Abstract
AIM: To study the production and secretion of corticotropin-releasing factor (CRF) by dendritic cells and the influence of commensal bacteria.
METHODS: JAWSII cells (ATCC CRL-11904), a mouse dendritic cell line, were seeded into 24-well culture plates and grown for 3 d. Commensal bacterial strains of Clostridium clostrodiiforme (JCM1291), Bacteroides vulgatus (B. vulgatus) (JCM5856), Escherichia coli (JCM1649), or Fusobacterium varium (F. varium) (ATCC8501) were added to the cells except for the control well, and incubated for 2 h. After incubation, we performed enzyme-linked immunosorbent assay for the cultured medium and reverse transcription polymerase chain reaction for the dendritic cells, and compared these values with controls.
RESULTS: The level of CRF secretion by control dendritic cells was 40.4 ± 6.2 pg/mL. The CRF levels for cells incubated with F. varium and B. vulgatus were significantly higher than that of the control (P < 0.0001). CRF mRNA was present in the control sample without bacteria, and CRF mRNA levels in all samples treated with bacteria were above that of the control sample. F. varium caused the greatest increase in CRF mRNA expression.
CONCLUSION: Our results suggest that dendritic cells produce CRF, a process augmented by commensal bacteria.
Keywords: Commensal bacteria, Corticotrophin-releasing factor, Dendritic cell, Fusobacterium varium, Irritable bowel syndrome
INTRODUCTION
Corticotropin-releasing factor (CRF), a 41-amino acid peptide, is produced mainly in the paraventricular nucleus of the hypothalamus. CRF is a key regulator of the hypothalamic-pituitary adrenal axis. Release of CRF leads to pituitary production of adrenocorticotropic hormone, followed by glucocorticoid secretion by the adrenal cortex[1-5]. Stress induces the release of CRF by the hypothalamus[6]. Stress is known to delay gastric emptying, accelerate colonic transit and evoke colonic motility[7-9], and to decrease the visceral threshold[10] resulting in the development of abdominal symptoms. These events can be triggered by intracerebroventricular or intravenous administration of CRF[8,9,11-13], while stress-induced gastrointestinal dysmotility and visceral hypersensitivity can be improved by central or peripheral administration of a non-selective CRF receptor antagonist[14-16]. Accordingly, stress can induce changes in gastrointestinal motility and visceral perception through CRF. Stressful events are more common in irritable bowel syndrome (IBS) patients[17]. Gastrointestinal dysmotility and visceral hypersensitivity, which are often observed in IBS patients, play an important role in the pathogenesis of IBS[18,19]. Therefore, it appears that CRF is involved in the pathogenesis of IBS.
The risk of developing IBS is increased after an episode of bacterial gastroenteritis. Rodoríguez et al[20] reported that the risk of developing IBS after bacterial gastroenteritis increased by 14.4-fold compared to the general population. Three months after development of bacterial gastroenteritis, patients with IBS symptoms and those without IBS symptoms appeared to be negative for macroscopic colitis, but only patients with IBS symptoms showed increases in the number of chronic inflammatory cells[21]. Moreover, intraepithelial lymphocytosis is observed in some IBS patients without history of bacterial gastroenteritis[22]. Accordingly, inflammation is also involved in the pathogenesis of IBS. The colitis that develops spontaneously in animal models[23-26] requires the presence of intestinal flora, since the disease fails to develop under germ-free conditions. Thus, it appears that commensal bacteria affect the intestinal mucosa and may be required for chronic intestinal inflammation. Dendritic cells (DCs) recognize luminal antigens (such as bacteria or viruses) through toll-like receptors (TLRs), activate natural immunity, and act as antigen-presenting cells resulting in the activation of acquired immunity. Consequently, it is possible that DCs react to common intestinal microbes.
CRF is present not only in the brain but also in several other human tissues, such as the spinal cord, adrenal medulla, lung, liver, pancreas, placenta, and gastrointestinal tract[27-31], and in arthritic joints of patients with rheumatoid arthritis[32]; however, other tissues may also serve as a source of CRF. Lymphocytes appear to be an important source of immunoreactive CRF[33-35], and normal human colonic mucosal enterochoromaffin cells have also been reported to produce CRF[36]. Moreover, CRF receptor expression in DCs was recently reported[37]; however, the possibility that CRF is present in DCs has not yet been investigated.
We hypothesize that (1) DCs produce and secrete CRF; (2) that some commensal bacteria augment these processes; and (3) that secreted CRF influences gut motor function and visceral perception prior to the development of IBS. In the present study, we examined whether CRF is produced or secreted by DCs, and whether production or secretion of CRF from DCs is augmented by commensal bacteria.
