Abstract
AIM: To identify whether there could have been changes in survival if lymph node ratio (N ratio) had been used.
METHODS: We assessed 334 gastric adenocarcinoma cases retrospectively between 2001 and 2009. Two hundred and sixteen patients out of 334 were included in the study. Patients were grouped according to disection1 (D1) or dissection 2 (D2) dissection. We compared the estimated survival and actual survival determined by Pathologic nodes (pN) class and N ratio, and SPSS 15.0 software was used for statistical analysis.
RESULTS: Ninety-six (44.4%) patients underwent D1 dissection and 120 (55.6%) had D2 dissection. When groups were evaluated, 23 (24.0%) patients in D1 and 21 (17.5%) in D2 had stage migration (P = 0.001). When both D1 and D2 groups were evaluated for number of pathological lymph nodes, despite the fact that there was no difference in N ratio between D1 and D2 groups, a statistically significant difference was found between them with regard to pN1 and pN2 groups (P = 0.047, P = 0.044 respectively). In D1, pN0 had the longest survival while pN3 had the shortest. In D2, pN0 had the longest survival whereas pN3 had the shortest survival.
CONCLUSION: N ratio is an accurate staging system for defining prognosis and treatment plan, thus decreasing methodological errors in gastric cancer staging.
Keywords: Gastric cancer, Lymph node dissection, Node ratio, Tumor nodule metastasis
INTRODUCTION
Staging in gastric cancer is usually carried out according to Japanese Research Society for Gastric Cancer (JRSGC) or Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) systems. In JRSGC, staging depends on anatomical localization of the involved lymph nodes, whereas the UICC/AJCC system uses number of metastatic lymph nodes. The UICC/AJCC system requires removal of at least 15 lymph nodes[1-3]. One shortcoming of the UICC/AJCC system is presence of less than 15 nodes in the surgical specimen, which might cause inadequate staging and over-optimistic prediction of prognosis. Only 29%-31% of curative gastric resections include ≥ 15 nodes[4,5]. Low number of lymph node removal increases risk of shift migration.
In the Japanese system, lymph nodes are classified in stations according to their localization. Lymph node dissections performed according to these stations are expressed as D0, D1, D2, D3. Japanese surgeons generally recommend D2 dissection as gold standard. Experts from the USA and Europe state that there is not a survival difference between D1 and D2 dissections and that there is high postoperative morbidity and mortality rate in D2[6,7]. However, there are some surgeons in the Western world who support D2 dissection[8,9].
Lymph node ratio (N ratio) is defined as the ratio of positive lymph nodes to total number of lymph nodes examined[10]. In the latest publications it is shown that N ratio compared to UICC/AJCC pN class is a more independent prognostic factor and predicts survival more accurately[11,12]. Tumor nodule metastasis (TNM) classification stages gastric cancers 10%-15% lower than they should be, which causes errors in survival expectations and treatment planning[13-15]. When N ratio is used instead of pN, there is 10% upgrade in staging which results in a 8.14% decrease in 5 year survival[16]. When nodal staging in TNM classification is changed to N ratio, pN1 in TNM might become N ratio 2. This alters treatment planning as N ratio 2 requires adjuvant chemoradiation while pN1 does not. Accurate staging is necessary for an accurate adjuvant therapy planning, which can increase survival and quality of life.
In this study, we re-staged patients who previously had either D1 or D2 dissection according to N ratio and investigated whether there could have been a change in treatment and survival if N ratio had been used for staging.
MATERIALS AND METHODS
We retrospectively assessed 334 gastric adenocarcinoma cases who underwent either D1 or D2 lymph node dissection between May 2001 and October 2009. Exclusion criteria were distant metastasis (including macroscopically significant paraaortic, superior mesenteric artery and mid-colic artery lymph node metastasis), D3 and D0 dissections, previous history of gastric surgery, postoperative mortality (death within 30 d postoperatively) and palliative surgery. Two hundred and sixteen patients out of 334 were included in the study. Ninety-six (44.4%) of these patients had D1 dissection and 120 (55.6%) had D2 dissection. Forty-seven patients who were lost in follow up, 17 with insufficient pathology reports, 5 with gastric stump recurrence, 12 who died within 30 d postoperatively, 7 who were inoperable, 15 who had palliative surgery, 12 with D0 dissections, and 3 with D3 dissections made up the excluded 118 patients.
