Abstract
Mitochondria play an important role in cell death and cardioprotection. During ischemia, when ATP is progressively deleted, ion pumps cannot function resulting in a rise in calcium (Ca2+), which further accelerates ATP depletion. The rise in Ca2+ during ischemia and reperfusion leads to mitochondrial Ca2+ accumulation, particularly during reperfusion when oxygen is reintroduced. Reintroduction of oxygen allows generation of ATP; however damage to electron transport chain results in increased mitochondrial generation of reactive oxygen species (ROS). Mitochondrial Ca2+ overload, and increased ROS can result in opening of the mitochondrial permeability transition pore, which further compromises cellular energetics. The resultant low ATP and altered ion homeostasis result in rupture of the plasma membrane and cell death. Mitochondria have long been proposed as central players in cell death, since the mitochondria are central to synthesis of both ATP and ROS and since mitochondrial and cytosolic Ca2+ overload are key components of cell death. Many cardioprotective mechanisms converge on the mitochondria to reduce cell death. Reducing Ca2+ overload and reducing ROS have both been reported to reduce ischemic injury. Preconditioning activates a number of signaling pathways that reduce Ca2+ overload and reduce activation of the mitochondrial permeability transition pore. The mitochondrial targets of cardioprotective signals will be discussed in detail.
I. Introduction
Myocardial cell death due to ischemia-reperfusion is a major cause of morbidity and mortality in western nations. In the past few decades, it has become clear that the myocardial response to ischemia-reperfusion can be manipulated to delay injury, which has motivated intense study of the mechanisms of cardioprotection. The cardioprotective strategy of ischemic preconditioning (PC), first described in 1986, provided an indication of the magnitude of the possible cardioprotective effect and stimulated considerable research into the mechanisms involved (176). Over the past two decades we have learned a great detail about the signaling pathways involved in preconditioning. More recently, activation of signaling kinases at reperfusion, either by agonist addition (100) or by postconditoning (287), has been shown to be cardioprotective. In spite of the identification of these signaling pathways, the precise mechanism by which these pathways reduce cell death is only beginning to be understood. In parallel with the intense study of cardioprotective mechanisms, the past few decades have also seen intense research into mechanisms involved in regulating apoptosis and necrosis (18, 51). It has recently been appreciated that necrosis is also regulated and can be inhibited by many “anti-apoptotic” agents. In the past few years, studies of both cardioprotection and cell death have focused on the role of the mitochondria as regulators of energetics and cell viability. This review will examine what protects against myocardial ischemia-reperfusion injury and what promotes injury, and what we can learn from the comparison. Section II will discuss mechanisms involved in cell death in cardiac ischemia-reperfusion. The relative roles of ischemia and reperfusion in irreversible injury will be discussed. The involvement of apoptosis and necrosis will also be considered. Section III will discuss alterations in calcium and ROS and their involvement in cell death. Section IV will discuss cardioprotective mechanisms with emphasis on the signaling pathways involved in pre and post-conditioning. This review will focus on acute cardioprotection, which is primarily mediated by activation of signaling pathways and post-translational modification of proteins. We will not discuss the mechanisms involved in the second window or delayed preconditioning, which are primarily mediated by gene induction and protein synthesis; the reader is referred to other excellent reviews on this topic (237, 282) Section V will summarize how the acute cardioprotective signaling pathways activated by pre- and postconditioning might reduce cell death. Section V will also consider future directions in cardioprotection.
II. Why myocytes die following ischemia-reperfusion?
A. Relative roles of Ischemia versus Reperfusion in Irreversible Myocyte Injury
It has been debated whether cardiomyocytes suffer irreversible injury primarily during ischemia, which may be revealed at the start of reperfusion, or whether additional injury occurs during reperfusion (reperfusion injury). This point has important clinical implications, because if additional injury occurs on reperfusion this would allow an opportunity to intervene with cardioprotective drugs on reperfusion. There have been a large number of basic studies which suggest that introduction of cardioprotective drugs or strategies at the very start of reperfusion can significantly reduce infarct size. Postconditioning (287), Na-H exchange inhibitors (128), activation of kinases (99), perfusion with erythropoietin (95), inhibition of PKCδ (114), inhibitors of the mitochondrial permeability transition pore (MPT) (98), inhibition of GSK-3β (89) and other interventions have been reported to protect when added at the start of reperfusion. Taken together these data suggest that events occurring at the start of reperfusion can impact cell fate, and that interventions at this time can be cardioprotective. However, as is clear from a number of failed clinical trials (30, 69, 250), it is imperative to initiate the intervention during the first seconds of reperfusion. Such early intervention is only practical before cardiac surgery or with angioplasty. It appears that the window of opportunity during reperfusion is very limited. Interventions initiated later than 5 to 10 minutes after the start of reperfusion do not appear to provide significant protection (279). This narrow window for intervention is consistent with the release of intracellular enzymes such as creatine kinase during early reperfusion. It is also of note that studies showing protection with interventions at the start of reperfusion typically employ relatively short durations of ischemia (248) that may not mimic the clinical setting in which patients present after longer durations of ischemia.
Although protection can be initiated at reperfusion, injury also occurs during ischemia, and the relative proportion of injury occurring during ischemia versus reperfusion likely depends on the duration of ischemia. Casapases are activated during ischemia (238) and ion dysfunction occurs during ischemia (236). If cardioprotective strategies can be initiated before or during ischemia, it is likely that they will enhance protection, especially with longer durations of ischemia. In addition, events during ischemia can enhance the opening of the MPT and thus the initiation of death at reperfusion. Thus, it is important to administer cardioprotective agents as soon as possible.
B. Mechanisms of Death: Role of Necrosis, Apoptosis and Autophagy
Cell death following ischemia-reperfusion has been reported to have features of apoptosis, autophagy, and necrosis. The precise proportion of each form of death may depend on the model (adult versus neonatal, cultured cells versus in vivo). The mechanisms regulating apoptosis, autophagy, and necrosis have been recently reviewed (51, 78, 83, 94, 127, 247, 290) and will not be covered in detail here.
Apoptotic cell death results in apoptotic bodies that contain cellular components, and these apoptotic bodies are phagocytized by other cells; because there is no release of intracellular components, there is little or no inflammatory response. Apoptosis as originally described by Kerr et al is characterized by chromatin condensation and fragmentation, cell shrinkage and plasma membrane budding with release of apoptotic bodies that are phagocytized (129). Although these morphological descriptions do not fully apply to the majority of cell death in the setting of ischemia-reperfusion, apoptotic mechanisms have been implicated in ischemia-reperfusion injury (8, 9, 51, 141). Apoptotic cell death typically occurs via activation of caspases which cleave DNA and other cell components. Deletion of pro-apoptotic proteins or increased expression of anti-apoptotic proteins have been shown to reduce ischemia-reperfusion mediated cell death. Mice lacking the pro-apoptotic protein Bax have been reported to have reduced ischemia-reperfusion injury (106). Mice that lack Fas have also been reported to have smaller infarcts following ischemia and reperfusion (141). Overexpression of the anti-apoptotic protein Bcl-2 has been shown in a number of studies to reduce infarct size (42, 113). However it is likely that anti-apoptotic proteins can reduce necrotic and autophagic death as well as apoptotic cell death (18, 38, 106, 113, 229). Studies have shown in wild type hearts subjected to 20 minutes of global ischemia and 2 hours of reperfusion that 38% of the heart was dead as measured by triphenyl-tetrazolium chloride staining, but only 4% of the death was apoptotic as assessed by TUNEL. Cardiac specific Bcl-2 overexpression reduced total cell death (from 38% to 18%) and apoptotic cell death from 4.3% to 1.2% (113). Thus, the decrease in apoptotic cell death is not sufficient to account for the reduction in cell death, suggesting that Bcl-2 overexpression reduces necrotic as well as apoptotic cell death. Similar data were obtained by others (158). Interestingly, cardiac specific overexpression of Bcl-2 has been shown to reduce the rate of fall in ATP during ischemia and to reduce ischemic acidification (113). The mechanisms responsible for these metabolic effects of Bcl-2 are not clear, but appear to involve effects of Bcl-2 on either reducing ATP entry into the mitochondria or reducing breakdown of ATP by the F1F0ATPase (see (173)).
Activation of caspases is thought to be a major mechanism of apoptotic cell death. The role of caspases in ischemia-reperfusion injury has been debated (14, 38, 52, 84). Some have reported that inhibition of caspases results in only a modest reduction in infarct size, less than that observed with overexpression of antiapoptotic proteins (84). However, a large number of studies have reported that addition of caspase inhibitors reduces infarct size, suggesting an important role for caspase activation in ischemia-reperfusion injury (164, 238, 280). Caspase 9 has been reported to be activated during ischemia, whereas caspase 8 and 9 are activated during reperfusion(238). It has also been suggested that inhibition of caspases promotes necrotic cell death (264), and autophagy (284).
Caspases cleave a large number of targets that contribute to cell death. However, cleavage of what substrate(s) would quickly (within 1 to 2 hours, a time of significant cell death) result in the immediate loss of cell viability and release of intracellular components such as creatine kinase and troponin? There has been a lot of focus on caspase cleave of DNA as an important mediator of cell killing. Although loss of DNA will eventually lead to cardiac cell death, it is not clear that cleavage of DNA is the immediate cause of cell death in cardiomyocytes which is observed within 1 to 2 hours of reperfusion following ischemia. Caspases target a number of mitochondria proteins and these targets could promote rapid cell death. Caspase cleavage of the cytoskeleton or plasma membrane constituents are also possible targets that could lead to rupture of the plasma membrane. Although as originally defined apoptosis does not result in rupture of the plasma membrane. However, in the setting of ischemia-reperfusion, where multiple forms of death occur, caspase cleavage of structural proteins could contribute to plasma membrane rupture.
