Abstract
AIM: To investigate relationships between colorectal adenoma incidence, metabolic syndrome (MS) components and lifestyle factors.
METHODS: We conducted a retrospective cohort study using data from individuals who had multiple sigmoidoscopies for colon cancer at the Health Promotion Center of Ulsan University Hospital in Korea from 1998 to 2007.
RESULTS: By multivariate analysis, the incidence of distal colon adenoma was increased by more than 1.76 times in individuals with at least one component of MS compared to those without a component of MS. After adjustment for age, gender, smoking, drinking, and physical exercise, only high body mass index (BMI) was significantly associated with the incidence of distal colon adenoma (Hazard ratio 1.66, 95% confidence interval 1.05-2.62).
CONCLUSION: Our results suggest that high BMI may increase the risk of colorectal adenoma in Korean adults.
Keywords: Body mass index, Distal colon adenoma, Korea, Lifestyle risk factor, Metabolic syndrome
INTRODUCTION
Colorectal cancer is a major cause of cancer death in developed Western countries such as the United States and nations of Europe[1]. However, the incidence of colorectal cancer has been increasing rapidly over the past two decades in Korea, which was previously known as a low-risk area. According to a report from the Ministry of Health and Welfare of Korea, colorectal cancer was the third most commonly diagnosed malignancy after stomach and lung cancer in 2005. From 1999 to 2005, the incidence of colorectal cancer rose from 26.2 to 39.6 cases per 100 000 population for men and from 16.4 to 22.2 per 100 000 for women[2].
As colorectal adenomas are recognized as precursors of colorectal cancer, identification of their risk factors would seem to be helpful in the prevention of colorectal cancer[3]. One of the main explanations given for the increasing incidence of colorectal cancer in Korea is the growing adoption of the westernized diet consisting of high fat and sugar. The western diet is also known to increase the risk of metabolic syndrome (MS)[4].
In several previously reported studies, MS or its individual components such as obesity, impaired glucose tolerance, hypertension, low high-density lipoprotein cholesterol, and hypertriglyceridemia were found to be associated with colorectal adenomas[5-13]. In addition, lifestyle factors such as alcohol drinking, cigarette smoking, and lack of physical exercise also have been shown to be associated with the incidence of colorectal adenoma[14-17]. However, most previous studies showed only a weak temporal relationship between exposure and disease occurrence.
To date, no cohort study has examined the association between the individual components of MS or lifestyle factors and the incidence of colorectal adenoma in Korea. In this study, we investigated the relationships between the incidence of distal colon adenoma and MS components and also lifestyle factors in a Korean population-based cohort.
MATERIALS AND METHODS
Study population
We conducted a retrospective cohort study using data from individuals who had multiple sigmoidoscopies at the Health Promotion Center of Ulsan University Hospital in Korea from 1998 to 2007. We recommend screening flexible sigmoidoscopy (SFS) to our patients according to guidelines for colorectal cancer screening issued by the American College of Gastroenterology[18]. Nevertheless, some patients within our study population had undergone two or more surveillance SFS examinations at short intervals. Perhaps some of these study participants had misunderstood the recommended guidelines or had lower gastrointestinal tract symptoms or risk factors for colorectal cancer such as family history; or there may be other reasons for the multiple examinations at short intervals.
A total of 15 353 asymptomatic adults underwent a flexible sigmoidoscopy. Of these, 13 566 were excluded because they had undergone only one sigmoidoscopy or data were missing from their records. Among the remaining 1787 individuals who had had at least two sigmoidoscopies during a period of two years, 225 individuals who were specific for tubular adenoma (n = 109 adults) or hyperplastic polyps (n = 116 adults) at the first sigmoidoscopic examination were excluded. Thus, a cohort of 1562 individuals was included in this study (Figure 1).
Figure 1.