In all patients, dissection was carried out according to JRSGC criteria, taking into account the anatomical localization of primary tumor and lymph nodes (n0 = no lymph node metastasis, n1 = metastasis to N1 lymph nodes, n2 = N2 lymph node metastasis, n3 = N3 lymph node metastasis)[17]. Metastatic lymph nodes were classified according to UICC/AJCC 2002 criteria. According to these criteria: N0 = no metastasis, N1 = 1-6 lymph node metastasis, N2 = 7-15 lymph node metastasis, N3 = more than 15 lymph nodes metastasis. N ratio was classified according to previously published studies as: N ratio 0 = 0%, N ratio 1 = 1%-10%, N ratio 2 = 11%-25%, N ratio 3 ≥ 25%. Patients with adjacent organ involvement or lymph node positivity in histopathological examination were referred to the oncology department.
Patients were grouped according to D1 or D2 dissection. Groups were analyzed for the significance of age (< 70, ≥ 70 years), gender, type of resection (total, subtotal), tumor localization (diffuse, upper 1/3, middle 1/3, lower 1/3), number of lymph nodes removed, number of metastatic lymph nodes, depth of invasion, N class, TNM stage, pathologic diagnosis, N ratio and Lauren classification.
Statistical analysis
SPSS 15.0 (SPSS Inc. Chicago, IL, United States) was used for statistical analysis. A P value < 0.05 was accepted as statistically significant. Independent two sample t test was used for quantitative variables (age, number of lymph nodes, number of pathological lymph nodes). χ2 test was used for categorical variables (type of surgery, gender, anatomical location of tumor, N ratio, TNM stage, pathological diagnosis).
For survival analysis in the D1 and D2 groups, Kaplan-Meier method was used. Log rank test was used to analyze differences between statistical significances. Log rank test was also used to assess each N class and N ratio crossed with D1 and D2 groups for 5 year survival analysis.
RESULTS
The characteristics of D1 and D2 groups are shown in Table 1. Groups were compared with univariate analysis for significant prognostic factors. There were statistically significant differences in age, localization of primary tumor, depth of invasion and TNM stage (P = 0.009, 0.007, 0.001, 0.001, respectively). In 72 (33.3%) cases, more than 15 lymph nodes were identified in pathology specimens. There was a statistically significant difference in D1 and D2 groups regarding > 15 lymph node removal [27 (28.1%), 45 (37.5%), respectively] (P < 0.001).
Table 1.
Case characteristics n (%)
| D1 | D2 | P value | |
| Gender | |||
| M | 61 (63.5) | 71 (59.2) | 0.512 |
| F | 35 (36.5) | 49 (40.8) | |
| Age (yr) | |||
| < 70 | 70 (72.9) | 86 (71.7) | 0.142 |
| ≥ 70 | 26 (27.1) | 34 (28.3) | |
| Surgical Procedure | |||
| TG | 79 (82.3) | 55 (45.8) | 0.001 |
| DSG | 17 (17.7) | 65 (54.2) | |
| Anatomical localization of primary tumor | |||
| Proximal | 9 (9.4) | 9 (7.5) | 0.087 |
| Middle | 39 (40.6) | 43 (35.8) | |
| Distal | 33 (34.4) | 59 (49.2) | |
| Diffuse | 15 (15.6) | 9 (7.5) | |
| Number of lymph nodes removed (mean ± SD) | 14.4 ± 6.1 | 23.5 ± 9.3 | 0.022 |
