profound in patients with strongly positive PrPc expression (P < 0.01) when compared with other patients.
Table 2.
Response to chemotherapy in patients with different prion protein expression
| PrPc expression | Case | CR | PR | NC | PD | CR + PR (%) |
| 0 | 59 | 7 | 25 | 18 | 9 | 32 (54.24) |
| 1 | 30 | 2 | 8 | 13 | 7 | 10 (33.33) |
| 2 | 39 | 1 | 9 | 19 | 10 | 10 25.64) |
| ≥ 3 | 57 | 0 | 11 | 21 | 25 | 11 (19.29) |
PrPc: Prion protein; CR: Complete remission; PR: Partial remission; NC: Stable disease; PD: Progressive disease.
Table 3.
Survival status of patients with different prion protein expression after chemotherapy
| PrPc expression | n | 6 mo | 1 yr | 2 yr | Death mortality n (%) |
| 0 | 59 | 0 | 11 | 45 | 3(5.10) |
| 1 | 30 | 0 | 8 | 20 | 2(6.67) |
| 2 | 39 | 3 | 7 | 17 | 7(17.95) |
| ≥ 3 | 57 | 5 | 12 | 25 | 17(29.82) |
PrPc: Prion protein.
Table 4.
Karnofsky performance scale score of patients with different prion protein expression levels
| PrPc expression | n | Before treatment | 2 yr later | Difference |
| 0 | 59 | 85.25 ± 11.40 | 73.90 ± 22.40 | 11.35 |
| 1 | 30 | 81.67 ± 17.34 | 69.33 ± 25.68 | 12.34 |
| 2 | 39 | 74.10 ± 18.77 | 60.77 ± 31.81 | 13.33 |
| ≥ 3 | 57 | 63.90 ± 18.87 | 45.93 ± 32.00 | 17.97 |
PrPC: Prion protein.
DISCUSSION
PrPc expression in gastric cancer cells and tissues
The expression of prion protein tends to occur in rapidly regenerating or poorly differentiated tissues[14-18]. Pammer et al[19] detected PrPc expression in gastrointestinal mucosa by immunohistochemistry, and results showed that PrPc expression was significantly increased in H. pylori-induced gastritis, suggesting that prion infection and replication could occur in gastrointestinal epithelial cells. In the present study, PrPc expression was detected through tissue microarray assays in normal gastric tissues, gastric cancers and precancerous lesions. Results revealed weak or no PrPc expression in normal gastric tissues, but hemocytes showed high PrPc expression; this observation could be attributed to normal tissues with some inflammation being used for detection, and positive expression was mainly found in infiltrated lymphocytes. PrPc expression was detected in gastric cancer tissues, adjacent normal tissues and normal tissues using tissue microarray assay based on tissue classification, e.g., negative group, positive group (+ to ++) and strongly positive group (+++ to ++++). χ2 tests revealed that PrPc expression in gastric cancers and precancerous lesions was dramatically increased when compared with normal tissues (P < 0.01). These findings imply that PrPc expression is closely associated with cell status. When cellular functions are active, including malignant transformation and excessive proliferation, PrPc expression is increased, which has been confirmed by Liang et al[9]. Moreover, PrPc expression was related to pathological types of gastric cancer. The proportion of PrPc-positive cells in undifferentiated cancer was significantly higher than that in gastric adenocarcinoma, suggesting that cancer differentiation is also correlated with PrPc expression, and the poorer the differentiation, the higher the level of PrPc expression. These results also indicate the relationship between cellular functions and PrPc expression: more active cellular function results in greater active proliferation and differentiation, and higher grades of malignancy mean higher levels of PrPc expression.
PrPc expression was detected by immunohistochemistry in 185 patients with gastric cancers, and PrPc expression in gastric cancers was significantly higher than that in normal gastric tissues. Strongly positive PrPc expression was found in 26 undifferentiated gastric cancers, while the well-differentiated adenocarcinoma had a low positive rate of PrPc expression (42.45%). However, negative or weak PrPc expression was also observed in gastric cancers, while positive, but not strongly positive, PrPc expression was detected in normal gastric tissues. These results were consistent with the tissue microarray assays.
PrPc expression was detected in different gastric cancer cell lines. The highest PrPc expression was found in an undifferentiated cancer cell line (HGC-27 cells) followed by a poorly differentiated cell line (KATO IIIcells). The lowest PrPc expression was detected in the immortalized normal human gastric epithelial cell line (GES cells).