Autophagy is another form of death which has been suggested to play a role in ischemia-reperfusion injury. Autophagy is a physiological mechanism that is used to remove damaged organs, such as mitochondria or endoplasmic reticulum. Autophagy is also initiated by starvation to provide nutrients. However, extensive autophagy can cause cell death. There are conflicting data as to whether the increased autophagy that occurs during ischemia and reperfusion is beneficial or detrimental. There are studies showing that inhibition of autophagy during ischemia or anoxia is detrimental, suggesting a beneficial role for stimulation of autophagy during ischemia (60, 277). However, in contrast to these studies a decrease in beclin1 (a protein that stimulates autophagy) reduces ischemia-reperfusion mediated autophagy and myocyte death (247). Also consistent with a detrimental role for autophagy in ischemia-reperfusion, in HL-1 cells an increase in beclin1, which increased autophagy, was also shown to decrease activation of BAX, and knockdown of beclin1 decreased activation of BAX (93). Thus the role of autophagy in cell death during ischemia-reperfusion is still somewhat unclear, although it seems that inhibition of autophagy during ischemia and reperfusion (as opposed to ischemia alone) is beneficial.
There are a number of connections between autophagy and other forms of cell death. An increase in calcium, as occurs during ischemia, has been shown to increase autophagy (107). Activation of calpain, a calcium activated protease has been reported to cleave Atg5, a protein involved in autophagy; cleaved Atg5 translocates to the mitochondria where it is reported to bind Bcl-2 and thereby stimulate apoptosis (94, 247). Taken together these data suggest that an increase in calcium will activate autophagy and at the same time convert some of the autophagy machinery to apoptosis. Activation of phosphoinositide-3-kinase (PI3K) class I, which is cardioprotective, decreases beclin1 leading to a decrease in autophagy. PI3K activation of the mammalian target of rapamycin (mTOR) results in inhibition of autophagy. Activation of class III PI3K, which is involved in regulating vesicular trafficking, stimulates autophagy. A decrease in Bcl-2 has been shown to result in an increase in beclin1. Also activation of JNK has been reported to increase beclin1. It is also clear that there is considerable cross-talk through calcium, calpain, caspases, Bcl-2 family members, and signaling kinases, between autophagy and the other death pathways active in ischemia-reperfusion.
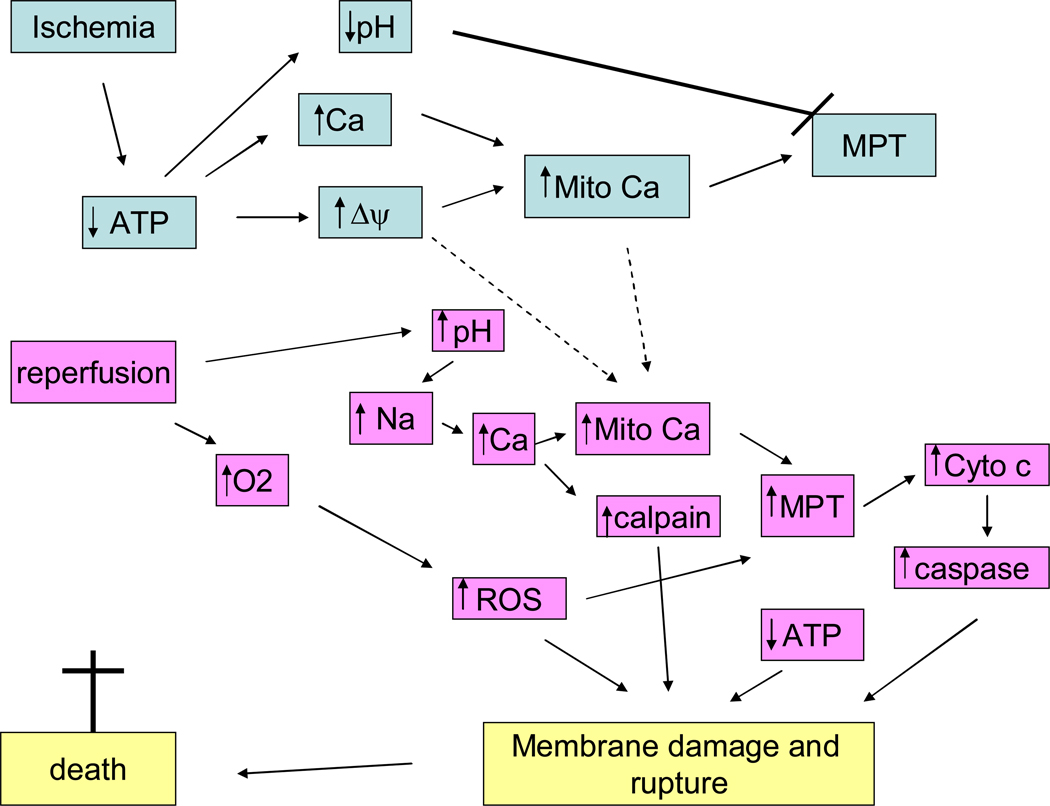
Necrotic cell death is characterized by cell swelling leading to irreversible rupture of the plasma membrane with release of cytosolic components, which result in an inflammatory response. An inflammatory response is an important component of ischemia-reperfusion injury. The release of troponin and creatine kinase that occur during ischemia-reperfusion are likely due to necrotic release of these intracellular components. Until recently, necrosis was thought to be an unregulated process, but recent studies suggest that necrosis can be regulated and that interventions can reduce necrotic cell death (83, 290). Since rupture of the plasma membrane is a prominent feature of necrosis and ischemia-reperfusion injury and is a lethal event, it is worth considering what might lead to rupture of the plasma membrane. Rupture of the plasma membrane could be facilitated by calpain or some other protease cleavage of the cytoskeleton. Complete loss of ATP would also inhibit ion pumps, which would result in swelling perhaps rupturing the plasma membrane, particularly if the cytoskeleton has been weakened. ATP falls to very low levels within about 15 to 20 minutes of global ischemia in a rat heart, a time when there is very little irreversible injury. Thus there is usually a poor correlation between ATP levels and irreversible injury. For example, Neely and Grotyohann reported that the ability of the heart to recover was inversely related to the build-up of metabolic endproducts (lactate, NADH etc) and not the levels of ATP (186). The lag between loss of ATP and irreversible injury is consistent with the loss of ATP initiating some process (or inhibiting some process) that ultimately results in cell death.
It is possible that a combination of protease activation with loss of ATP and ion dysregulation and cell swelling all conspire to rupture the plasma membrane. The question then arises as to what causes activation of proteases and the total loss of ATP? A rise in cytosolic free Ca2+ concentration ([Ca2+]i) has been consistently observed in ischemia and early reperfusion and most cardioprotective protocols reduce the rise in [Ca2+]i during ischemia. A rise in [Ca2+]i will lead to activation of calpains which could be involved in cleaving proteins leading to plasma membrane rupture. Calpain also activates the pro-apoptotic BID and calpain cleaves Atg5 shifting the balance from autophagy to apoptosis. An increase in [Ca2+]i and ROS can lead to activation of an inner mitochondrial large conductance channel known as the mitochondrial permeability transition (MPT). Opening of this channel would lead to loss of ATP and mitochondrial function, which would quickly lead to mitochondrial swelling and release of cytochrome c, which could activate apoptosis. If a large number of mitochondria in a cell undergo MPT the cell will lose the capacity to make ATP, the cell will lose ion homeostasis resulting in cell swelling, membrane rupture and cell death.
Death following ischemia-reperfusion injury appears to be a mixture of apoptotic, autophagy, and necrotic cell death and it can have features of all three. The distinction between the modes of death may not be important since recent data suggest that all three forms of cell death can be regulated and are inter-related (83, 290). With ischemia and reperfusion, it may therefore be more useful to discuss mechanisms of death without attempting to precisely define the mode of death. The important issue is that cell death during ischemia-reperfusion appears to be an active process, which can be inhibited with appropriate interventions. Taken together the mitochondria are emerging as an important mediator and regulator of all forms of cell death in ischemia-reperfusion. In particular, the MPT appears to be a major regulator of both apoptotic and necrotic cell death. Recent studies have shown that inhibition of MPT by knockdown of cyclophilin D (a regulator of the MPT) results in a significant reduction in infarct size in ischemia reperfusion, but loss of cyclophilin D did not block BAX mediated apoptosis. These and other data suggest a major role for the MPT in ischemia-reperfusion injury. The MPT is activated by elevated calcium and reactive oxygen species (ROS), and both of these are elevated during ischemia and reperfusion. In the next section we will focus on mechanisms that regulate Ca2+ and ROS during ischemia since Ca2+ and ROS appear to be the primary activators of MPT during ischemia-reperfusion.
III What Activates the Mitochondrial Permeability Transition?
A. Role of Calcium
1) Ca2+ rises during ischemia?