| Number of metastatic lymph nodes (mean ± SD) | 3.8 ± 2.2 | 6.1 ± 4.6 | 0.034 |
| T (Depth of invasion) | |||
| T1 | 8 (8.3) | 9 (7.5) | 0.056 |
| T2 | 9 (9.4) | 15 (12.5) | |
| T3 | 59 (61.5) | 75 (62.5) | |
| T4 | 20 (20.8) | 21 (17.5) | |
| N (According to AJCC) | |||
| 0 | 20 (20.8) | 26 (21.7) | 0.296 |
| 1 | 32 (33.3) | 47 (39.2) | |
| 2 | 40 (41.7) | 37 (30.8) | |
| 3 | 4 (4.2) | 10 (8.3) | |
| TNM stage | |||
| IA | 2 (2.1) | 6 (5.0) | 0.001 |
| IB | 12 (12.5) | 7 (5.8) | |
| II | 19 (19.8) | 30 (25) | |
| IIIA | 42 (43.8) | 58 (48.3) | |
| IIIB | 21 (21.9) | 19 (15.8) | |
| V | - | - | |
| Pathology | |||
| Adenocarcinoma | 77 (80.2) | 97 80.8) | NA |
| Signet ring cell | 4 (4.2) | 18 (15.0) | |
| MAC | 10 (10.4) | 2 (1.7) | |
| Carcinoid tumor | 2 (2.1) | - | |
| Lymphoma | 3 (3.1) | 3 (2.5) | |
| N ratio | |||
| 0 | 12 (12.5) | 19 (15.8) | 0.001 |
| 1 | 29 (30.2) | 34 (28.3) | |
| 2 | 39 (40.6.) | 23 (19.2) | |
| 3 | 16 (16.7) | 44 (36.7) | |
| Lauren classification | |||
| Diffuse | 47 (49.0) | 52 (53.3) | 0.424 |
| Intestinal | 49 (51.0) | 68 (56.7) |
D1: D1 lymphadenectomy; D2: D2 lymphadenectomy; M: Male; F: Female; N ratio: Node ratio; TG: Total gastrectomy; DSG: Distal subtotal gastrectomy; MAC: Mucinous adenocarcinoma; NA: Not available; AJCC: American Joint Committee on Cancer; TNM: Tumor nodule metastasis.
The total number of lymph nodes obtained in all cases in the D1 group was 1381 (14.4 ± 6.1) and in the D2 group this figure was 2823 (23.5 ± 9.3) (P = 0.022). Pathological lymph nodes in the D1 group numbered 374 (3.8 ± 2.2) whereas in the D2 group there were 732 (6.1 ± 4.6) (P = 0.034). There was no difference in pN0, N ratio 0, pN3 and N ratio 3 between groups. In the D1 group, N2 class had the highest number of cases while the same was true for pN1 class in the D2 group (P = 0.003, P = 0.011, respectively). In Table 2, five year survival rates of D1 and D2 dissections in the pN and N ratio groups are compared. None of the N ratio subgroups (N ratio 0, 1, 2, 3) demonstrated statistically significant differences in 5 year survival after D1 or D2 dissections (P = 0.389, P = 0.070, P = 0.192, P = 0.267, respectively). In the pN0 and pN3 subgroups, D1 or D2 dissection did not cause a statistically significant change in 5 year survival (P = 0.172, not available) while pN1 and pN2 did (P = 0.047, P = 0.044, respectively)
Table 2.
Comparison of 5 year survivals in dissection 1 and dissection 2 dissection groups depending on N and N ratio n (%)
| N | D | n | 5 YS | P value | N ratio | n | 5 YS | P value |
| N0 | D1 | 20 | 6 (30.0%) | 0.172 | 0 | 20 | 6 (30.0%) | 0.389 |
| D2 | 26 | 13 (50.0%) | 26 | 13 (50.0%) | ||||
| N1 | D1 | 32 | 4 (12.5%) | 0.047 | 1 | 29 | 4 (13.8%) | 0.070 |
| D2 | 47 | 15 (31.9%) | 44 | 13 (29.5%) | ||||
| N2 | D1 | 40 | 6 (15.0%) | 0.044 | 2 | 39 | 5 (12.8%) | 0.192 |
| D2 | 37 | 1 (2.7%) | 32 | 3 (9.4%) | ||||
| N3 | D1 | 4 | NA | NA | 3 | 16 | 1 (6.3%) | 0.267 |
| D2 | 10 | NA | 44 | 0 |
D1: D1 lymphadenectomy; D2: D2 lymphadenectomy; N ratio: Node ratio; 5 YS: Five years survival; NA: Not available.