MATERIALS AND METHODS
Dendritic cell cultures
Mouse dendritic cells (JAWSII; ATCC CRL-11904) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, United States). JAWSII cells were grown in alpha minimum essential medium with ribonucleosides + deoxyribonucleosides (Gibco, Invitrogen Co., NY, United States) containing 20% fetal bovine serum (Thermo Electron Corp. Melbourne, AU) and 5 ng/mL murine GM-CSF (PeproTech EC, London, United Kingdom). 1 × 105 JAWII cells per well were added to 24-well culture plates (Corning Inc., Corning, NY, United States) and incubated for 72 h under 5% CO2 at 37 °C in a humidified incubator.
Bacterial strains and culture conditions
We used 4 type strains: Clostridium clostrodiiforme (C. clostrodiiforme) (JCM1219; Japan Collection of Microorganisms, RIKEN, Wako, Japan), Bacteroides vulgatus (B. vulgatus) (JCM5826), Escherichia coli (E. coli) (JCM1649), and Fusobacterium varium (F. varium) (ATCC8501; ATCC, Rockville, MD, United States). These strains have been reported to be pathogens for inflammatory diseases such as ulcerative colitis[38-40]. The bacterial strains were harvested in GAM agar plates (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) at 37 °C for 72 h. Aerobic bacteria (E. coli) were incubated under 5% CO2 in a humidified incubator. Anaerobic bacteria (C. clostrodiiforme, B. vulgatus, and F. varium) were incubated in an anaerobic chamber (Forma Scientific, Marietta, Ohio) containing 10% CO2, 10% H2, and 80% N2. After collection of colonies with a disposable plastic loop, they were suspended at 1 × 105 cells/mL in the same medium as used for dendritic cell culture and used immediately.
Sample preparation
JAWSII cells were grown to near confluence before infection with bacteria. Bacteria were added into each well, except the control well, at a cell to bacteria ratio of 1:1000 and incubated for 2 h under 5% CO2 at 37 °C. Following incubation, conditioned medium and floating cells were collected using a pipette and attached cells were removed from the wells using a cell scraper. All cells with medium were pooled and centrifuged at 125 × g for 7 min according to the manufacturer’s instructions. The pelleted cells were resuspended in 200 μL of Isogen (Nippon Gene, Inc., Japan) and frozen at -80 °C. Conditioned medium was also stored at -80 °C.
Detection of CRF by ELISA
To examine whether dendritic cells secrete CRF, enzyme-linked immunosorbent assays (ELISAs) were conducted for the conditioned medium using commercial assay kits (CRF EIA kit; Phoenix Pharmaceuticals Inc., CA, United States). Data represent the averages of 8 independent experiments.
Detection of CRF mRNA by real-time reverse transcription polymerase chain reaction
Total RNA was isolated from cells using an RNeasy Mini Kit (QIAGEN, Inc., Chatsworth, CA), and treated with an RNase-free DNase Set (QIAGEN) following the manufacturer’s instructions. RT for synthesizing the first-strand cDNAs was carried out using SuperScriptII RT (Invitrogen) according to the manufacturer’s instructions. The resultant cDNA corresponding to 10-100 ng of total RNA in a 25 μL final volume was examined by real time PCR using the ABI PRISM 7700 sequence detection system (Applied Biosystems, CA, United States) with Taqman Universal PCR Master Mix together with TaqMan gene expression assay products (Assay ID Mm01293920-s1; Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression was examined as an internal reference and CRF gene expression was examined by means of the comparative CT method. CT values reflect the PCR cycle number for which the amount of amplified target reaches a fixed threshold. The CT for the target gene and reference gene was calculated for each sample. ΔCT is the difference in CT between the target and reference genes. ΔΔCT is the difference between the ΔCT of the sample and the ΔCT of the calibrator (the control sample). CRF mRNA expression normalized to an internal reference and expressed relative to the control sample treated without bacteria is given by 2-ΔΔCT. Data represent the averages of 4 independent experiments.
Statistical analysis
All the data were expressed as mean values ± SEM. Statistical analysis was carried out with analysis of variance and Fisher's protected least significant difference. Differences were considered statistically significant at P < 0.05.