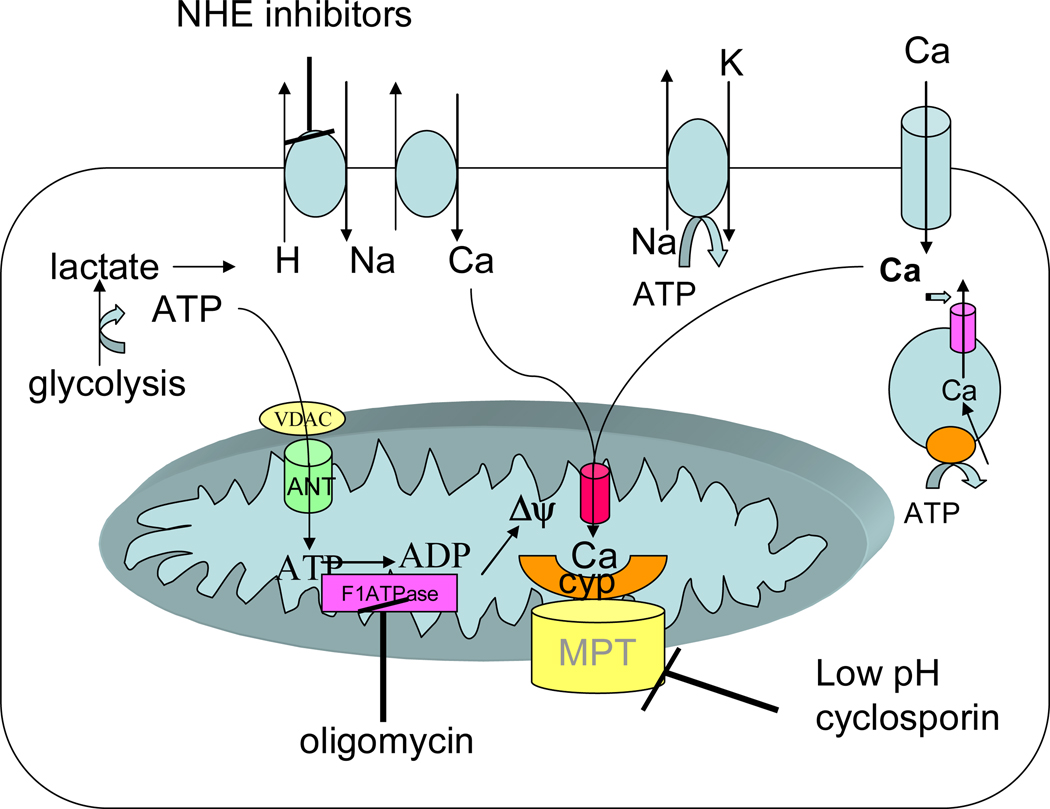
Studies from over 2 decades ago showed that a rise in cytosolic [Ca2+]i preceded irreversible myocardial injury and that drugs or protocols that reduced or delayed the rise in cytosolic [Ca2+]i also reduced or delayed myocardial death (112, 138, 174, 196, 234–236). Although some early studies reported that a rise in [Ca2+]i was not important in the genesis of injury (44, 133), the majority of recent studies have shown that a rise in [Ca2+]i precedes irreversible injury and that mechanisms that block the rise in [Ca2+]i attenuate or delay the onset of irreversible injury (155, 162, 183, 196, 234). Each systole is initiated by Ca2+ entry via the L-type Ca2+ channel resulting in Ca2+ induced Ca2+ release from the sarcoplasmic reticulum, and the combined increase in [Ca2+]i leads to contraction. The Ca2+ that enters via L-type Ca channel is removed from the cell primarily by Na-Ca exchange (NCX) with a very small contribution from the sarcolemmal Ca2+ ATPase (25, 67). The Ca2+ released from the sarcoplasmic reticulum is reaccumulated into the sarcoplasmic reticulum (SR) via SR/ER Ca2+ ATPase (SERCA). Studies show that much of the rise in cytosolic Ca2+ during ischemia is due to Ca2+ entry by reverse mode NCX secondary to the rise in [Na+]i during ischemia (see figure 1) (112). This occurs because of increased generation of protons during ischemia, which are extruded from the cell via Na-H exchange (NHE), resulting in an increase in intracellular Na+ (174). Na has also been shown to enter the cell during ischemia on non-inactivating Na+ channels (172). This rise in intracellular Na+, coupled with the depolarized plasma membrane results in a reversal of NCX to bring Ca2+ into the myocyte. NHE inhibitors, such as amiloride derivatives and the more specific NHE inhibitor, cariporide, have been shown in animal studies to attenuate the rise in [Na+]i during ischemia and the subsequent rise in [Ca2+]i, and to reduce ischemia-reperfusion injury. The animal data clearly showed that these inhibitors were beneficial when administered prior to ischemia (128, 174). Inhibition of NCX has also been shown to be protective (174, 183), and hearts from mice lacking the NNH or mice lacking the NCX have been shown to have reduced ischemia-reperfusion injury (112, 269). In addition to NCX, Ca2+ entry via the L-type Ca2+ channel can contribute to the rise in Ca2+ during ischemia (41).
Figure 1.
Changes in ions and metabolites during ischemia. During ischemia ATP declines resulting in a decrease in pH due to anaerobic glycolysis. The increase in proton stimulates Na-H exchange and Na-Ca exchange resulting in an increase in cytosolic Ca. Much of the ATP generated by glycolysis is consumed by the reverse mode of the mitochondrial F1F0ATPase which uses the energy to generate Δψ. The Δψ can then be used to take up Ca into the mitochondria. This increase in mitochondrial Ca can activate the MPT, but the low pH due ischemia inhibits MPT so that it is not activated until reperfusion when the pH is restored to normal.
2) Ca2+ during reperfusion
During ischemia intra- and extracellular pH are acidic; however during reperfusion extracellular pH rapidly returns to normal. However, initially the intracellular pH is still acidic and this pH gradient facilitates extrusion of H+ from the cell in exchange for Na+ on NHE. The increased cytosolic Na+ can be extruded by Na-K-ATPase or NCX in exchange for Ca2+, thereby raising (at least transiently) [Ca2+]i. Arrhythmias can be triggered by altered Ca2+ homeostasis and are a major cause of death in ischemia-reperfusion. In the absence of arrhythmias, [Ca2+]i, returns quickly to near normal levels in myocytes that survive. However, there can be [Ca2+]i, oscillation (64, 198), which can lead to hypercontracture which will contribute to further decline in ATP and deterioration of the myocyte. In some myocytes, [Ca2+]i, remains high during reperfusion; this condition typically results in irreversible injury to the myocyte. What determines whether [Ca2+]i, returns to normal, oscillates or stays elevated? This depends on myocyte ATP levels (which may in turn depend on activation of MPT), intracellular Na+ levels, and damage to Ca2+ handling proteins such as the ryanodine release channel in the SR. Thus if there is SR dysfunction, [Ca2+]i, oscillations can occur, which further deplete ATP and can contribute to arrhythmias. The Ca2+ released from the SR can also be taken up by the mitochondria leading to activation of the MPT. Improving SR Ca2+ handling has been shown to be an important target for reducing ischemic injury (55, 197, 291). Interestingly, adenoviral mediated overexpression of SERCA was shown to reduce infarct size and improve function following ischemia (55).
Treatment with NHE inhibitors given immediately at the start of reperfusion provides some protection, although usually somewhat less than that observed when NHE inhibitors are given during ischemia as well as reperfusion (172). A large clinical trial was performed to test the beneficial effects of cariporide (an NHE inhibitor). Consistent with the animal studies, NHE inhibitors were protective only in the setting of CABG surgery (30, 250). A follow up study to specifically test the effects of NHE inhibitors was undertaken using a high dose of the drug and was discontinued due to toxicity (30).
3) Role of mitochondrial calcium
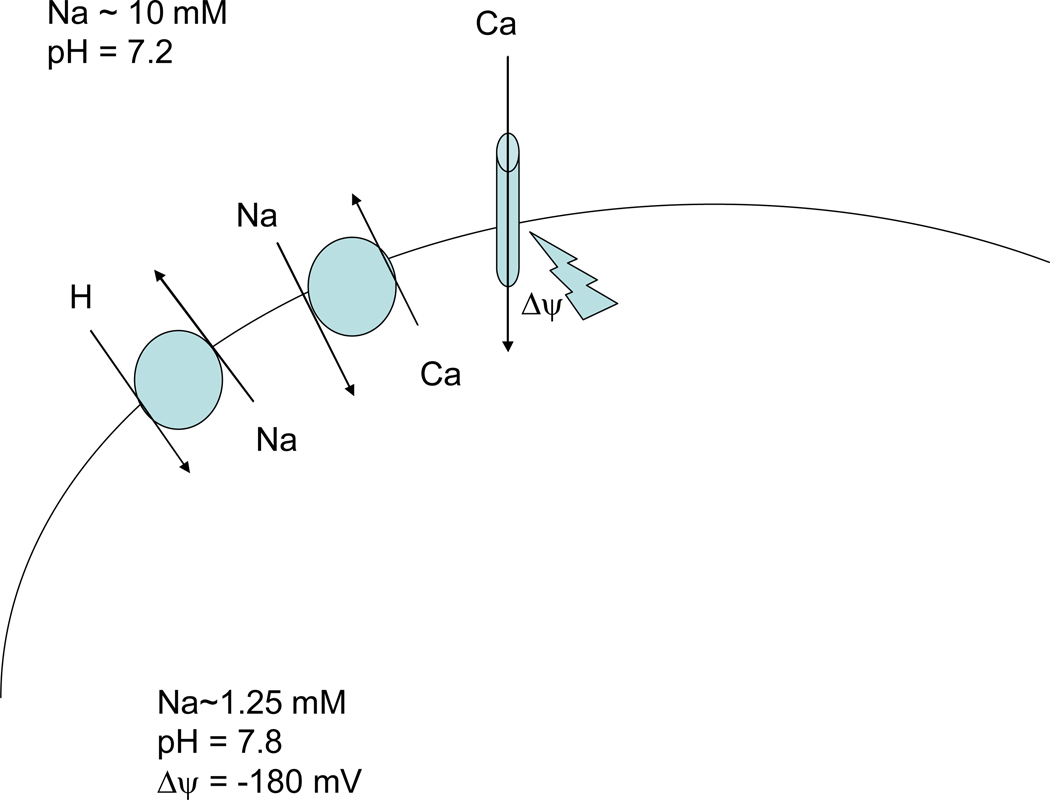
Ca2+ uptake into mitochondria occurs via the mitochondrial calcium uniporter, which uses the energy of mitochondrial membrane potential (Δψ) (see figure 2); thus Δψ used to transport Ca2+ is not available to synthesize ATP from ADP. The mitochondrial uniporter is inhibited by ruthenium red and related compounds. Surprisingly, the Ca2+ uniporter has not been cloned. Ca2+ uptake into the mitochondria will act to dissipate Δψ, unless electron transport is activated to re-synthesize the gradient. Thus during ischemia, with inhibited electron transport (due to lack of oxygen), one would expect Ca2+ uptake into the mitochondria to dissipate Δψ, ultimately limiting Ca2+ uptake into the mitochondria. Ca2+ is released from the mitochondria in exchange for Na+ (on the mitochondrial NCX; see figure 2). The matrix Na+ level has been reported to be regulated by mitochondrial NHE and thus the pH gradient across the inner mitochondrial membrane. In energized mitochondria, the Na+ gradient is the inwardly directed and [Na+]i is reported to be lower in the matrix (58, 124).