In the D1 group, 20 patients who were deemed pN0 (no metastasis) were found to be N ratio 0. Twenty-one out of 32 cases (65.6%) with 1-6 lymph node metastasis (pN1) were found to be N ratio 1, while the remaining 11 (34.4%) were classed as N ratio 2. Twenty-eight (70%) of pN2 patients (n = 40) were classified as N ratio 2; 12 were classified as N ratio 3. Four patients who had ≥ 16 positive nodes were found to be N ratio 3.
In the D2 group, 26 patients who were classified as pN0 were N ratio 0. Thirty-seven (78.7%) out of 47 pN1 patients were N ratio 1, six of them (12.8%) were N ratio 2 and 4 of them (8.5%) were N ratio 3. Thirty-seven cases were pN2; of which 26 (70.3%) were N ratio 2, and 11 were (29.7%) N ratio 3. All pN3 cases (n = 10) were found to be N ratio 3 (Table 3).
Table 3.
Node ratios vs node classes n (%)
| N ratio (metastatic/total number of lymph nodes removed) | |||||
| 0(0) | 1(0-9) | 2(10-25) | 3(> 25) | Total | |
| D1 group | |||||
| N0 (0) | 20 (100) | 20 | |||
| N1 (1-6) | 21 (65.6) | 11 (34.4) | 32 | ||
| N2 (7-15) | 28 (70.0) | 12 (30.0) | 40 | ||
| N3 (> 15) | 4 (100) | 4 | |||
| D2 group | |||||
| N0 (0) | 26 (100) | 26 | |||