RESULTS
Secretion of CRF and augmentation of secretion upon infection with commensal bacteria
The level of CRF secretion by DCs was 40.4 ± 6.2 pg/mL [95% confidence interval (CI), 25.7-55.2]. When commensal bacteria of the strains C. clostrodiiforme JCM1291, B. vulgatus JCM5826, E. coli JCM1649 and F. varium ATCC8501 were added, the level of CRF secretion by DCs was 41.9 ± 4.9 pg/mL (95% CI, 30.4-53.4), 230.7 ± 46.7 pg/mL (95% CI, 120.3-341.1), 67.9 ± 8.0 pg/mL (95% CI, 49.0-86.7) and 316.9 ± 46.4 pg/mL (95% CI, 207.1-426.6), respectively (Figure 1). The CRF levels for both F. varium and B. vulgatus were significantly higher than that of the control (P < 0.0001). When CRF levels for different bacterial strains were compared, the levels for both F. varium and B. vulgatus were significantly higher than for E. coli (P < 0.0001, P = 0.0005) and C. clostrodiiforme (P < 0.0001, P < 0.0001), respectively.
Figure 1.
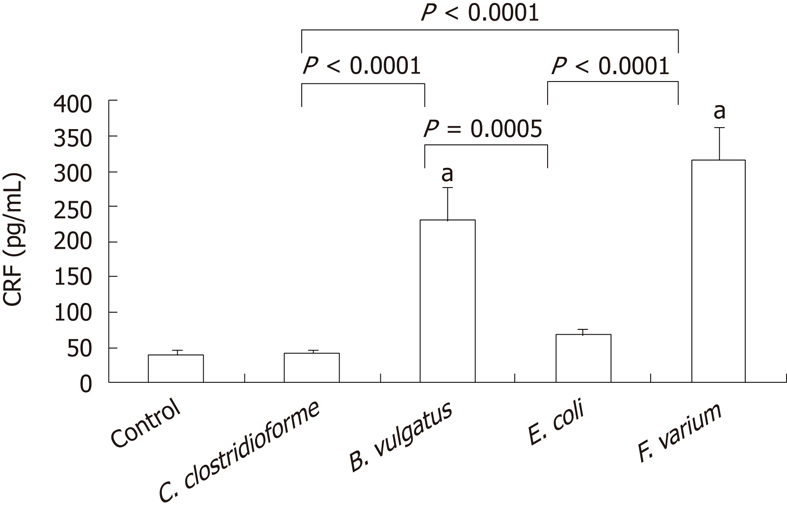
Detection of corticotropin-releasing factor by enzyme-linked immunosorbent assay. JAWSII cells were treated with indicated bacteria for 2 h and conditioned medium was examined by enzyme-linked immunosorbent assay. Data represent the mean ± SEM of 8 independent experiments. Statistical analysis was carried out using analysis of variance and Fisher's protected least significant difference. aP < 0.0001 vs control. CRF: Corticotropin-releasing factor; C. clostrodiiforme: Clostridium clostrodiiforme; B. vulgatus: Bacteroides vulgatus; E. coli: Escherichia coli; F. varium: Fusobacterium varium.
Induction of CRF mRNA upon infection with commensal bacteria
CRF mRNA was present in all samples. Mean CT values were as follows: control, 27.44; C. clostridioforme, 25.70; B. vulgatus, 25.96; E. coli, 26.27; F. varium, 24.63. F. varium caused the greatest increase in CRF mRNA expression (3.7 ± 0.2 fold increase; 95% CI, 3.1-4.3 fold), followed by E. coli (2.4 ± 0.4; 1.3-3.6), B. vulgatus (2.2 ± 0.3; 1.2-3.2), and C. clostrodiiforme (1.6 ± 0.1; 1.1-1.2). The 95% CI for CRF mRNA levels in all samples treated with the indicated bacteria were above that of the control sample without bacteria, indicating that the bacteria significantly stimulated CRF mRNA expression (Figure 2). A comparison of CRF mRNA levels in different bacterial strains showed that levels were significantly higher with F. varium than with the other bacteria (P < 0.0001 vs C. clostridioforme, P < 0.0018 vs B. vulgatus, P < 0.006 vs E.coli). The CRF mRNA level for E. coli was significantly higher than for C. clostrodiiforme (P = 0.0398).
Figure 2.