Figure 2.
Regulation of mitochondrial Ca2+. Ca2+ enters mitochondria on the uniporter, driven by the negative mitochondrial membrane potential. Ca2+ efflux from the mitochondrial matrix is via the mitochondrial Na-Ca exchanger.
During simulated ischemia, most studies suggest that there is a small rise in mitochondrial Ca2+ (87, 163, 170, 183, 208). Interestingly, Griffiths et al observed that the rise in mitochondrial Ca2+ during ischemia was inhibited by clonazepam (an inhibitor of mitochondrial NCX), thus suggesting a role for mitochondrial NCX operating in the reverse mode to increase mitochondrial matrix Ca2+ (87). It is suggested that the Na+ gradient decreases as a result of the decrease in pH gradient and this reduced inwardly directed Na+ gradient, along with the rise in [Ca2+]i, may contribute to Ca2+ entry into the mitochondria on Na-Ca2+ exchange. However clonazepam increased the rise in mitochondrial [Ca2+] during reperfusion, suggesting that during reperfusion when the mitochondrial pH gradient and the Na+ gradient is restored, the mitochondrial NCX extrudes Ca2+ from the mitochondria (87). Interestingly Bcl-2 has been reported to modulate mitochondrial NCX (288). Cardioprotective agents such as diazoxide and sarcolemmal NCX inhibitors are reported to reduce mitochondrial Ca2+ overload (170, 183).
Griffiths et al (88) reported that 0.1 µM cyclosporin A (CsA), an MPT inhibitor, protected cells subjected to simulated ischemia and reperfusion; protection was defined as ability to recover rod shape and respond to electrical stimulation. Interestingly, the mitochondrial Ca2+ concentration in the surviving myocytes was higher in the CsA treated myocytes than in untreated; these data are consistent with the hypothesis that CsA mediated protection is by reducing the Ca2+ activation of the MPT. Under these conditions, CsA does not reduce the Ca2+ loading of mitochondria, it reduces the Ca2+ sensitivity of the MPT. The myocytes were able to survive with higher levels of mitochondrial Ca2+. Higher concentrations of CsA were found to reduce mitochondrial Ca2+ during simulated ischemia, an effect that was attributed to CsA inhibition of calcineurin.
Miyamae et al (162) measured mitochondrial [Ca2+] in perfused hearts with indo-1 and surface fluorescence, using Mn2+ to quench cytosolic Ca2+. They found an inverse correlation between mitochondrial [Ca2+] during ischemia and recovery of LVDP on reperfusion. Miyamae et al reported that ruthenium red attenuated the increase in mitochondrial [Ca2+] and improved recovery of LVDP. However the effects of ruthenium red on mitochondrial [Ca2+] have been very diverse, perhaps reflecting the fact that ruthenium red inhibits many Ca2+ channels in the cell. Taken together, reducing the rise in cytosolic and mitochondrial [Ca2+] during ischemia has been shown to be cardioprotective. However, cardioprotection can also be achieved, as in the case of CsA, not by lowering mitochondrial [Ca2+] but by inhibition of Ca2+ activation of the MPT. Thus there are multiple pathways to cardioprotection.
On reperfusion, when oxygen is re-introduced and Δψ is regenerated, if [Ca2+]i is elevated, one would expect significant Ca2+ uptake into the mitochondria via the Ca2+ uniporter. Consistent with this, on re-oxygenation (removal of metabolic inhibition), there appears to be an initial additional rise in mitochondrial [Ca2+], which then falls toward baseline (87). As discussed, this rise in mitochondrial [Ca2+ during reperfusion has been suggested to be a trigger for activation of the MPT.
B. Role of reactive oxygen species (ROS)
1) Does ROS rise during ischemia?
The role of reactive oxygen species (ROS) in ischemia-reperfusion injury has been extensively studied. ROS has been measured by electron spin resonance using spin traps added to the perfusate to trap the short lived radicals that are released into the perfusate (13, 31, 79, 293). Electron spin resonance has also been used to measure signals from the spin traps in the perfused heart (293). Free radicals have also been measured directly by electron spin resonance by grinding frozen samples (294). However caution must be used since radicals can be generated artifactually by the grinding (231). Other studies have used fluorescent indicators to measure ROS (23, 130, 263); these studies have typically been performed on isolated myocytes using simulated ischemia (23, 263) although some studies have used surface fluorescence of perfused hearts (130). These studies have consistently demonstrated a burst of ROS generation on the start of reperfusion (13, 31, 79, 130, 263, 293, 294). Whether there is an increase in ROS during ischemia in an intact heart, however, is less clear. Studies in isolated myocytes have shown an increase in ROS generation during anoxia or simulated ischemia (23); however it is difficult to totally remove oxygen from solutions. Oxygen levels available are a balance between supply and demand. There is usually some oxygen in the buffer, but in a perfused heart with global ischemia the high density of cells rapidly consumes the small amount of oxygen available. In isolated myocytes the oxygen demand is low (due to low density of cells) and can be met in part by the low contaminant oxygen in the surrounding buffer (which in many cases is quite large). Thus the generation of ROS in hypoxic isolated myocytes could be due to small amounts of oxygen not present in intact ischemic myocardium. ROS has also been measured during global ischemia using surface fluorescent measurement of dihydroethidium (130). The surface of the heart would be exposed to ambient oxygen and the surface has been shown to behave differently than the interior core of the heart during ischemia (37). ROS has also been measured during low flow ischemia (13), but again oxygen is present during low flow ischemia. An early study using ESR measured ROS during ischemia in frozen ground heart and observed an increase in ROS during ischemia (294). Taken together these data suggest that generation of ROS in heart during ischemia is likely to be heterogeneous depending on the level of tissue oxygenation, which will depend on factors such as collateral flow. Even in a global model of ischemia oxygen does not immediately fall to zero so there is initially some oxygen to generate ROS. However the levels of ROS generated during ischemia are low and the pathological significance of them is uncertain.
2) ROS during Reperfusion
During reperfusion, with the return of oxygen, a large burst of ROS has been consistently shown to occur (13, 31, 79, 130, 263, 293, 294). The increase in ROS during ischemia and reoxygenation is thought to be due to damage to electron transport chain components resulting in inefficient transfer of electrons, generating superoxide. It was proposed that ROS, particularly ROS generated during early reperfusion, would lead to extensive oxidative damage to the cell resulting in loss of cell viability. This hypothesis was extensively tested in the 1980s. Studies consistently demonstrated a role for ROS, particularly ROS generated during reperfusion, in myocardial stunning. Treatment of in vivo and in vitro hearts with antioxidants reduced ROS and stunning (29). In contrast to the general agreement regarding ROS and stunning, there is considerable controversy and disagreement regarding the role of ROS in reducing infarct size. Although a number of studies find that addition of antioxidants or scavengers such as superoxide dismutase and catalase reduce infarct size (6, 43, 132, 184), there are a similar number of studies that find no reduction in infarct size (62, 77, 187, 190, 261, 267). Others have suggested that antioxidants can delay but not prevent manifestations of infarction (159). Based on the beneficial effects reported in some of these studies, clinical trials were initiated to test whether superoxide dismutase would be beneficial in patients undergoing angioplasty following acute myocardial ischemia. These clinical trials failed to show a beneficial effect of superoxide dismutase on ventricular function (69, 171). The negative studies do not necessary mean that ROS is not an important determinant of infarct size; only that agents such as superoxide dismutase and catalase administered intravascularly are not protective. It is likely that ROS generated during reperfusion initiates injury before it can be scavenged by exogenous superoxide dismutase and catalase. Also consistent with the hypothesis, overexpression of manganese superoxide dismutase reduced infarct size in mice (122). Kim et al have recently reported that ROS generated during early reperfusion is the primary activator of the MPT and cardiomyocyte death (133). It has been reported that some recently developed, intracellularly targeted scavengers provide some reduction in infarct size (2, 27, 228). Antioxidants such as vitamins C and E have also been suggested to scavenge ROS and reduce ischemic injury (199), although the result of clinical trials do not suggest reduced overall mortality with vitamin E supplementation (1, 151, 285).