| N1 (1-6) | 37 (78.7) | 6 (12.8) | 4 (8.5) | 47 | |
| N2 (7-15) | 26 (70.3) | 11 (29.7) | 37 | ||
| N3 (> 15) | 10 (100) | 10 | |||
D1: D1 lymphadenectomy; D2: D2 lymphadenectomy; N: Groups with respect to number of lymph nodes removed.
When both D1 and D2 groups were evaluated, 23 (24.0%) patients in D1 and 21 (17.5%) in D2 had stage migration (P = 0.001).
In D1, pN0 had the longest survival while pN3 had the shortest. In D2, pN0 had the longest survival whereas pN3 had the shortest survival (Figure 1). In D1, N ratio 0
Figure 1.
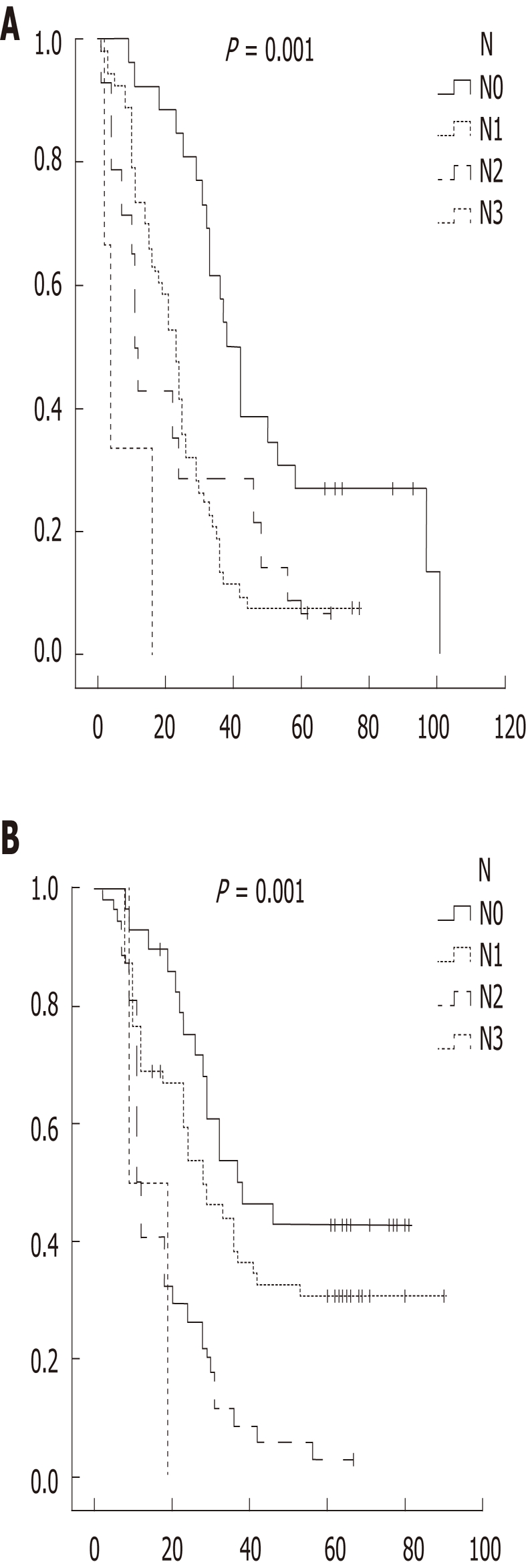
Kaplan meier survival analysis of dissection 1 (A) and dissection 2 (B) group with N class.
had the longest survival while N ratio 2 had the shortest (24.3 mo for N ratio 2, 24.8 mo for N ratio 3). In D2, N ratio 1 had the longest survival whereas N ratio 3 had the shortest survival. Overall, 5 year survival was 20.8%
(n = 45). Survival in the D2 (24.2%) group was longer than in the D1 group (13.3%) (P < 0.001) (Figure 2).
Figure 2.
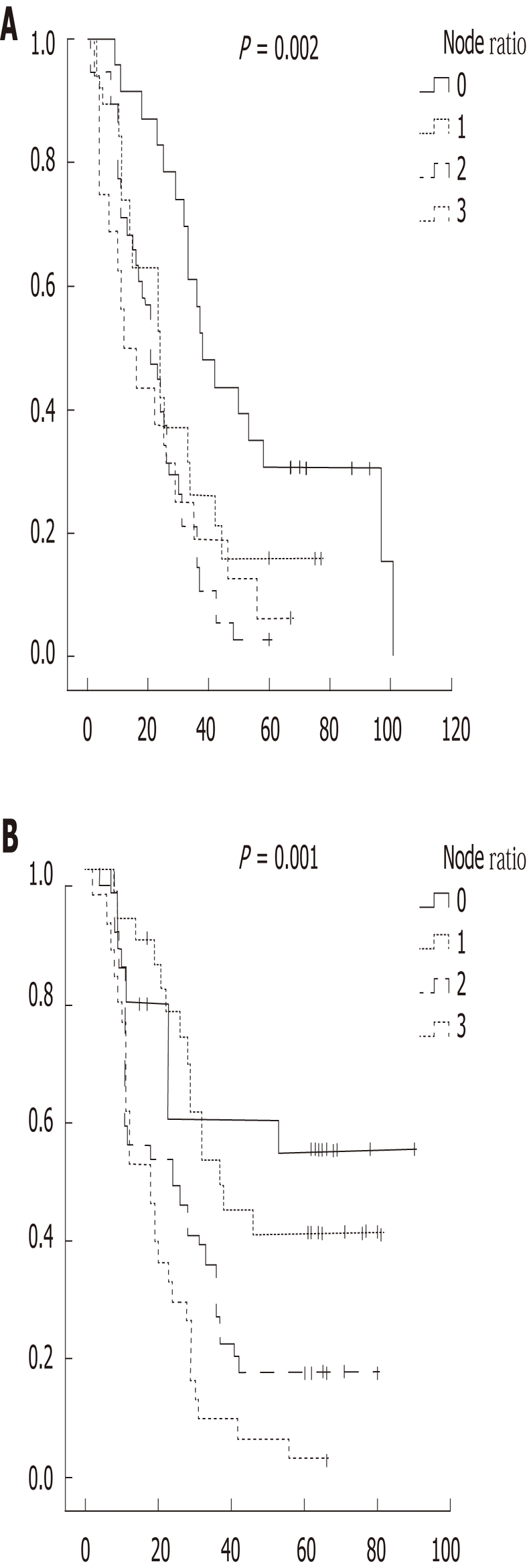
Kaplan meier survival analysis of dissection 1 (A) and dissection 2 (B) group with N ratio.