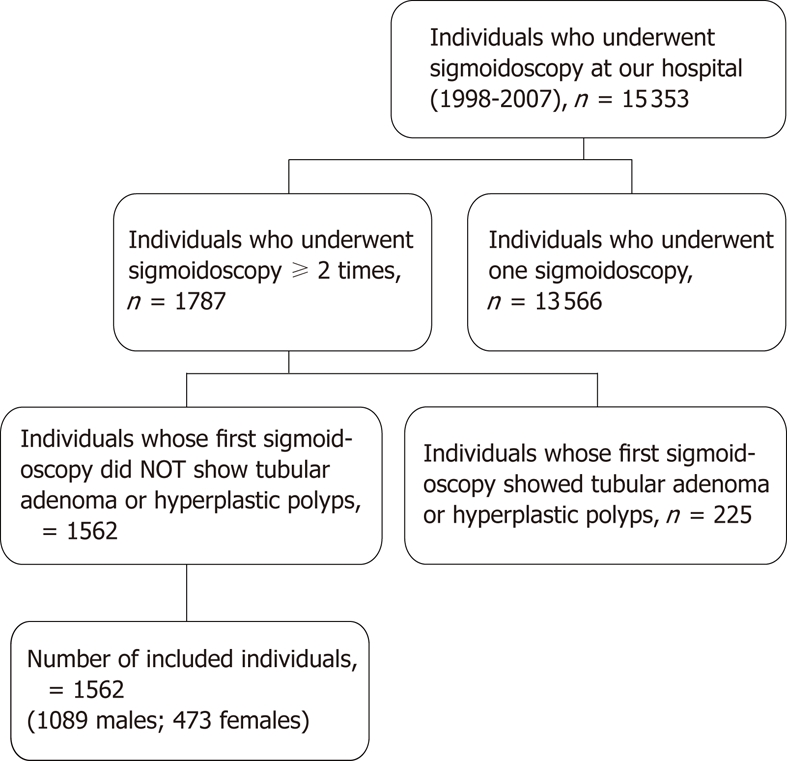
Case selection.
The following parameters were collected from medical records and from self-administered questionnaires: height, body weight, systolic and diastolic blood pressures, serum biochemistry, flexible sigmoidoscopy results, and past medical history. The study protocol was approved by the Institutional Review Board of Ulsan University Hospital.
Questionnaire information
Using a structured, self-administered questionnaire, patients were asked about their smoking and drinking habits, physical activity, medical history and socioeconomic status.
Smoking was categorized as never, ex-smoker and current smoker. An individual who reported smoking within the past 30 d was classified as a current smoker. An ex-smoker was defined as an individual who had smoked at least one pack-year and was distinguished from someone who had never smoked. Current drinkers were defined as individuals who had consumed alcohol at least once per week over a period of at least 1 year. For alcohol consumption, individuals were classified as never or current drinkers.
Physical activity was classified as regular, irregular, and none based on the regularity, regardless of the type of exercise or intensity level. Individuals who reported having exercised regularly within the past year were classified into the regular exercise group. The irregular exercise group comprised individuals who had exercised irregularly within the past year, and were distinguished from those who had never exercised in the past year. The regular and irregular exercise groups were classified as the exercise “yes” group in the analysis.
Anthropometric and laboratory measurements
Anthropometric measurements were made by well-trained examiners in individuals wearing light clothing and without shoes. Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg using Inbody 2.0 (Biospace, Seoul, Korea). Body mass index (BMI) values were calculated by dividing weight (kg) by height squared (m2). Blood pressure was measured by well-trained nurses using a mercury sphygmomanometer in the sitting position after at least a 10-min rest period. Following an overnight fasting, blood samples were obtained and analyzed on a Hitachi Modular DPE system (Roche Diagnostics, Germany). The fasting plasma glucose (FPG) level was measured using a hexokinase UV method. Triglycerides (TG) were measured by an enzymatic calorimetric method. HDL-cholesterol (HDL-C) level was determined by the homogeneous enzymatic colorimetric method.