These studies reinforce the importance of localization and timing in cardioprotection. The concept that ROS is an important contributor to ischemia-reperfusion injury is likely to be correct; however the ability to translate this knowledge into a clinical therapy is very complex. Delivery of the antioxidants (superoxide dismutase) or NHE inhibitors to the right compartment in the right time period is very difficult to achieve in controlled animal studies and even more difficult in patients. As a result of the controversy in the animal studies and the failed clinical trials it is often concluded that inhibition of ROS will not influence infarct size. A more realistic assessment is that to have a significant benefit in reducing infarct size requires the correct delivery (at the start of reperfusion) of mitochondrial targeted antioxidants perhaps in conjunction with other therapies (reduction in Ca, inhibition of MPT etc). However, oxidants can also have a cardioprotective signaling function, and antioxidants can inhibit cardioprotection (40, 71, 191).
3) Mitochondrial ROS
Mitochondria are thought to be both a major source of ROS as well as a major target for ROS damage. Mitochondrial electron transport is one of the primary sources of reactive oxygen species (ROS) in the cell. ROS is generated during electron transport at complex I and III. There is a positive correlation between mitochondrial membrane potential (Δψ) and production of ROS by electron transport. It has been suggested that even though only low levels of ROS are generated during ischemia, this ROS can lead to damage to the electron transport chain (143, 209); this damage is then thought to lead to increased ROS production because of inefficient transfer of electrons. Inhibition of electron transport at complex I during ischemia reduces ROS generation during ischemia. Inhibition of electron transport (particularly complex I) and mitochondrial uncouplers have been shown to reduces ischemia-reperfusion injury (143, 209); it is proposed that this protection is mediated by reduction in ROS during ischemia.
However, not all ROS is detrimental and ROS generation has been shown to be part of the protective signaling pathway of preconditioning (40). Diazoxide, an activator of the mitoKATP channel has been shown to increase ROS (71, 191), and addition of antioxidants has been shown to block the protection afforded by diazoxide as well as PC.
The burst of ROS at the start of reflow will also lead to damage to the electron transport chain. ROS generation on reflow has also been shown to be a major activator of MPT. Taken together it appears that low levels of ROS generation are important in signaling cardioprotection. However high levels of ROS, as occur at the start of reperfusion, can lead to damage to the electron transport chain and activation of the MPT. As discussed later, S-nitrosation of proteins, as can occur with PC, can protect proteins from further oxidative damage (244).
IV. What protects and how does it protects?
A. Preconditioning
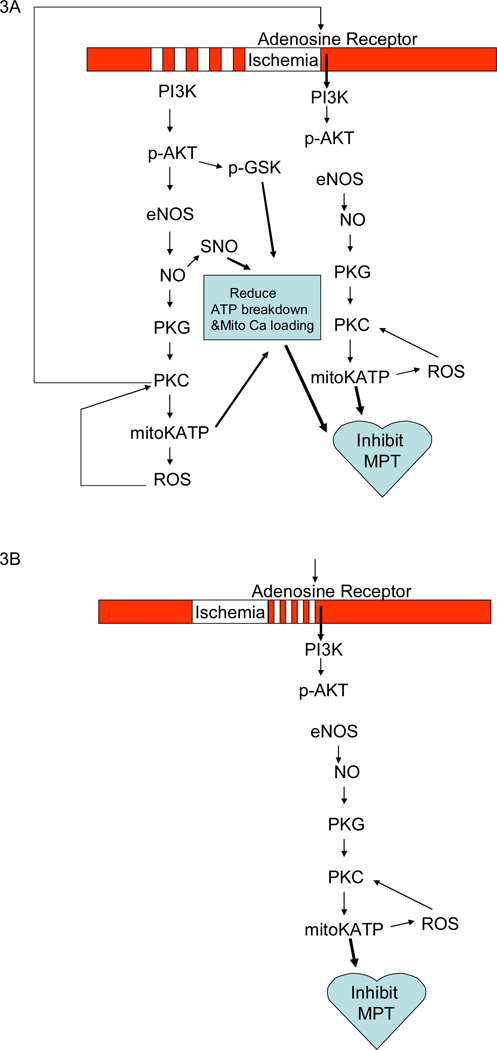
PC, originally described by Murry et al, consists of 4 cycles of 5 minutes of ischemia and 5 minutes of reperfusion just prior to a sustained period of ischemia (see figure 3) (176). Others have used different protocols, all of which have in common brief periods of ischemia separated by brief periods of reperfusion. PC has been shown to reduce infarct size, reduce the generation of lactate and to reduce the rate of fall in ATP. Later studies, by other groups, showed that PC also reduced arrhythmias and reduced contractile dysfunction. As discussed below, over the subsequent 20 years, we have learned a lot about the signaling pathways activated by PC. However we are just beginning to understand how activation of these signaling pathways leads to protection.
Figure 3.
Panel 3A illustrates the signaling pathways activated by PC during ischemia and at reperfusion. Panel 3B show the signaling pathways activated by postconditioning.
There are several interesting aspects of PC that provide potential insight into the mechanisms. The protection afforded by PC is lost if the time between the initial PC protocol and the sustained period of ischemia is extended beyond ~1 hour. A “second window” of preconditioning, which involves upregulation of genes, occurs ~24 hours after preconditioning (203, 281). The initial “early” preconditioning does not appear to depend on new protein synthesis because of the rapid onset and because since inhibition of protein synthesis does not block early PC (251); although this may need to be re-examined (239). Because the second window is mediated by new gene expression rather than post-translational modification of proteins, the mechanisms of the second window are different and will not be covered in this review. The reader is referred to other excellent reviews on this area (237, 281).
In the original characterization of PC it was noted that PC hearts have less anaerobic glycolysis during the sustained period of ischemia; that is, they make less lactate and have less ischemic acidosis (i.e. a higher intracellular pH during ischemia) (178, 236). Interestingly, cardiac specific overexpression of the antiapoptotic protein Bcl-2, addition of diazoxide, which mimics PC reportedly by opening a mitochondrial KATP channel, and adenosine pretreatment have also been shown to reduce acidosis during ischemia (71, 73, 113). The mechanism responsible for reduced ischemic acidosis by these cardioprotective agents is unknown. In addition to reduced anaerobic glycolysis, the rate of ATP consumption during ischemia is slower in PC hearts. There is an initial decrease in ATP during the PC protocol, such that PC hearts start with a lower ATP than non PC hearts. However, ATP falls more slowly in PC hearts (178, 236). Taken together these data suggest that PC reduces the rate of ATP hydrolysis during ischemia, since they have less anaerobic glycolysis to generate ATP (which is the primary source of ATP during global ischemia) and ATP levels fall more slowly. The mechanism by which PC reduces ATP breakdown during ischemia is still unknown. Interestingly, cardiac specific overexpression of the antiapoptotic protein Bcl-2, overexpression of the cardioprotective PKC-ε, and adenosine pretreatment have all been shown to slow the rate of ATP breakdown during ischemia (50, 73, 113).
An early hypothesis to account for the reduced ATP hydrolysis was that PC might inhibit ATP breakdown by reverse mode of the F1F0ATPase. Under normoxic conditions, the protons extruded from the mitochondrial matrix by electron transport re-enter the matrix via the F1F0ATPase which couples the proton gradient to synthesis of ATP. When the mitochondrial membrane potential (Δψ) falls, the F1F0ATPase can reverse and consume ATP to generate Δψ. It has been reported that as much as 35–50% of the ATP generated by glycolysis during ischemia is consumed by the reverse mode F1F0ATPase (see figure 1) (90, 118). Di Lisa et al used the fluorescent potential sensitive dye, JC1 to measure mitochondrial Δψ in anoxic rat cardiomyocytes and showed a biphasic decline in Δψ (56). Di Lisa et al showed that glycolytically generated ATP was used to maintain Δψ, since Δψ was shown to decline more rapidly during ischemia in the presence of oligomycin, an inhibitor of the F1F0ATPase (see Figure 1). Leyssens et al obtained similar results using JC1 to measure Δψ in rat cardiomyocytes metabolically inhibited with cyanide and 2-deoxyglucose (144). They found Δψ declined in metabolically inhibited myocytes, but the decline in Δψ was enhanced when the F1F0ATPase was inhibited with oligomycin. These data support the conclusion that the F1F0ATPase is a major consumer of ATP during ischemia and/or metabolic inhibition and they further demonstrate that this consumption of glycolytic ATP is used to maintain Δψ. Consistent with the hypothesis that PC slows the rate of ATP breakdown by reverse mode of the F1F0ATPase, Ylitalo et al have shown that during ischemia Δψ depolarized to a greater extent in PC hearts than in non-PC hearts (283).
Measurements of mitochondrial membrane potential in perfused rat hearts during ischemia and reperfusion have recently been reported (157). These studies show a decrease in Δψ during ischemia confirming studies of simulated ischemia in isolated myocytes. They further reported that on reperfusion a larger number of cells undergo loss of Δψ, consistent with activation of MPT on reperfusion.
There are data suggesting that during ischemia a mitochondrial ATPase inhibitor subunit (IF1) binds to and inhibits the mitochondrial ATPase thus conserving ATP (205–207). It was proposed that perhaps PC promoted earlier binding of the IF1 to the F1F0ATPase. However, studies by two different groups, using submitochondrial particles, found no evidence supporting inhibition of the ATPase in PC hearts (85, 266). However, other groups have reported that PC and diazoxide enhance IF1 binding to the F1F0ATPase (4, 46, 47). A recent study has also reported that pharmacological PC with adenosine results in increased phosphorylation of the beta subunit of the ATP synthase; however the functional effects of phosphorylation of the ATPase were not addressed (12).