DISCUSSION
Staging is of paramount importance in decision making for treatment of cancer. This staging should be universally accepted and standardized. Currently, the most commonly used staging system for gastric cancer is the AJCC/UICC system. For an accurate staging in this system at least 15 nodes are required, thus at least a D2 dissection should be performed (pN3 class requires at least 16 lymph nodes in the specimen). A lesser lymph node dissection is inadequate for predicting survival and making a treatment plan. Sun et al[18], in 2159 curative resections, showed a correlation between total number of lymph nodes and metastatic lymph nodes. In our study, total lymph node number dissected in the D1 group was 1 381 (14.4 ± 6.1) and 2823 (23.5 ± 9.3) in the D2 group. Pathological lymph nodes in the D1 and D2 groups were 374 (3.8 ± 2.2) and 732 (6.1 ± 4.6), respectively. This might mean that in D1 dissection an average of 2.3 lymph nodes are not included in specimens, which could end up in recurrence.
Multivariate analysis shows that N ratio is independent of the number of lymph nodes dissected (even less than 10 lymph nodes is enough for classification)[11]. Thus, there was no change in N ratio or stage regardless of overall number of dissected lymph nodes. It was Okusa et al[19] who first showed that N ratio in addition to number of positive lymph nodes is a prognostic factor affecting survival in gastric cancer. In contrast to previous study, Bilici et al[20] concluded that in 202 curative gastric resections N ratio and pN were independent prognosticators and did not have superiority over each other. Pedrazzani et al[21], in their study of 526 gastric cancer patients, showed that survival was not different between pN1 and pN2 patients while patients classified as N ratio 1 and 2 had different mortality and survivals.
The aim of lymphadenectomy in gastric adenocarcinoma is to prolong survival. All metastatic lymph nodes should be removed for this purpose. The wider the lymphatic dissection, the higher the chance of removing all metastatic lymph nodes. However, this must be achieved with low mortality and morbidity rates. In their multicenter study, Marchet et al[22] found that in 1853 cases when D2 dissection was performed, patients who had D1 dissection and were staged as pN1 would turn out to be pN2 or pN3. Bando et al[15] stated in their study that if their 228 patients with lymph node metastasis (who actually had D2 gastrectomy) had D1 gastrectomy, 103 of them would have been understaged. The authors also stated that using the N ratio would have decreased this shift in staging. Nitti et al[11] suggested that D2 dissection should be performed to define number of metastatic, reactional or normal lymph nodes and to also decrease stage migration. In our study, in the D1 group, N ratio 0 had the longest survival while N ratio 2 had the shortest. In the D2 group, N ratio 1 had the longest survival despite shortest survival in the N ratio 3 group. There was a statistically significant difference between both N1 and N2 in both the D1 and D2 groups (P = 0.047, P = 0.044, respectively). N ratio groups did not show any significant difference for either D group.
Another target in gastric cancer treatment is maintaining locoregional control. Wide lymph node dissection is necessary for this. In one study, it was shown that higher number of lymph nodes provides better staging and more accurate prediction of prognosis[23]. Marchet et al[22] stated that 15 lymph nodes was the cut off point for statistical significance for survival in corresponding N ratio groups. In 257 patients with D1 gastrectomy, Maduekwe et al[24] obtained an average of 14 lymph nodes and showed that cases with > 15 lymph node removal had better overall survival than cases with < 15 nodes in pathology specimen. Karpeh et al[25] showed in 1038 gastric cancer patients that there was an increase in median survival in patients with ≥ 15 lymph node removal. When the same patients were staged with N ratio independent from number of lymph nodes, there was no difference in survival. In our study, we resected ≥ 15 nodes in 27 (28.1%) patients in the D1 group and 45 (32.5%) patients in the D2 group (P < 0.001). When each group was analyzed separately, stage migration was less in D2 when compared to D1 (17.5% and 24.0%, respectively).