Diagnosis of distal colon adenoma
The sigmoidoscopies were performed by gastroenterologists who observed the entire procedure on a video monitor. The procedure was intended to screen the distal colon, including the descending and sigmoid colon, and the rectum. When fully inserted, the 60-cm sigmoidoscope reached to the mid-descending colon. Endoscopic findings were recorded in a computer database. All visualized lesions were biopsied and histologically assessed by an experienced pathologist. The size of each polyp was estimated by the use of 8-mm-diameter open-biopsy forceps. Histological assessment of the polyps was performed by a single pathologist blinded to each patient’s status. In this study, the adenomas were classified as tubular, serrated, villo-tubular, high-grade dysplasia, and adenocarcinoma types according to the World Health Organization classification[19]. For multiple primary adenomas in the distal colon at different times, the earliest diagnosis was applied, and for those occurring simultaneously, the most advanced and most invasive diagnosis was applied.
Definition of MS
MS was defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria, with BMI used in place of waist circumference[20]. BMI was calculated as described above (kg/m2). The definition of obesity was BMI ≥ 25, as recommended by the Korean Ministry of Health and Welfare in 2006[21]. The diagnosis of MS in this study was made when at least 3 of the following 5 characteristics were present: (1) BMI ≥ 25 kg/m2; (2) TG ≥ 150 mg/dL, or on drug treatment for elevated TG; (3) HDL-C < 40 mg/dL in males and < 50 mg/dL in females, or on drug treatment for reduced HDL-C; (4) blood pressure (BP) ≥ 130/85 mmHg, or on drug treatment for hypertension; and (5) FPG ≥ 110 mg/dL, or on drug treatment for elevated glucose.
Statistical analysis
Multivariate Cox proportional hazards analysis was applied to assess the effect of prognostic variables in MS and non-MS individuals. Results of the Cox proportional hazards model were presented as the hazard ratio (HR) and the 95% confidence interval (95% CI). All P values were two-sided, and all statistical analyses were performed using SPSS version 14.0.
RESULTS
A total of 1562 individuals (1089 men and 473 women) were included in the study. Of these, 229 (14.7%) subjects (197 men and 32 women) were found to have distal colon adenomas.
Table 1 shows the age- and gender-specific prevalence of MS in the study population. The prevalence of MS in males was slightly higher than in females (18% and 14%, respectively). Hypertension was the most common metabolic abnormality in both sexes.
Table 1.
Age- and gender-specific prevalence of metabolic syndrome in the study population n (%)
| Age group(yr) |
Males |
Females |
||||||||||||
| n |
MS criteria |
n |
MS criteria |
|||||||||||
| BMI ≥ 25 | FBS ≥ 110 | HBP | TG ≥ 150 | HDL< 40 | MS ≥ 3 | BMI ≥ 25 | FBS ≥ 110 | HBP | TG ≥ 150 | HDL< 50 | MS ≥ 3 | |||
| 20-29 | 4 | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (25) | 0 (0) | 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| 30-39 | 81 | 29 (36) | 8 (10) | 14 (17) | 29 (36) | 22 (27) | 18 (22) | 26 | 4 (15) | 0 (0) | 2 (8) | 2 (8) | 12 (46) | 0 (0) |
| 40-49 | 451 | 154 (34) | 68 (15) | 137 (30) | 131 (29) | 120 (27) | 76 (17) | 191 | 34 (18) | 15 (8) | 40 (21) | 20 (10) | 91 (48) | 18 (9) |
| 50-59 | 466 | 164 (35) | 91 (20) | 195 (42) | 123 (26) | 101 (22) | 80 (17) | 200 | 52 (26) | 28 (14) | 61 (31) | 28 (14) | 104 (52) | 34 (17) |
| ≥ 60 | 87 | 21 (24) | 21 (24) | 46 (53) | 28 (32) | 14 (16) | 20 (23) | 54 | 20 (37) | 10 (19) | 26 (48) | 14 (26) | 30 (56) | 16 (30) |
| Sub-total | 1089 | 368 (34) | 188 (17) | 393 (36) | 311 (29) | 258 (24) | 194 (18) | 473 | 110 (23) | 53 (11) | 129 (27) | 64 (14) | 239 (51) | 68 (14) |
BMI: Body mass index (kg/m2); FBS: Fasting blood sugar (mg/dL); HBP: High blood pressure (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg); TG: Triglyceride (mg/dL); HDL: High density lipoprotein (mg/dL); MS: Metabolic syndrome.