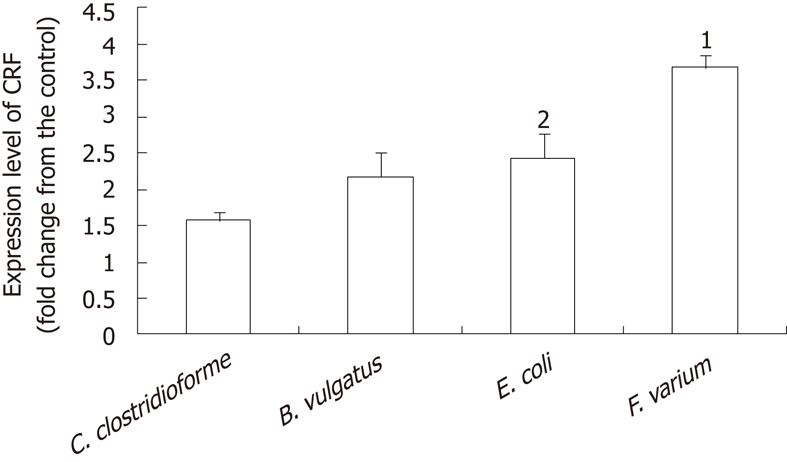
Corticotropin-releasing factor expression determined by comparative CT method. JAWSII cells were treated with the indicated bacteria for 2 h. After treatment, total RNA was isolated from cells. CT value reflects the polymerse chain reaction cycle number for which the amount of amplified target reached a fixed threshold. Data represent the mean ± SEM of 4 independent experiments. Statistical analysis was carried out using analysis of variance and Fisher's protected least significant difference. 1Significantly higher than other indicated bacteria [P < 0.0001 vs Clostridium clostrodiiforme (C. clostridioforme), P < 0.0018 vs Bacteroides vulgatus, P < 0.006 vs Escherichia coli]. 2significantly higher than C. clostridioforme (P = 0.0398). CRF: Corticotropin-releasing factor; C. clostrodiiforme: Clostridium clostrodiiforme; B. vulgatus: Bacteroides vulgatus; E. coli: Escherichia coli; F. varium: Fusobacterium varium.
DISCUSSION
The results from this study demonstrate that DCs can secrete CRF protein. Moreover, secretion of the CRF protein and expression of CRF mRNA were stimulated after challenge with commensal bacteria.
The term “commensal” generally refers to living in a relationship in which one organism derives food or other benefits from another organism without hurting or helping it; therefore, intestinal microorganisms have been referred to as commensal bacteria. Studies with animal models of mucosal inflammation, such as the interleukin-10 knockout mouse model[24,26] and the T cell receptor alpha-chain-deficient mouse model[25], suggested that the presence of intestinal flora was required in order for colitis to develop spontaneously, since the disease failed to develop under germ-free conditions. Bacterial overgrowth in the small intestine is associated with the severity of symptoms of IBS associated with increased intestinal gas and immune responses, and antibiotic therapy has been shown to attenuate IBS symptoms in human patients[41]. Thus, intestinal flora that have been described as commensal flora may actually have several harmful effects on the intestinal mucosa.
DCs recognize luminal antigens, and consequently trigger mucosal inflammation. In such instances, DCs may react to common intestinal microbes and may increase the output of CRF. In the present study, the commensal bacteria F. varium and B. vulgatus resulted in a significant increase in CRF protein levels as compared with control samples that were not exposed to bacteria. All 4 bacterial strains caused an increase in the expression of CRF mRNA as compared with control samples. Thus, all of the bacterial strains examined in this study appear to have the ability to increase the output of CRF from DCs. F. varium appeared to have the strongest effect, since CRF mRNA levels in the presence of F. varium were significantly higher than in the presence of the other bacteria. C. clostridioforme had the lowest ability to increase both CRF protein and CRF mRNA from DCs. C. clostridioforme are the only gram-positive bacteria of the 4 strains examined in this study. Gram-negative bacteria activate immune cells through TLR4 of DCs, while gram-positive bacteria initiate their effect through TLR2[42]. Differences in such pathways may lead to different results. In further work, another intestinal microbe such as probiotic bacteria should be examined. As probiotic bacteria should have a potentially beneficial effect for the host[43], it would be of great interest to similarly examine the response of DCs with regard to CRF levels.
JAWSII cells, a type of DC, are derived from the bone marrow. In the murine Peyer’s patch, myeloid DCs are present in the subepithelial dome region. While little is known about the phenotype of colonic DCs, it was recently reported that myeloid DCs made up the largest population[44]. Thus, JAWSII cells are thought to have the character of colonic DCs. Additional studies with human colonic DCs would be valuable, as would further studies to delineate the processes by which commensal bacteria increase the amount of CRF from DCs.