Because the protection afforded by PC is lost within 1–2 hours (149, 161, 177) it was suggested that PC was mediated by some time dependent intermediate, which is either lost or regenerated with time. It was proposed that perhaps glycogen was the memory in PC (273). Glycogen is significantly reduced during the PC protocol, so anaerobic glycolysis is limited and less lactate can be generated during the sustained period of ischemia in PC hearts (236). Less anaerobic glycolysis results in less acid generation during ischemia, which would reduce the rise in Na (via Na-H exchange) and reduce the rise in Ca (via Na-Ca exchange) (174). If the time between PC and the sustained ischemia was extended, glycogen would be resysnthesized and the protection lost. However, a number of studies showed that depletion of glycogen per se does not result in cardioprotection (135, 232, 265).
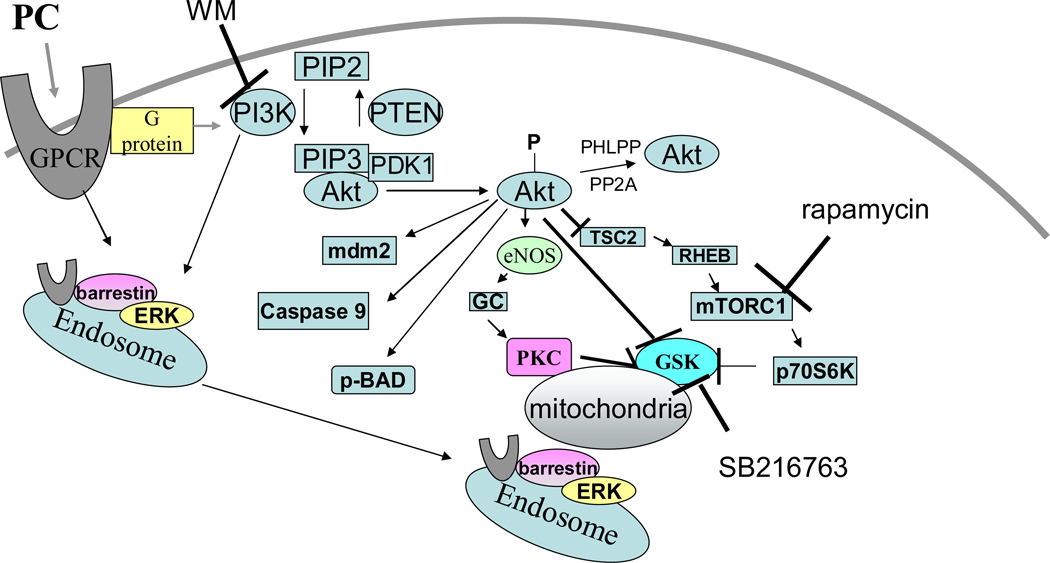
Recent studies suggest that PC activates a number of signaling pathways and that the activation of these pathways likely decays with time, leading to loss of protection. We will therefore consider the signaling cascades in detail with attention to how each component might lead to protection. PC causes release of agonists such as adenosine, bradykinin, and opioids which bind to G-protein coupled receptors (GPCR) and activate a cardioprotective signaling cascade. As shown in figure 4, activation of GPCRs leads to activation of the phosphoinositide-3-kinase (PI3K) pathway, which via phosphoinositide-dependent protein kinase 1 (PDK1) results in activation of downstream targets such as Akt, p70S6K, and signaling through βarrestin and endosomal signaling which results in activation of extracellular regulated kinase (ERK) and other kinases and phosphatases. In addition Akt, p70S6K, protein kinase C (PKC) and ERK all modulate a number of downstream signaling pathways, which are discussed in detail.
Figure 4.
Details of the signaling pathways activated by PC. PC leads to release of agonist that activate GPCR, leading to activation of PI3K, which in turn activates AKT and P70S6K. Internalization of GPCRs involves sequential binding of β-arrestin, the clathrin adaptor (adaptor protein 2; AP-2), and clathrin. PI3K and its phosphoinositide products play a critical role in recruitment of β-arrestin GPCR complexes to endosomes. Many of these pathways converge on the mitochondria where the result in activation of the mitoKATP channel and inhibition of the MPT.
1) Phosphoinositide-3-kinase (PI3K)
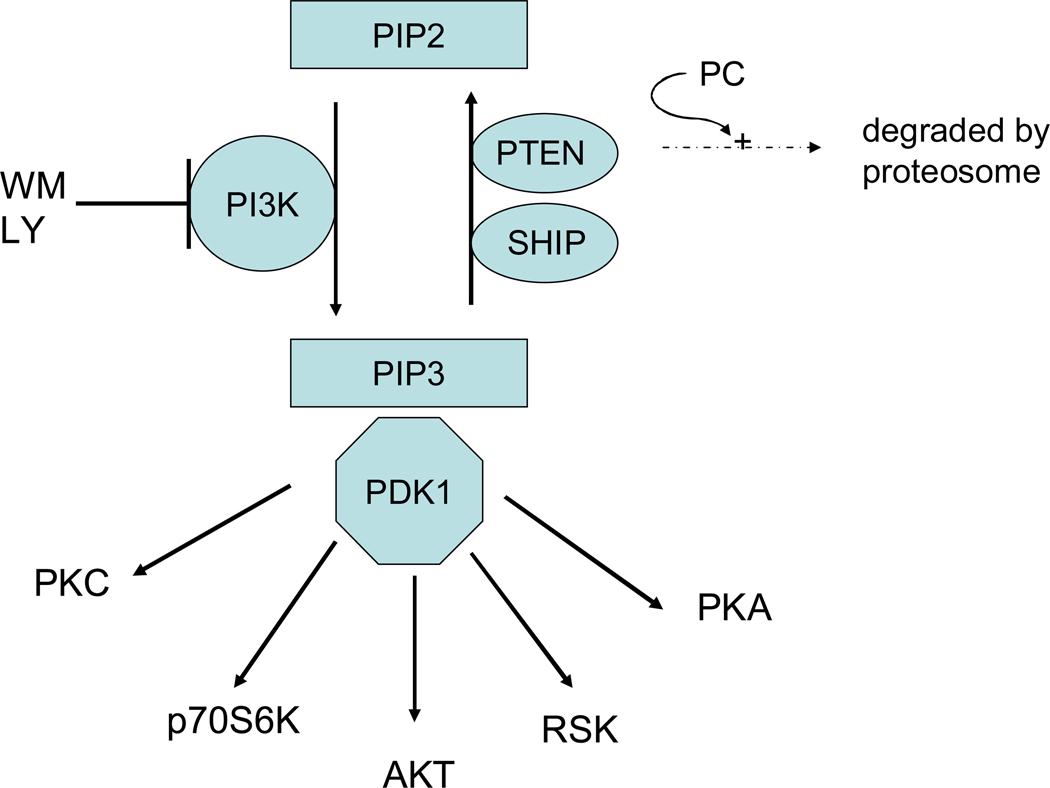
PI3K is a lipid and protein kinase. As a lipid kinase, PI3K catalyzes the phosphorylation of the inositol ring of phosphoinositides at the D3 position. As a protein kinase, PI3K has been shown to phosphorylate non-muscle tropomyosin which is involved in β-adrenergic receptor endocytosis (179). Activation of PI3K has been associated with cytoprotection and reduced apoptosis. PI3K is inhibited by wortmanin (WM) and LY294002 (see figure 5). Inhibition of PI3K blocks the protective effect of PC (254).
Figure 5.
PI3K signaling. PI3K generates PIP3 which recruits AKT to the membrane where it can be phosphorylated by PDK. Newly synthesized PKC is attached to the plasma membrane where it is phosphorylated by PDK1. PDK1 phoshorylation of PKCs in the activation loop is required for PKC activation and stability. However, for most PKC isoforms, the rate of PKC processing by PDK1 is not dependent on phosphoinositides generated by PI3K and thus the rate of PKC processing is not inhibited by wortmannin (188). PDK can also phosphorylate and activate PKA, RSK and p70S6K.
What are the targets of PI3K and how might it mediate protection?
The action of PI3K is usually attributed to generation of phosphatidylinositol-3,4,5-triphosphate (PIP3) which facilitates PDK1 phosphorylation of substrates (see figure 5). PDK1 has been reported to phosphorylate Akt, p70S6K, PKC, serum and glucocorticoid regulated kinase (SGK), p90 ribosomal S6 kinase (RSK), and protein kinase A (PKA). It is not clear that all of these targets are phosphorylated in vivo. Akt, p70S6K, and RSK were not phosphorylated in response to agonist in cells lacking PDK1, suggesting that they are in vivo substrates of PDK1 (272). Despite its name, PDK1 is reported to be constitutively active (188). PIP3 generated by PI3K is thought to bring Akt to the membrane and to alter Akt conformation to facilitate phosphorylation by constitutively active PDK1. The details of how PI3K enhances PDK1 mediated phosphorylation of other substrates such as p70Sk6 or SGK is not clearly understood (see (168)). PDK1 phosphorylation of p70S6K and SGK appears to be dependent on prior phosphorylation of a hydrophobic motif and PI3K appears to somehow enhance phosphorylation at this site. However, there are also data showing that PI3K can enhance the activity of PDK1 (271).