The associations between the incidence of distal colon adenomas and the number of MS components are shown in Table 2. In our cohorts, 263 (16.8%) of the individuals were diagnosed with MS according to NCEP-ATP III criteria. Among the MS subjects, 182 (69.2%) had three components, 64 (24.3%) had four components, and 17 (6.5%) had all five. After adjusting for age and gender, the presence of any component of MS showed a positive association with the incidence of distal colon adenoma. According to multivariate analysis, the risk of distal colon adenoma was increased in patients with even one component of MS compared to patients without any component of MS, but this result was not statistically significant.
Table 2.
Hazard ratios for distal colon adenomas according to the number of metabolic syndrome components
| Number of MS diagnostic criteria present | Cases | Person years | Age-, sex-adjusted hazard ratio | 95% CI | Multivariate hazard ratio1 | 95% CI | ||
| 0 | 434 | 1446 | 1.00 | 1.00 | ||||
| 1 | 504 | 1697 | 1.18 | 0.83 | 1.67 | 1.76 | 0.94 | 3.29 |
| 2 | 361 | 1272 | 1.02 | 0.70 | 1.50 | 1.69 | 0.86 | 3.29 |
| 3 | 182 | 610 | 1.36 | 0.88 | 2.10 | 1.48 | 0.66 | 3.36 |
| 4 | 64 | 224 | 1.35 | 0.73 | 2.47 | 2.14 | 0.81 | 5.63 |
| 5 | 17 | 62 | 1.52 | 0.54 | 4.28 | 2.92 | 0.62 | 13.73 |
Adjusted for age, sex, smoking status, drinking status, exercise status. CI: Confidence Interval; MS: Metabolic syndrome.
Among the MS components, diastolic blood pressure, systolic blood pressure, BMI, HDL and FBS were positively associated with the risk of distal colon adenoma; however, only the association with BMI was statistically significant (Table 3). Among the lifestyle factors examined in our study, smoking and drinking were positively associated with the risk of distal colon adenoma whereas exercise showed a negative correlation, but these results were not statistically significant.
Table 3.
Hazard ratios for distal colon adenomas according to individual components of metabolic syndrome and lifestyle factors
| Characteristics | Cases | Person years | Age-, sex-adjustedhazard ratio | 95% CI | Multivariate hazard ratio2 | 95% Cl | ||
| DBP | ||||||||
| < 85 | 1237 | 4106 | 1.00 | 1.00 | ||||
| ≥ 85 | 325 | 1206 | 0.75 | 0.54 | 1.04 | 0.68 | 0.35 | 1.29 |
| SBP | ||||||||
| < 130 | 1085 | 3648 | 1.00 | 1.00 | ||||
| ≥ 130 | 477 | 1664 | 0.97 | 0.73 | 1.29 | 1.50 | 0.82 | 2.73 |
| BMI | ||||||||
| < 25 | 1084 | 3688 | 1.00 | 1.00 | ||||
| ≥ 25 | 478 | 1623 | 1.39 | 1.07 | 1.82 | 1.66 | 1.05 | 2.62 |
| HDL | ||||||||
| ≥ 40 (≥ 501) | 1065 | 3641 | 1.00 | 1.00 | ||||
| < 40 (< 501) | 497 | 1671 | 1.19 | 0.88 | 1.60 | 1.04 | 0.57 | 1.88 |
| TG | ||||||||
| < 150 | 1187 | 4025 | 1.00 | 1.00 | ||||
| ≥ 150 | 375 | 1287 | 0.96 | 0.72 | 1.29 | 0.76 | 0.45 | 1.27 |
| FBS6 | ||||||||
| < 110 | 1321 | 4457 | 1.00 | 1.00 | ||||
| ≥ 110 | 241 | 855 | 1.13 | 0.82 | 1.58 | 1.32 | 0.77 | 2.27 |
| Smoking | ||||||||
| Never smoked | 606 | 2048 | 1.0 | 1.00 | ||||
| Ex-smoker | 218 | 769 | 0.86 | 0.57 | 1.29 | 1.34 | 0.70 | 2.54 |
| Current smoker | 262 | 1072 | 0.81 | 0.56 | 1.17 | 1.39 | 0.79 | 2.44 |
| Drinking | ||||||||
| No drinking | 396 | 1307 | 1.00 | 1.00 | ||||
| Current drinking | 772 | 2695 | 1.59 | 1.02 | 2.47 | 1.82 | 0.92 | 3.60 |
| Exercise | ||||||||
| Never | 409 | 1587 | 1.00 | 1.00 | ||||
| Irregular | 398 | 1549 | 1.09 | 0.73 | 1.62 | 0.96 | 0.58 | 1.58 |
| Regular (≥ 1 time/wk) | 273 | 1077 | 1.22 | 0.78 | 1.90 | 0.88 | 0.49 | 1.60 |
Female;
Adjusted for sex, age, systolic and diastolic blood pressure. DBP: Diastolic blood pressure; SBP: Systolic blood pressure; BMI: Body mass index; HDL: High density lipoprotein; TG: Triglyceride; FBS: Fasting blood sugar; HDL: High density lipoprotein; CI: Confidence interval.