Curative gastric resections include total gastrectomy or subtotal gastrectomy. Total gastrectomy should be performed for patients with proximal, middle or diffusely located cancers. Prospective, randomized studies have proven that in distally-located tumors, total gastrectomy does not have an advantage over distal subtotal gastrectomy[26]. The crucial point is achieving tumor-free distal and proximal margins. Extent of gastric resection does not affect the predictive power of N ratio. Huang et al[10] showed that in 634 distal gastrectomy patients, N ratio was more accurate in predicting survival than pN stage, thus better for treatment planning.
It might not be radical to say that until a reliable staging with less stage migration is proposed, N ratio could be used for staging. Regardless of the lymph node staging used, a wide lymph node dissection should be done to prevent errors in staging. The shortcomings of our study were mainly the number of cases involved and the retrospective design. Prospective, multicenter trials are needed to better define whether N ratio should replace pN staging in gastric cancer.
In conclusion, N ratio is an accurate and up-to-date staging system for defining prognosis and treatment plan, thus decreasing methodological errors in gastric cancer staging. We believe that, in order to better evaluate prognosis and define a treatment plan, D2 dissection should be the preferred option instead of D1 dissection.
COMMENTS
Background
Staging in gastric cancer is usually carried out according to Japanese Research Society for Gastric Cancer or Union for International Cancer Control /American Joint Committee on Cancer systems. The authors tried to identify whether there could have been change in survival if Node ratio had been used for classification of gastric cancer.
Research frontiers
In this study, the authors re-staged patients who previously had either dissection 1 and dissection 2 according to N ratio and investigated whether there could have been a change in treatment and survival if N ratio had been used for staging.
Innovations and breakthroughs
N ratio is an accurate and up-to-date staging system for defining prognosis and treatment plan, thus decreasing methodological errors in gastric cancer staging. In order to better evaluate prognosis and define a treatment plan, D2 dissection should be the preferred option instead of D1 dissection.
Peer review
This study retrospectively analyzed relationships between n-factors and prognosis among gastric cancer patients who underwent D1 or D2 resection, and concluded that N ratio, which is defined as the ratio of positive lymph nodes to total number of lymph nodes examined, is an accurate staging system for defining prognosis and treatment plan.
Footnotes
Peer reviewer: Shogo Kikuchi, MD, PhD, Professor, Department of Public Health, Aichi Medical University School of Medicine, 21 Karimata, Yazako, Nagakute-cho, Aichi-gun, Aichi 480-1195, Japan
S- Editor Tian L L- Editor Logan S E- Editor Xiong L
References
- 1.Greene FL. TNM staging for malignancies of the digestive tract: 2003 changes and beyond. Semin Surg Oncol. 2003;21:23–29. doi: 10.1002/ssu.10018. [DOI] [PubMed] [Google Scholar]
- 2.Sayegh ME, Sano T, Dexter S, Katai H, Fukagawa T, Sasako M. TNM and Japanese staging systems for gastric cancer: how do they coexist? Gastric Cancer. 2004;7:140–148. doi: 10.1007/s10120-004-0282-7. [DOI] [PubMed] [Google Scholar]
- 3.Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94:2862–2866. doi: 10.1002/cncr.10550. [DOI] [PubMed] [Google Scholar]
- 4.Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143–2151. doi: 10.1002/cncr.22229. [DOI] [PubMed] [Google Scholar]