The phosphoinositides such as PIP3 generated by PI3K are degraded by the phosphatases PTEN (phosphatase and tensin homolog deleted on chromosome ten)(154) and SHIP (SH2 domain-containing inositol polyphosphate 5-phosphatase) (268). PTEN and SHIP thus oppose the action of PI3K (see figure 5). Regulation of these phosphatases is another means by which PC might mediate protection. PTEN has been suggested to be involved in cardioprotection (167). PTEN is degraded during a PC protocol in the rat heart consisting of 15 min of ischemia and 30 minutes of reperfusion (36). The loss of PTEN, which increases phosphorylation of Akt (by increasing PIP3 levels), appears to be mediated by the proteosome and dephosphorylation since loss of PTEN is blocked by a proteosome inhibitor MP32 and a phosphatase inhibitor, okadaic acid. It is also interesting that receptor internalization, which has been shown to be important in PC (256), is blocked by PTEN (63). Degradation of PTEN during PC might be involved in stimulating receptor recycling. PTEN is regenerated with extended reperfusion; this would be consistent with the loss of PC with extended reperfusion.
2) Akt
It has been shown that PC results in increased phosphorylation of Akt and that PC activation of Akt is inhibited by WM and LY294002, suggesting a role for PI3K in activation of Akt during PC (254). Recent data have shown that in hearts with reduced PDK1 activity, PC does not result in an increase in phosphorylation of Akt and does not afford protection (34).
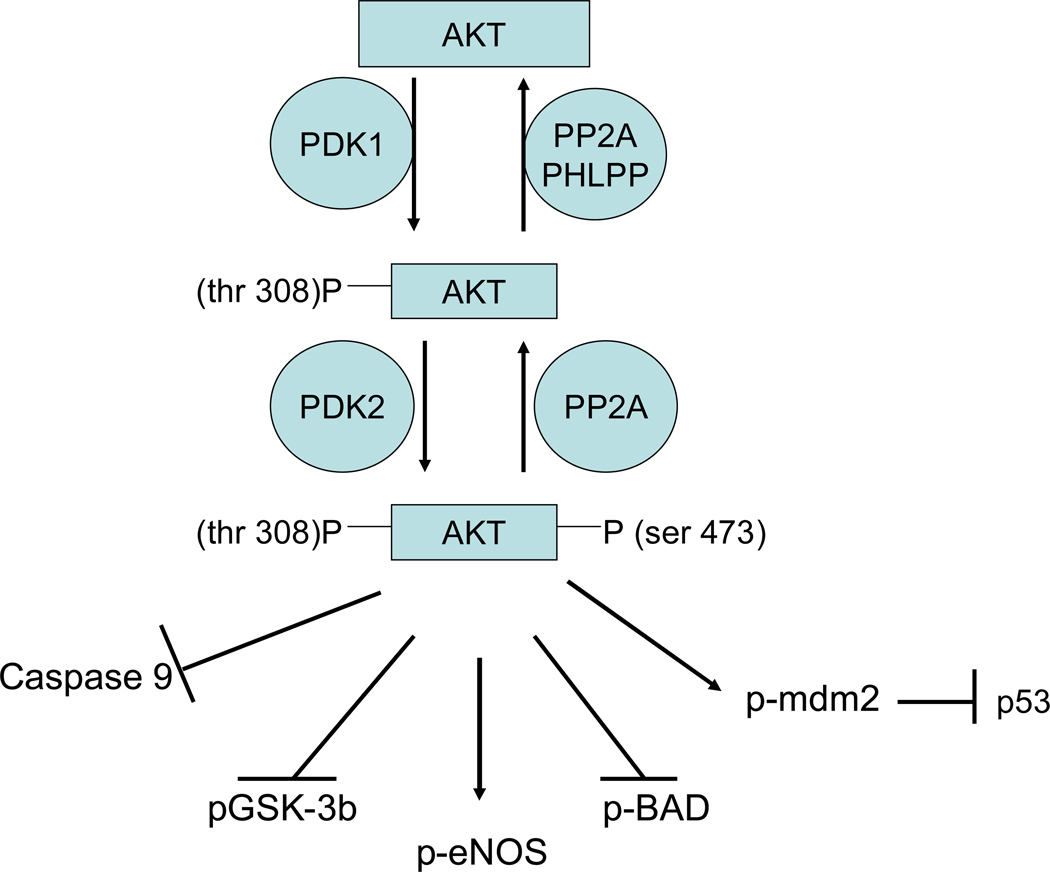
What are the targets of Akt and how might it mediate protection?
Akt is thought to mediate protection by phosphorylation of a number of target proteins (see figure 6) such as glycogen synthase kinase (GSK)-3β, endothelial nitric oxide synthase (eNOS), the pro-apoptotic Bcl-2 family member BAD, caspase 9, the ubiquitin ligase murine double minute 2 (mdm2), and others (see(68)). Phosphorylation of BAD targets it to 14-3-3 protein where it is sequestered, thereby blocking its pro-apoptotic role. Overexpression of Akt blocked hypoxia mediated activation of caspase 3 and 9(260). Akt also phosphorylates and activates a ubiquitin ligase, mdm2. Myocytes overexpressing mdm2 have been reported to be resistant to hypoxiareoxygenation cell death (257). It has been reported that the pro-apoptotic p53 can be inactivated by binding to phosphorylated mdm2 (166). Mdm2 is also recruited to a βarrestin signaling complex (82, 227), and the βarrestin signaling complex appears to be involved in the protection afforded by preconditioning (256). Akt phoshorylates and activates eNOS resulting in an increase in nitric oxide (NO) production(254). Akt also phosphorylates and inactivates GSK-3β (254). Inactivation of GSK-3β has been reported to be important in cardioprotection in a number of models (89, 123, 255); this will be discussed in detail below.
Figure 6.
AKT signaling. Akt is activated by phosphorylation of thr 308 by PDK1 and by phosphorylation of ser 473 by a poorly defined kinase. Phosphorylation of Akt at thr 308 is mediated by binding of PI3K generated lipid (PIP3) to the pleckstrin homology domain of Akt thereby facilitating translocation and association of Akt with PDK1. PIP3 binding to Akt also exposes the PDK1 phosphorylation site thus facilitating phosphorylation of Akt (188). A large number of kinases have been proposed to phosphorylate ser 473, including Akt autophosphorylation, PDK1, ILK1, MAPKAPK2, PKCbII, PIKK, ATM, DNA-PK, and TORC2 (see (68)). Increasing data seem to suggest a role for TORC2 in phosphorylating ser 473 on Akt (116). Akt can also be regulated by dephosphorylation, and PP2a (202) and PHLPP (33) have been reported to be important phosphatases regulating Akt phosphorylation.
3) P70 Ribosomal S6 kinase (P70S6K)
P70S6K is another major target of PDK1, which appears to be activated by PC in a WM sensitive manner (256). As shown in figure 7, P70S6K is phosphorylated on a number of sites, and there are data suggesting that kinases from different pathways such as PKC, ERK, mammalian target of rapamycin (mTOR), Akt and PDK1 are involved in the phosphorylation and activation of p70S6K. Thus, p70S6K has a role in integrating signals from diverse pathways.
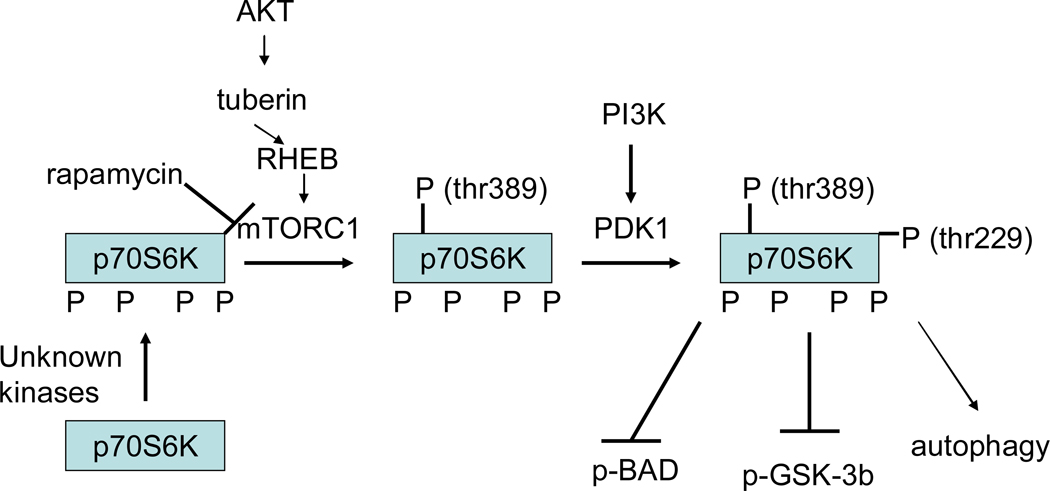
Figure 7.