DISCUSSION
In this study, we confirmed a positive association between MS and distal colon adenoma in a Korean population. We found that, compared to persons without any component of MS, the risk of distal colon adenoma positively increased with the presence of even one component of MS. These results are consistent with those of Lee et al[8]. Moreover, we found only BMI to be an independent risk factor of distal colon adenoma among the five components of MS examined.
Although NCEP-ATP III requires waist circumference for diagnosis of MS, we used BMI as an indicator of obesity because the cut-off points of waist circumference in Koreans differ from those currently recommended by the NCEP-ATP III. The cut-off points of waist circumference for central obesity in Koreans are 90cm for men and 85cm for women[22]. Moreover, currently there is no equivalent method for the measurement of waist circumstance, whereas BMI is an easy and accurate index. Waist circumference measurements show strong between-observer differences, and should, where possible, be carried out by one observer. Weight and height are the most precisely measured variables, and it is entirely appropriate that they continue to be the predominant measure of choice in the vast majority of nutritional anthropometric studies[23]. Therefore, most medical institutions in Korea use BMI as an indicator of obesity, defined by The Korean Ministry of Health and Welfare as BMI ≥ 25[21].
Several studies have reported that MS is associated with colorectal adenoma and that obesity is a risk factor for the lesion, but the relationship between BMI and the prevalence of colorectal adenoma has remained controversial[5-9,16,24,25]. Recently, a retrospective study of the effect of body weight changes on the development of new colorectal adenomas was reported by Yamaji et al[24]. In their cross-sectional study, they found that the prevalence of colorectal adenoma increased proportionally with increasing BMI. According to their results, the prevalence of colorectal adenoma was 15.4%, 20.6%, 22.7%, and 24.2%, respectively, in the first (BMI < 21.350 kg/m2), second (BMI 21.350 ≤ BMI < 23.199 kg/m2), third (BMI 23.199 ≤ BMI < 25.156 kg/m2), and fourth (BMI ≥ 25.156 kg/m2) quartiles. Compared with the first quartile, the adjusted odds ratios (ORs) were 1.15 (95% CI, 0.97-1.37; P = 0.10) for the second quartile, 1.19 (95% CI, 1.01-1.41; P = 0.04) for the third quartile, and 1.32 (95% CI, 1.12-1.56; P = 0.001) for the fourth quartile. Their result is similar to our finding of a positive relationship between BMI and the prevalence of distal colon adenoma. In our study we confirmed that persons with high BMI (≥ 25) had a 66% higher incidence of distal colon adenoma compared to persons with BMI < 25. The study by Lee et al[8] also revealed that the occurrence of adenomatous colonic polyps was significantly associated with increased BMI levels. In contrast, Willett and colleagues found no significant association between BMI and colorectal adenoma[16,25].
Another controversy remains regarding whether gender differences exist in the relationship between BMI and colorectal adenoma. Some authors reported that BMI was associated with a higher risk of colorectal adenomas among men but not among women[26,27]. However, others reported an increased risk and prevalence for significant colorectal neoplasia in women as BMI increased, but not in men[17,28].