- 5.Mullaney PJ, Wadley MS, Hyde C, Wyatt J, Lawrence G, Hallissey MT, Fielding JW. Appraisal of compliance with the UICC/AJCC staging system in the staging of gastric cancer. Union Internacional Contra la Cancrum/American Joint Committee on Cancer. Br J Surg. 2002;89:1405–1408. doi: 10.1046/j.1365-2168.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 8.Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Winchester D, Osteen R. Cancer of the stomach: a patient care evaluation study by the American College of Surgeons. Ann Surg. 1993;218:583–592. doi: 10.1097/00000658-199321850-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hundahl SA, Macdonald JS, Benedetti J, Fitzsimmons T. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol. 2002;9:278–286. doi: 10.1007/BF02573066. [DOI] [PubMed] [Google Scholar]
- 10.Huang CM, Lin JX, Zheng CH, Li P, Xie JW, Lin BJ, Wang JB. Prognostic impact of metastatic lymph node ratio on gastric cancer after curative distal gastrectomy. World J Gastroenterol. 2010;16:2055–2060. doi: 10.3748/wjg.v16.i16.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–108512. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 12.Sianesi M, Bezer L, Del Rio P, Dell’Abate P, Iapichino G, Soliani P, Tacci S. The node ratio as prognostic factor after curative resection for gastric cancer. J Gastrointest Surg. 2010;14:614–619. doi: 10.1007/s11605-009-1142-x. [DOI] [PubMed] [Google Scholar]
- 13.de Manzoni G, Verlato G, Roviello F, Morgagni P, Di Leo A, Saragoni L, Marrelli D, Kurihara H, Pasini F. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer. 2002;87:171–174. doi: 10.1038/sj.bjc.6600432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol. 1995;13:19–25. doi: 10.1200/JCO.1995.13.1.19. [DOI] [PubMed] [Google Scholar]
- 15.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 16.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127–139. doi: 10.1007/BF02468883. [DOI] [PubMed] [Google Scholar]
- 17.Therneau TM, Grambsh , PM , Flemming TR. Martingale based residuals for survival models. Biometrika. 1990;77:147–160. [Google Scholar]
- 18.Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, Xu Y, Li DM, Wang ZN, Xu HM. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897–905. doi: 10.1093/annonc/mdn707. [DOI] [PubMed] [Google Scholar]
- 19.Okusa T, Nakane Y, Boku T, Takada H, Yamamura M, Hioki K, Yamamoto M. Quantitative analysis of nodal involvement with respect to survival rate after curative gastrectomy for carcinoma. Surg Gynecol Obstet. 1990;170:488–494. [PubMed] [Google Scholar]
- 20.Bilici A, Ustaalioglu BB, Gumus M, Seker M, Yilmaz B, Kefeli U, Yildirim E, Sonmez B, Salepci T, Kement M, et al. Is metastatic lymph node ratio superior to the number of metastatic lymph nodes to assess outcome and survival of gastric cancer? Onkologie. 2010;33:101–105. doi: 10.1159/000277927. [DOI] [PubMed] [Google Scholar]
- 21.Pedrazzani C, Sivins A, Ancans G, Marrelli D, Corso G, Krumins V, Roviello F, Leja M. Ratio between metastatic and examined lymph nodes (N ratio) may have low clinical utility in gastric cancer patients treated by limited lymphadenectomy: results from a single-center experience of 526 patients. World J Surg. 2010;34:85–91. doi: 10.1007/s00268-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 22.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estes NC, MacDonald JS, Touijer K, Benedetti J, Jacobson J. Inadequate documentation and resection for gastric cancer in the United States: a preliminary report. Am Surg. 1998;64:680–685. [PubMed] [Google Scholar]
- 24.Maduekwe UN, Lauwers GY, Fernandez-Del-Castillo C, Berger DL, Ferguson CM, Rattner DW, Yoon SS. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol. 2010;17:1267–1277. doi: 10.1245/s10434-010-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than Number? An analysis of 1,038 patients. Ann Surg. 2000;232:362–371. doi: 10.1097/00000658-200009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Crose N, Gennari L. Total versus subtotal gastrectomy: surgical morbidity and mortality rates in a multicenter Italian randomized trial. The Italian Gastrointestinal Tumor Study Group. Ann Surg. 1997;226:613–620. doi: 10.1097/00000658-199711000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]