In our study, PrPc expression was detected in different types of gastric cancer by immunohistochemistry and western blot and in different gastric cancer tissues from patients and different gastric cancer cell lines using tissue microarray assay. In this study, PrPc expression was associated with cell status and differentiation, which is consistent with previous reports[9,20].
Dynamic changes in PrPc in the process of anti-cancer drug resistance
PrPc is closely related to tumor development. PrPc expression has been shown to be related not only to cancer cell proliferation but also to drug resistance and cancer cell metastasis[21]. Meslin et al[22] showed that PrPc gene silencing in ADM-resistant MCF-7 breast cancer cells increased the sensitivity of those cells to TRAIL-induced apoptosis. In addition, Bcl-2 expression was inhibited and pro-apoptotic Bax expression was increased following PrPc gene silencing, resulting in enhanced apoptosis. Our in vitro experiments further validated this result. After pulse induction of SGC7901 cells with different concentrations of adriamycin, PrPc, Bcl-2 and Bax expression and apoptosis were determined in adriamycin-resistant cells. Results showed that PrPc expression increased and apoptosis decreased with the increase in resistance to adriamycin. Our experiments not only show the dynamic changes in PrPc, Bcl-2 and Bax expression in gastric cancer cells resistant to various concentrations of adriamycin, but they also reveal that PrPc over-expression decreases apoptosis in cancer cells and reduces their sensitivity to drugs. It has been confirmed that PrPc is an anti-apoptotic gene that can cause synergistic effects with Bcl-2. PrPc has been shown to be able to dimerize with the C-terminus of Bcl-2[23,24]. When PrPc expression is up-regulated, the Bcl-2/Bax ratio is increased and is accompanied by the suppression of p53 and Bax, resulting in anti-apoptosis[25-29]. Bcl-2 is expressed in almost all cell types, while PrPc expression is rarely observed in normal cells. This observation also determines the significance of PrPc expression in clinical practice. Our results confirmed the above findings[2,10].
In another study, Meslin et al[22] detected PrPc expression in breast cancer patients that were non-responsive to an estrogen receptor inhibitor. Results of the study of the efficacy of chemotherapy indicated that patients with suppressed PrPc expression show favorable sensitivity to chemotherapy. This study was very important in monitoring the sensitivity of cancers to chemotherapy and drug resistance. In addition, detection of PrPc expression may become an indicator in diagnosing cancers and in monitoring the therapeutic efficacy. Our results confirmed that PrPc over-expression predicts gastric cancer cell drug resistance.
In addition, PrPc expression is closely related to the apoptosis pathway. Up-regulation of PrPc expression reduces apoptosis by increasing Bcl-2 expression[2,3]. A study showed that the N-terminus of PrPc in metastatic gastric cancer with high PrPc expression could promote invasion and metastasis, which was partially associated with extracellular signal-regulated kinase/mitogen extracellular kinase pathway activation and subsequent transcriptional activation of MMP1[5]. Furthermore, PrPc over-expression promoted the proliferation of gastric cancer cells and was strongly related to the incidence and development of gastric cancer[6,7]. These in vitro experiments confirmed that the up-regulation of PrPc expression predicted enhanced proliferation and reduced apoptosis in gastric cancer cells, which resulted in drug resistance.
The role of PrPc expression in monitoring the therapeutic efficacy of gastric cancer
In the present study, positive PrPc expression was associated with the pathological type of gastric cancer. Mixed gastric cancer and undifferentiated gastric cancer had relatively high PrPc expression, which was consistent with tissue microarray assay results and the detection of PrPc expression in different gastric cancer cell lines. Results from the follow-up revealed that the patients with strongly positive PrPc expression showed poor sensitivity to chemotherapy and that the 2-year survival rates and KPS scores were significantly decreased. However, those with negative PrPc expression showed high sensitivity to chemotherapy, and the 2-year survival rates and KPS scores were remarkably higher than those with positive PrPc expression. Thus, PrPc could be a marker for the prognosis and response to chemotherapy in gastric cancer patients. In the National Center for Biotechnology Information gene bank (http://www.ncbi.nlm.nih.gov/geo/), results show PrPc expression in gastric cancer is higher than that in normal tissues (high-density oligonucleotide microarray, P < 0.05), and researchers speculate PrPc may be a useful resource for future development of therapeutic targets and diagnostic markers for gastric cancer (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE2685)[30].This result also confirms our previous findings that PrPc expression in gastric cancer can promote multidrug resistance and metastasis[2,3] and that PrPc over-expression in gastric cancer patients predicts tolerance to chemotherapy and a low survival rate. Long-term follow-up should be conducted to investigate the chemotherapeutic efficacy.