In the present study, current and past smoking and current drinking behaviors all showed a positive association with the occurrence of distal colon adenoma, whereas physical activity showed a negative association. Our results are similar to those of other studies, but none of the associations in any of the studies achieved statistical significance[29-32]. Prior to this study, we hypothesized that individuals with unhealthy lifestyle behaviors, such as cigarette smoking, alcohol drinking, and physical inactivity, would be at greater risk of distal colon adenoma. The lack of statistical significance in the current study could be due to the short observation period and the interaction between MS components.
The strengths of our study include the cohort design and the relatively large sample size. Furthermore, we investigated the association of each component of MS, which provided the opportunity to examine the difference in incidence rate of distal colon adenoma between MS and non-MS individuals. Additionally, lifestyle factors related to MS, including cigarette smoking, alcohol consumption, and physical exercise, were examined. One other advantage of our study is that actual measurements of height and weight were made by trained individuals. This is preferred over self-reported data, which have been used frequently in a number of well-known, large-scale cohort studies, because heavier individuals tend to under-report their weight. Furthermore, our study simultaneously measured anthropometrics, FBS, TG, HDL-C, blood pressure, and adenoma incidence in a population of individuals at average adenoma risk undergoing sigmoidoscopy. Moreover, subjects in both the adenoma and normal groups were confirmed as having or not having the lesion through the same diagnostic procedure of sigmoidoscopy. Therefore, it is highly unlikely that the study results might be affected by misclassification bias, and this hospital-based retrospective study is internally valid because the normal and recurrent adenoma groups were selected from the same source population.
There were several limitations in our study which need to be addressed. Firstly, the information on cigarette smoking, alcohol drinking and physical activity was self-reported, thus allowing for recall bias. This may explain why a sedentary lifestyle and cigarette smoking were not associated with an increased risk of distal colon adenoma. Secondly, the study population may not be representative of the general population because the subjects were not randomly selected. Also, there may have been some heterogeneity in fasting status and the time of day that blood was collected. Finally, only the adenomas of the distal colon, not the entire colon, were screened using sigmoidoscopy. For the detection of colon adenoma, colonoscopy may be more accurate than sigmoidoscopy.
In conclusion, only BMI, but no other individual component of MS, was positively associated with distal colon adenoma risk. In contrast with previous cross-sectional studies, we found that other MS components did not have a synergistic effect on development of distal colon adenoma[3,4]. However, this may be attributable to the limitations mentioned above. One possible explanation for our findings is that each component of MS may promote or prevent adenoma development via different mechanisms that do not act in an additive or synergistic manner. Further molecular biological research and epidemiological studies are needed to explore this topic.
To our knowledge, the present study is the first cohort study supporting a relationship between BMI and new development of distal colon adenoma in a Korean population. BMI appears to be a better predictor than the MS cluster, and therefore BMI may be the only component needed.
ACKNOWLEDGMENTS
We thank the members of the Ulsan University Hospital Health Promotion Center and all subjects who participated in this study.
COMMENTS
Background
In Korea, as in the developed countries, the incidence of colorectal cancer has been increasing rapidly over the past two decades with the adoption of westernized lifestyles. Because the western diet increases the risk of metabolic syndrome (MS) and colorectal adenomas are precursors of colorectal cancer, some researchers have investigated the relationships between the incidence of colorectal adenoma and MS components and lifestyle factors.
Research frontiers
Several studies have reported that MS is associated with colorectal adenoma and that obesity is a risk factor for the lesion. However the relationship between body mass index (BMI) as a diagnostic tool of obesity and the prevalence of colorectal adenoma has remained controversial. Moreover, to date, no cohort study has examined the association between the individual components of MS or lifestyle factors and the incidence of colorectal adenoma in Korea.