In conclusion, DCs produce and secrete CRF, and commensal bacteria augment production or secretion of CRF from DCs. The results of the present study suggest that the secretion of CRF from DCs is stimulated by commensal bacteria in the gut, and that CRF derived from DCs may play a role in the pathogenesis of IBS.
COMMENTS
Background
Corticotropin-releasing factor (CRF) influencing gut motor function and visceral perception appears to be involved in the pathogenesis of irritable bowel syndrome (IBS). Intestinal inflammation is associated with post-infectious IBS, and also observed in some IBS patients without history of bacterial gastroenteritis. Intestinal inflammation may be triggered by dendritic cell-mediated immune responses to commensal bacteria, which may involve CRF generation by dendritic cells (DCs).
Research frontiers
Spontaneously developing colitis requires intestinal flora, since colitis fails to develop under germ-free conditions. DCs recognize luminal exogenous antigens and activate natural and acquired immunity. It is possible that DCs react to common intestinal microbes. CRF is present in several human tissues, and CRF receptor expression in DCs was recently reported. There has been no report on the production and secretion of CRF from DCs.
Innovation and breakthroughs
This is the first report that DCs produce and secrete CRF and that commensal bacteria augment production or secretion of CRF from DCs.
Applications
DCs produce and secrete CRF and some commensal bacteria augment these processes. Secreted CRF may influence gut motor function and visceral perception prior to the development of IBS. Accordingly, the results of this study may shed light on the pathogenesis of irritable bowel syndrome.
Terminology
CRF is produced mainly in the paraventricular nucleus of the hypothalamus, but recently has been reported to be present not only in the brain but also in several other human tissues. CRF is a key regulator of the hypothalamic-pituitary adrenal axis, and it appears that CRF is involved in the pathogenesis of IBS.
Peer review
The manuscript reports the effect on CRF secretion in short term cultures (3 d) by a murine dendritic cell line following incubation with three different commensal bacterial strains. The methodology appears generally well conceived and the manuscript is well written. The conclusions that dendritic cells produce and secrete CRF and that commensal bacteria augment production or secretion of CRF from dendritic cells are straightforward and justified by the results obtained, although the biological significance of the observations remains obscure.
Footnotes
Supported by Grants in Aid for Scientific Research (C) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, No. 17590679
Peer reviewer: Jorgen Rask-Madsen, MD, FRCP, Professor of Gastroenterology, Department of Gastroenterology, Herlev Hospital, Skodsborg Strandvej 280A, 2942, Herlev DK-2730, Denmark
S- Editor Tian L L- Editor Logan S E- Editor Xiong L
References
- 1.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci USA. 1983;80:4851–4855. doi: 10.1073/pnas.80.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibahara S, Morimoto Y, Furutani Y, Notake M, Takahashi H, Shimizu S, Horikawa S, Numa S. Isolation and sequence analysis of the human corticotropin-releasing factor precursor gene. EMBO J. 1983;2:775–779. doi: 10.1002/j.1460-2075.1983.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulte HM, Chrousos GP, Gold PW, Booth JD, Oldfield EH, Cutler GB, Loriaux DL. Continuous administration of synthetic ovine corticotropin-releasing factor in man. Physiological and pathophysiological implications. J Clin Invest. 1985;75:1781–1785. doi: 10.1172/JCI111890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 6.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 7.Barquist E, Zinner M, Rivier J, Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992;262:G616–G620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- 8.Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716–723. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- 9.Gue M, Junien JL, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964–970. doi: 10.1016/0016-5085(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 10.Ford MJ, Camilleri M, Zinsmeister AR, Hanson RB. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology. 1995;109:1772–1780. doi: 10.1016/0016-5085(95)90743-2. [DOI] [PubMed] [Google Scholar]
- 11.Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology. 1988;95:1510–1517. doi: 10.1016/s0016-5085(88)80070-2. [DOI] [PubMed] [Google Scholar]