In summary, we conclude that: (1) PrPc expression is associated with gastric cancer pathological type and cancer cell differentiation; (2) PrPc exerts synergistic effects with Bcl-2 to decrease apoptosis, resulting in drug resistance; (3) Gastric cancer patients with PrPc over-expression are resistant to chemotherapy and have a poor prognosis. We speculate that PrPc might be one of the markers used for monitoring the response to chemotherapy and the prognosis of gastric cancer.
COMMENTS
Background
Prion Protein (PrPc or PrP) is a pathogen causing bovine spongiform encephalopathy (BSE, also known as “mad cow disease”). PrPc is encoded by host prion gene but not by the prion gene. In 2003, the authors’ study showed that PrPc was associated with multi-drug resistance of gastric cancer and thereafter a series of studies have been conducted to explore the role of PrPc in the differentiation, proliferation and metastasis of cancer cells and to investigate the structural domains of PrPc. The present study aimed to investigate the PrPc expression in gastric cancer cell lines and the role of PrPc expression in predicting outcome of gastric cancer patients undergoing chemotherapy.
Research frontiers
PrPc is closely related to tumor development. Study has demonstrated PrPc expression is related with not only cancer cell proliferation, but also the drug resistance and metastasis of cancer cells associated with multi-drug resistance of gastric cancer. When PrPc expression is up-regulated, Bcl-2/Bax ratio is increased accompanied by suppression of p53 and Bax resulting in anti-apoptosis. Up-regulation of PrPc expression predicted enhanced proliferation and anti-apoptosis of gastric cancer cells resulting in drug resistance. In the National Center for Biotechnology Information gene bank, results show PrP expression in gastric cancer is higher than that in normal tissues, and researchers speculate PrP may be a useful resource for future development of therapeutic targets and diagnostic markers for gastric cancer.
Innovations and breakthroughs
In the article “Over-expression of PrPC and its antiapoptosis function in gastric cancer” to be published on March 24, 2006, the authors found that PrPc might play a role as an effective antiapoptotic protein through Bcl-2-dependent apoptotic pathways in gastric cancer cells. According to results from this article, the authors conclude that PrPc and Bcl-2 have synergistic anti-apoptotic effects leading to increased resistance to chemotherapy. The PrPc expression level might be a valuable marker in predicting the efficacy of chemotherapy and prognosis of gastric cancer patients receiving chemotherapy. The findings were based on a series of studies which have been conducted to explore the role of PrPc in the differentiation, proliferation and metastasis of cancer cells and to investigate the structural domains of PrPc.
Applications
PrPc expression could be used as a marker for predicting the efficacy of chemotherapy and prognosis of gastric cancer. PrPc over-expression in gastric cancer patients predicts poor chemosensitivity and poor prognosis. Therefore, the PrPc expression level might be a valuable marker in predicting the efficacy of chemotherapy and prognosis of gastric cancer patients receiving chemotherapy.
Terminology
PrPc is a pathogen causing BSE (also known as “mad cow disease”). The prion (from proteinaceous infectious only) is devoid of informational nucleic acids and consists of an `infectious’ protein that is capable of converting a normal host protein termed PrPc, or simply PrP, into a likeness of itself. PrPc is encoded by host prion gene but not by prion gene.
Peer review
The authors explore the dynamic changes of PrPc in the process of gastric cancer drug-resistance and the role of PrPc expression in the prognosis of gastric cancer patients receiving chemotherapy. The study revealed that: (1) PrPc expression is associated with gastric cancer pathological type of and cancer cell differentiation; (2) PrPc exerts synergistic effects with Bcl-2 to decrease apoptosis, resulting in drug resistance; and (3) Gastric cancer patients with PrPc over-expression are resistant to chemotherapy and have a poor prognosis. These findings suggest that PrPc might be one of the markers used for monitoring the response to chemotherapy and the prognosis of gastric cancer.