Innovations and breakthroughs
In our study, we found that the incidence of distal colon adenoma was increased by more than 1.76 times in individuals with at least one component of MS compared to those without a component of MS. Among the five components of MS examined, only BMI was the independent risk factor of distal colon adenoma. Persons with high BMI (> 25) had a 66% higher incidence of distal colon adenoma compared to persons with BMI < 25. These results suggest that high BMI may increase the risk of colorectal adenoma in Korean adults. To our knowledge, this is the first cohort study with a relatively large sample size.
Applications
Reducing the BMI can be one of the best ways to prevent colorectal cancer in Korean.
Peer Review
This is the first cohort study to demonstrate an association between the individual components of metabolic syndrome and the incidence of colorectal adenoma in Korean. It revealed that only a high BMI was associated with a high risk of colorectal adenoma. The results are interesting and may contribute to reduce the risk of developing colorectal cancer.
Footnotes
Supported by the Biomedical Research Center Promotion Fund of the Ulsan University Hospital (UUH-2008-08)
Peer reviewer: Dr. Benjamin Perakath, Professorx, Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India
S- Editor Sun H L- Editor Logan S E- Editor Zhang DN
References
- 1.Boyle P, Langman JS. ABC of colorectal cancer: Epidemiology. BMJ. 2000;321:805–808. doi: 10.1136/bmj.321.7264.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009;24:995–1003. doi: 10.3346/jkms.2009.24.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Offerhaus GJ, Giardiello FM, Tersmette KW, Mulder JW, Tersmette AC, Moore GW, Hamilton SR. Ethnic differences in the anatomical location of colorectal adenomatous polyps. Int J Cancer. 1991;49:641–644. doi: 10.1002/ijc.2910490502. [DOI] [PubMed] [Google Scholar]
- 4.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 5.Morita T, Tabata S, Mineshita M, Mizoue T, Moore MA, Kono S. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces health study. Asian Pac J Cancer Prev. 2005;6:485–489. [PubMed] [Google Scholar]
- 6.Kim JH, Lim YJ, Kim YH, Sung IK, Shim SG, Oh SO, Park SS, Yang S, Son HJ, Rhee PL, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. 2007;16:1543–1546. doi: 10.1158/1055-9965.EPI-07-0199. [DOI] [PubMed] [Google Scholar]
- 7.Wang YY, Lin SY, Lai WA, Liu PH, Sheu WH. Association between adenomas of rectosigmoid colon and metabolic syndrome features in a Chinese population. J Gastroenterol Hepatol. 2005;20:1410–1415. doi: 10.1111/j.1440-1746.2005.03971.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee GE, Park HS, Yun KE, Jun SH, Kim HK, Cho SI, Kim JH. Association between BMI and metabolic syndrome and adenomatous colonic polyps in Korean men. Obesity (Silver Spring) 2008;16:1434–1439. doi: 10.1038/oby.2008.216. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Kim Y, Lee S. An association between colonic adenoma and abdominal obesity: a cross-sectional study. BMC Gastroenterol. 2009;9:4. doi: 10.1186/1471-230X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marugame T, Lee K, Eguchi H, Oda T, Shinchi K, Kono S. Relation of impaired glucose tolerance and diabetes mellitus to colorectal adenomas in Japan. Cancer Causes Control. 2002;13:917–921. doi: 10.1023/a:1021967301138. [DOI] [PubMed] [Google Scholar]
- 11.Brauer PM, McKeown-Eyssen GE, Jazmaji V, Logan AG, Andrews DF, Jenkins D, Marcon N, Saibil F, Cohen L, Stern H, et al. Familial aggregation of diabetes and hypertension in a case-control study of colorectal neoplasia. Am J Epidemiol. 2002;156:702–713. doi: 10.1093/aje/kwf112. [DOI] [PubMed] [Google Scholar]