- 12.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- 13.Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Tache Y, Sytnik B, Munakata J, Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol Motil. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 14.Mönnikes H, Schmidt BG, Raybould HE, Taché Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 1992;262:G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- 15.Bonaz B, Taché Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- 16.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanazawa M, Endo Y, Whitehead WE, Kano M, Hongo M, Fukudo S. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig Dis Sci. 2004;49:1046–1053. doi: 10.1023/b:ddas.0000034570.52305.10. [DOI] [PubMed] [Google Scholar]
- 18.Kumar D, Wingate DL. The irritable bowel syndrome: a paroxysmal motor disorder. Lancet. 1985;2:973–977. doi: 10.1016/s0140-6736(85)90525-2. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez LA, Ruigómez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ. 1999;318:565–566. doi: 10.1136/bmj.318.7183.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 23.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 24.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishi D, Takahashi I, Kai Y, Tamagawa H, Iijima H, Obunai S, Nezu R, Ito T, Matsuda H, Kiyono H. Alteration of V beta usage and cytokine production of CD4+ TCR beta beta homodimer T cells by elimination of Bacteroides vulgatus prevents colitis in TCR alpha-chain-deficient mice. J Immunol. 2000;165:5891–5899. doi: 10.4049/jimmunol.165.10.5891. [DOI] [PubMed] [Google Scholar]
- 26.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suda T, Tomori N, Tozawa F, Mouri T, Demura H, Shizume K. Distribution and characterization of immunoreactive corticotropin-releasing factor in human tissues. J Clin Endocrinol Metab. 1984;59:861–866. doi: 10.1210/jcem-59-5-861. [DOI] [PubMed] [Google Scholar]
- 28.Petrusz P, Merchenthaler I, Maderdrut JL, Vigh S, Schally AV. Corticotropin-releasing factor (CRF)-like immunoreactivity in the vertebrate endocrine pancreas. Proc Natl Acad Sci USA. 1983;80:1721–1725. doi: 10.1073/pnas.80.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibasaki T, Odagiri E, Shizume K, Ling N. Corticotropin-releasing factor-like activity in human placental extracts. J Clin Endocrinol Metab. 1982;55:384–386. doi: 10.1210/jcem-55-2-384. [DOI] [PubMed] [Google Scholar]
- 30.Nieuwenhuijzen Kruseman AC, Linton EA, Lowry PJ, Rees LH, Besser GM. Corticotropin-releasing factor immunoreactivity in human gastrointestinal tract. Lancet. 1982;2:1245–1246. doi: 10.1016/s0140-6736(82)90105-2. [DOI] [PubMed] [Google Scholar]
- 31.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, et al. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 32.Crofford LJ, Sano H, Karalis K, Friedman TC, Epps HR, Remmers EF, Mathern P, Chrousos GP, Wilder RL. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol. 1993;151:1587–1596. [PubMed] [Google Scholar]
- 33.Ekman R, Servenius B, Castro MG, Lowry PJ, Cederlund AS, Bergman O, Sjögren HO. Biosynthesis of corticotropin-releasing hormone in human T-lymphocytes. J Neuroimmunol. 1993;44:7–13. doi: 10.1016/0165-5728(93)90262-w. [DOI] [PubMed] [Google Scholar]
- 34.Muglia LJ, Jenkins NA, Gilbert DJ, Copeland NG, Majzoub JA. Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J Clin Invest. 1994;93:2066–2072. doi: 10.1172/JCI117201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kravchenco IV, Furalev VA. Secretion of immunoreactive corticotropin releasing factor and adrenocorticotropic hormone by T- and B-lymphocytes in response to cellular stress factors. Biochem Biophys Res Commun. 1994;204:828–834. doi: 10.1006/bbrc.1994.2534. [DOI] [PubMed] [Google Scholar]
- 36.Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology. 1994;106:859–865. doi: 10.1016/0016-5085(94)90743-9. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Kwon YS, Park CO, Oh SH, Lee JH, Wu WH, Chang NS, Lee MG, Lee KH. Corticotropin-releasing factor decreases IL-18 in the monocyte-derived dendritic cell. Exp Dermatol. 2009;18:199–204. doi: 10.1111/j.1600-0625.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 38.Burke DA, Axon AT. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. BMJ. 1988;297:102–104. doi: 10.1136/bmj.297.6641.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda H, Fujiyama Y, Andoh A, Ushijima T, Kajinami T, Bamba T. Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulcerative colitis. J Gastroenterol Hepatol. 2000;15:61–68. doi: 10.1046/j.1440-1746.2000.02045.x. [DOI] [PubMed] [Google Scholar]
- 40.Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003;52:79–83. doi: 10.1136/gut.52.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 43.O'Hara AM, O’Regan P, Fanning A, O’Mahony C, Macsharry J, Lyons A, Bienenstock J, O’Mahony L, Shanahan F. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology. 2006;118:202–215. doi: 10.1111/j.1365-2567.2006.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruickshank SM, English NR, Felsburg PJ, Carding SR. Characterization of colonic dendritic cells in normal and colitic mice. World J Gastroenterol. 2005;11:6338–6347. doi: 10.3748/wjg.v11.i40.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]