Footnotes
Supported by National Natural Science Foundation of China, No. 30672063; China Postdoctoral Science Foundation Funded Project, No. 20080431404; and China Postdoctoral Special Fund, No. 200801038
Peer reviewer: Jean Paul Galmiche, MD, Professor, Department of Gastroenterology and Hepatology, Hôpital Hôtel Dieu, Nantes cedex 44093, France
S- Editor Sun H L- Editor O’Neill M E- Editor Xiong L
References
- 1.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Du JP, Jin XH, Shi YQ, Zhao YQ, Liu CJ, Cao YX, Qiao TD, Chen BJ, Fan DM. [The over-expression of prion protein in drug resistant gastric cancer cell line SGC7901/ADR and its significance] Zhonghua YiXue ZaZhi. 2003;83:328–332. [PubMed] [Google Scholar]
- 3.Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, Liu N, Qiao T, Fan D. Over-expression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer. 2005;113:213–220. doi: 10.1002/ijc.20570. [DOI] [PubMed] [Google Scholar]
- 4.Liang J, Ge F, Guo C, Luo G, Wang X, Han G, Zhang D, Wang J, Li K, Pan Y, et al. Inhibition of PI3K/Akt partially leads to the inhibition of PrP(C)-induced drug resistance in gastric cancer cells. FEBS J. 2009;276:685–694. doi: 10.1111/j.1742-4658.2008.06816.x. [DOI] [PubMed] [Google Scholar]
- 5.Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu N, Zhang G, Jin H, Gao J, Xie H, et al. Cellular prion protein promotes invasion and metastasis of gastric cancer. FASEB J. 2006;20:1886–1888. doi: 10.1096/fj.06-6138fje. [DOI] [PubMed] [Google Scholar]
- 6.Liang J, Pan YL, Ning XX, Sun LJ, Lan M, Hong L, Du JP, Liu N, Liu CJ, Qiao TD, et al. Over-expression of PrPC and its antiapoptosis function in gastric cancer. Tumour Biol. 2006;27:84–91. doi: 10.1159/000092488. [DOI] [PubMed] [Google Scholar]
- 7.Liang J, Wang JB, Pan YL, Wang J, Liu LL, Guo XY, Sun L, Lin T, Han S, Xie HH, et al. High frequency occurrence of 1-OPRD variant of PRNP gene in gastric cancer cell lines and Chinese population with gastric cancer. Cell Biol Int. 2006;30:920–923. doi: 10.1016/j.cellbi.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Liang J, Bai F, Luo G, Wang J, Liu J, Ge F, Pan Y, Yao L, Du R, Li X, et al. Hypoxia induced over-expression of PrP(C) in gastric cancer cell lines. Cancer Biol Ther. 2007;6:769–774. doi: 10.4161/cbt.6.5.4001. [DOI] [PubMed] [Google Scholar]
- 9.Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J, Chen Y, Wang X, Liu J, Guo X, et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J. 2007;21:2247–2256. doi: 10.1096/fj.06-7799com. [DOI] [PubMed] [Google Scholar]
- 10.Du JP, Chen P, Fei HX, Yang MH, Chen ZH. Establishment of adriamyciniamycin-resistant and cisplatin-resistant gastric carcinoma cell lines and assessment on their sustainability of drug resistance. Chin J Gastroenterol Hepatol. 2007;4:368–372. [Google Scholar]
- 11.Liang J, Wang J, Luo G, Pan Y, Wang X, Guo C, Zhang D, Yin F, Zhang X, Liu J, et al. Function of PrPC (1-OPRD) in biological activities of gastric cancer cell lines. J Cell Mol Med. 2009;13:4453–4464. doi: 10.1111/j.1582-4934.2009.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J, Luo G, Ning X, Shi Y, Zhai H, Sun S, Jin H, Liu Z, Zhang F, Lu Y, et al. Differential expression of calcium-related genes in gastric cancer cells transfected with cellular prion protein. Biochem Cell Biol. 2007;85:375–383. doi: 10.1139/o07-052. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, You H, Liu F, An H, Shi Y, Yu Q, Fan D. Differentially expressed gene profiles between multidrug resistant gastric adenocarcinoma cells and their parental cells. Cancer Lett. 2002;185:211–218. doi: 10.1016/s0304-3835(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 14.Ramljak S, Asif AR, Armstrong VW, Wrede A, Groschup MH, Buschmann A, Schulz-Schaeffer W, Bodemer W, Zerr I. Physiological role of the cellular prion protein (PrPc): protein profiling study in two cell culture systems. J Proteome Res. 2008;7:2681–2695. doi: 10.1021/pr7007187. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge J, Walker KB. The normal cellular form of prion protein modulates T cell responses. Immunol Lett. 2005;96:147–150. doi: 10.1016/j.imlet.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Massimino ML, Ferrari J, Sorgato MC, Bertoli A. Heterogeneous PrPC metabolism in skeletal muscle cells. FEBS Lett. 2006;580:878–884. doi: 10.1016/j.febslet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Shmakov AN, Bode J, Kilshaw PJ, Ghosh S. Diverse patterns of expression of the 67-kD laminin receptor in human small intestinal mucosa: potential binding sites for prion proteins? J Pathol. 2000;191:318–322. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH640>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Diarra-Mehrpour M, Arrabal S, Jalil A, Pinson X, Gaudin C, Piétu G, Pitaval A, Ripoche H, Eloit M, Dormont D, et al. Prion protein prevents human breast carcinoma cell line from tumor necrosis factor alpha-induced cell death. Cancer Res. 2004;64:719–727. doi: 10.1158/0008-5472.can-03-1735. [DOI] [PubMed] [Google Scholar]
- 19.Pammer J, Cross HS, Frobert Y, Tschachler E, Oberhuber G. The pattern of prion-related protein expression in the gastrointestinal tract. Virchows Arch. 2000;436:466–472. doi: 10.1007/s004280050474. [DOI] [PubMed] [Google Scholar]
- 20.Lima FR, Arantes CP, Muras AG, Nomizo R, Brentani RR, Martins VR. Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. J Neurochem. 2007;103:2164–2176. doi: 10.1111/j.1471-4159.2007.04904.x. [DOI] [PubMed] [Google Scholar]
- 21.Mehrpour M, Codogno P. Prion protein: From physiology to cancer biology. Cancer Lett. 2010;290:1–23. doi: 10.1016/j.canlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Meslin F, Hamaï A, Gao P, Jalil A, Cahuzac N, Chouaib S, Mehrpour M. Silencing of prion protein sensitizes breast adriamycin-resistant carcinoma cells to TRAIL-mediated cell death. Cancer Res. 2007;67:10910–10919. doi: 10.1158/0008-5472.CAN-07-0512. [DOI] [PubMed] [Google Scholar]
- 23.Kurschner C, Morgan JI. Analysis of interaction sites in homo- and heteromeric complexes containing Bcl-2 family members and the cellular prion protein. Brain Res Mol Brain Res. 1996;37:249–258. doi: 10.1016/0169-328x(95)00323-k. [DOI] [PubMed] [Google Scholar]
- 24.Demeule M, Jodoin J, Gingras D, Béliveau R. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466:219–224. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- 25.Meslin F, Conforti R, Mazouni C, Morel N, Tomasic G, Drusch F, Yacoub M, Sabourin JC, Grassi J, Delaloge S, et al. Efficacy of adjuvant chemotherapy according to Prion protein expression in patients with estrogen receptor-negative breast cancer. Ann Oncol. 2007;18:1793–1798. doi: 10.1093/annonc/mdm406. [DOI] [PubMed] [Google Scholar]
- 26.Sisó S, Puig B, Varea R, Vidal E, Acín C, Prinz M, Montrasio F, Badiola J, Aguzzi A, Pumarola M, et al. Abnormal synaptic protein expression and cell death in murine scrapie. Acta Neuropathol. 2002;103:615–626. doi: 10.1007/s00401-001-0512-6. [DOI] [PubMed] [Google Scholar]
- 27.Park SK, Choi SI, Jin JK, Choi EK, Kim JI, Carp RI, Kim YS. Differential expression of Bax and Bcl-2 in the brains of hamsters infected with 263K scrapie agent. Neuroreport. 2000;11:1677–1682. doi: 10.1097/00001756-200006050-00017. [DOI] [PubMed] [Google Scholar]
- 28.Roucou X, Giannopoulos PN, Zhang Y, Jodoin J, Goodyer CG, LeBlanc A. Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells. Cell Death Differ. 2005;12:783–795. doi: 10.1038/sj.cdd.4401629. [DOI] [PubMed] [Google Scholar]
- 29.Kim BH, Lee HG, Choi JK, Kim JI, Choi EK, Carp RI, Kim YS. The cellular prion protein (PrPC) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation. Brain Res Mol Brain Res. 2004;124:40–50. doi: 10.1016/j.molbrainres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Hippo Y, Taniguchi H, Tsutsumi S, Machida N, Chong JM, Fukayama M, Kodama T, Aburatani H. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 2002;62:233–240. [PubMed] [Google Scholar]