- 12.Bayerdörffer E, Mannes GA, Richter WO, Ochsenkühn T, Seeholzer G, Köpcke W, Wiebecke B, Paumgartner G. Decreased high-density lipoprotein cholesterol and increased low-density cholesterol levels in patients with colorectal adenomas. Ann Intern Med. 1993;118:481–487. doi: 10.7326/0003-4819-118-7-199304010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Tabuchi M, Kitayama J, Nagawa H. Hypertriglyceridemia is positively correlated with the development of colorectal tubular adenoma in Japanese men. World J Gastroenterol. 2006;12:1261–1264. doi: 10.3748/wjg.v12.i8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JC, Alpern Z, Sethi G, Messina CR, Martin C, Hubbard PM, Grimson R, Ells PF, Shaw RD. Prevalence and risk of colorectal neoplasia in consumers of alcohol in a screening population. Am J Gastroenterol. 2005;100:2049–2055. doi: 10.1111/j.1572-0241.2005.41832.x. [DOI] [PubMed] [Google Scholar]
- 15.Almendingen K, Hofstad B, Trygg K, Hoff G, Hussain A, Vatn MH. Smoking and colorectal adenomas: a case-control study. Eur J Cancer Prev. 2000;9:193–203. [PubMed] [Google Scholar]
- 16.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7:253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. In: Kleihues P, Sobin LH, editors. World Health Organization Classification of Tumours. Lyon: IARC Press; 2000. pp. 103–143. [Google Scholar]
- 20.Park HS, Shin ES, Lee JE. Genotypes and haplotypes of beta2-adrenergic receptor and parameters of the metabolic syndrome in Korean adolescents. Metabolism. 2008;57:1064–1070. doi: 10.1016/j.metabol.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Giovannucci E, Kelsey K, Rimm EB, Stampfer MJ, Colditz GA, Spiegelman D, Willett WC, Hunter DJ. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996;56:4862–4864. [PubMed] [Google Scholar]
- 22.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–177. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 24.Yamaji Y, Okamoto M, Yoshida H, Kawabe T, Wada R, Mitsushima T, Omata M. The effect of body weight reduction on the incidence of colorectal adenoma. Am J Gastroenterol. 2008;103:2061–2067. doi: 10.1111/j.1572-0241.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959–2967. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs ET, Ahnen DJ, Ashbeck EL, Baron JA, Greenberg ER, Lance P, Lieberman DA, McKeown-Eyssen G, Schatzkin A, Thompson PA, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. Am J Epidemiol. 2009;169:657–666. doi: 10.1093/aje/kwn401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato Y, Nozaki R, Yamada K, Takano M, Haruma K. Relation between obesity and adenomatous polyps of the large bowel. Dig Endosc. 2009;21:154–157. doi: 10.1111/j.1443-1661.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JC, Messina CR, Dakhllalah F, Abraham B, Alpern Z, Martin C, Hubbard PM, Grimson R, Shaw RD. Body mass index: a marker for significant colorectal neoplasia in a screening population. J Clin Gastroenterol. 2007;41:285–290. doi: 10.1097/01.mcg.0000247988.96838.60. [DOI] [PubMed] [Google Scholar]
- 29.Lee WC, Neugut AI, Garbowski GC, Forde KA, Treat MR, Waye JD, Fenoglio-Preiser C. Cigarettes, alcohol, coffee, and caffeine as risk factors for colorectal adenomatous polyps. Ann Epidemiol. 1993;3:239–244. doi: 10.1016/1047-2797(93)90025-y. [DOI] [PubMed] [Google Scholar]
- 30.Todoroki I, Kono S, Shinchi K, Honjo S, Sakurai Y, Wakabayashi K, Imanishi K, Nishikawa H, Ogawa S, Katsurada M. Relationship of cigarette smoking, alcohol use, and dietary habits with sigmoid colon adenomas. Ann Epidemiol. 1995;5:478–483. doi: 10.1016/1047-2797(95)00064-x. [DOI] [PubMed] [Google Scholar]
- 31.Cope GF, Wyatt JI, Pinder IF, Lee PN, Heatley RV, Kelleher J. Alcohol consumption in patients with colorectal adenomatous polyps. Gut. 1991;32:70–72. doi: 10.1136/gut.32.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez ME, McPherson RS, Annegers JF, Levin B. Cigarette smoking and alcohol consumption as risk factors for colorectal adenomatous polyps. J Natl Cancer Inst. 1995;87:274–279. doi: 10.1093/jnci/87.4.274. [DOI] [PubMed] [Google Scholar]