Regulation of p70S6K phosphorylation and signaling. P70S6K is phosphorylated by PDK1 on thr 229 and by mTOR on thr 389. Prior phosphorylation of thr 389 is reported to be necessary before PDK1 can phosphorylate thr 229. Phosphorylation of thr 389 appears to be rate-limiting and correlates with activity; however activation of mTOR depends on Akt activation. P70S6K is also phosphorylated on the pseudosubstrate or auto-inhibitory domain at ser 411, ser 418, thr 421 and ser 424, but the kinases responsible for phosphorylating these sites are debated. Phosphorylation of these sites appears to be necessary but not sufficient for activation. The activation of mTOR appears to be mediated by activation of Akt. Akt phosphorylates and inhibits tuberin (TSC2), a GTPase, which results in activation of RHEB, which results in phosphorylation and activation of mTOR. Akt is also reported to directly phosphorylate mTOR on serine 2448, but phosphorylation of this site does not appear to regulate activity of mTOR. mTOR activity is also inhibited by AMP-kinase; thus under conditions of increased AMP (decreased ATP) activation of AMP-kinase will lead to inhibition of mTOR
Inhibition of PI3K with WM blocks PC mediated phosphorylation of p70S6K and blocks protection (256). Although inhibition of PI3K blocks PC, the relative role of PDK1 activation of p70S6K, versus other targets, such as Akt, have not been delineated. Rapamycin, an inhibitor of mTOR (which phosphorylates p70S6K on thr 389) has been used to examine the effects of p70S6K (see figure 7). Rapamycin added before ischemia has been reported to block opioid mediated cardioprotection (89). Addition of rapamycin alone prior to ischemia had no effect on infarct size (89). Studies have also reported that rapamycin added at the start of reperfusion blocks PC (99). In another study, addition of insulin at the onset of reperfusion reduced infarct size and resulted in an increase in phosphorylation of p70S6K and Akt; the protection was abrogated by addition of rapamycin concomitant with insulin (120). In contrast to these finding, a recent study has reported that acute treatment with rapamycin per se is cardioprotective (131). Khan et al suggested that perhaps acute addition of rapamycin might activate Akt due to crosstalk between the pathways.
These studies with rapamycin have largely been taken as evidence for a role for p70S6K in PC; however the effects of rapamycin on PC may also be mediated by mTOR. In support of a direct role of p70S6K in protection, Jonassen et al have shown that insulin mediated protection in Girardi cells was blocked by anti-sense oligodeoxynucleotides targeted against p70S6K (119). Additional studies on the role of p70S6K and mTOR in PC will be needed.
What are the targets of p70S6K and how might it mediate protection?
As shown in figure 7, GSK3β can be phosphorylated by p70S6K (245). P70S6K has also been reported to phosphorylate BAD (97). Autophagy has been recently suggested to play a role in ischemic injury (93) and p70S6K, has recently been suggested to positively regulate autophagy, in contrast to the inhibition of autophagy by mTOR (223).
4) Mammalian target of rapamycin (mTOR)
As discussed above, rapamycin, an inhibitor of mTOR, has been shown to block the protective effects of PC and its effects have largely been attributed to inhibition of phosphorylation of p70S6K by mTOR. However, mTOR has some effects that could be important in cardioprotection, which are not related to its effects on phosphorylation of p70S6K. mTOR is activated by growth factors which activate PI3K. mTOR appears to function in a complex with either raptor (mTORC1; a rapamycin sensitive complex) or rictor (rapamycin insensitive companion of TOR) (mTORC2). These two complexes appear to have different functions. As mentioned, mTOR phosphorylates and contributes to the activation of p70S6K, and rapamycin has been shown to block the phosphorylation of thr 389 of p70S6K. However there are p70S6K mutants lacking the carboxyl terminal domain that can be phosphorylated on thr 389 in a rapamycin insensitive manner (5, 211). Data have shown that rapamycin blocks phosphorylation of p70S6K by mTOR complexed with raptor (mTORC1), but rapamycin does not block phosphorylation of p70s6K by mTOR complexed with rictor (mTORC2)(5). mTORC2 (mTOR complexed with rictor) has been reported to phosphorylate Akt on ser473 (the putative PDK2 site) (213).
What are the targets of mTOR and how might it mediate protection?
mTOR phosphorylates and inactivates eukaryotic translation initiation factor eIF4E-binding protein-1 (4EBP1) resulting in an increase in protein synthesis; this may be involved in delayed PC, but since inhibition of protein synthesis does not consistently block acute PC, this increase in protein synthesis is not likely to be required for acute PC protection. mTOR has been reported to phosphorylate PKCε. Recent studies in Jurkat cells have shown that mTOR complexed with raptor increases mitochondrial oxygen consumption and mitochondrial Δψ, whereas mTOR complexed with rictor decreases mitochondrial oxygen consumption (217, 218). The mTOR-raptor complex also regulates the balance between ATP generated by oxidative phosphorylation versus glycolysis. Addition of rapamycin to Jurkat cells for 12 hours decreased oxygen consumption and reduced phosphorylation of several mitochondrial proteins such as the voltage dependent anion channel (VDAC) and several mitochondrial dehydrogenases (218). mTOR was also shown to localize with mitochondria (218). Whether rapamycin would alter oxygen consumption, Δψ and phosphorylation of proteins in a more rapid time scale (consistent with time frame of PC) is unknown. Many cardioprotective agents appear to decrease Δψ and oxygen consumption; it is therefore somewhat difficult to reconcile that rapamycin, which reduces mitochondrial membrane potential and oxygen consumption, blocks PC. However, as mentioned, acute addition of rapamycin has been reported to be protective (131). Prolonged rapamycin treatment has been reported to inhibit assembly of TORC2 and inhibit phosphorylation of Akt (212). It will be important to better define the time dependent changes mediated by rapamycin on Akt, mitochondrial function and other signaling pathways important in cardioprotection. Clearly this is a complex area, but one that warrants further study.
5) Glycogen synthase kinase (GSK)
As shown in figure 4, GSK-3β is phosphorylated and inhibited during PC in a WM sensitive manner (255). The activity of GSK-3β is regulated by phosphorylation and by interaction with proteins. In unstimulated cells, GSK-3β is active and phosphorylates downstream substrates (see figure 8). Phosphorylation of GSK3β results in its inactivation. As shown in figure 8, Akt, p70S6K, PKC, PKA and p90RSK have all been reported to phosphorylate and inactivate GSK-3β. FrzA, a secreted antagonist of the Wnt/Frizzled pathway is reported to decrease phosphorylation of GSK-3β, independent of activity of Akt. Overexpression of FrzA has been reported to block the PC mediated increase in phosphorylation of GSK-3β and block the protection afforded by PC (21). ERK has recently been reported to phosphorylate GSK-3β and prime it for phosphorylation and inactivation by p70S6K (57). GSK-3β is also regulated by dephosphorylation and protein phosphatase 2A is suggested to be the primary phosphatase regulating dephosphorylation of GSK-3β.
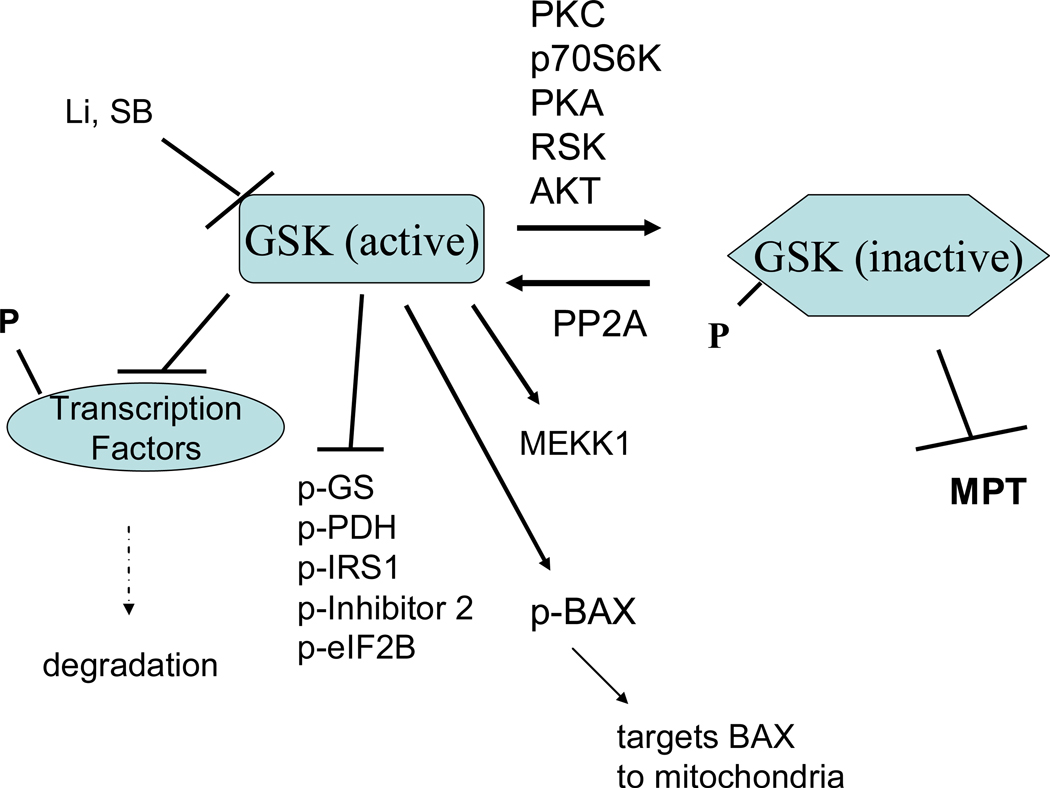
Figure 8.
GSK-β signaling in cardioprotection. In unstimulated cells, GSK-3β is unphosphorylated and active and it phosphorylates and usually inactivates its downstream targets. GSK-3β is phosphorylated and inactivated by a large number of kinases. Inactive GSK-3β has been suggested to be cardioprotective.
Phosphorylation and inactivation of GSK-3β has been reported to be antiapoptotic. Tong et al showed that PC results in increased phosphorylation of GSK-3β, which is blocked by inhibitors of PI3K (254). Tong et al also showed that addition of inhibitors of GSK-3β prior to ischemia was as protective as PC. Using a cardiomyocyte model, Juhaszova et al showed that many different cardioprotective agents result in increased phosphorylation of GSK-3β (123). Gross et al showed that inhibition of GSK-3β is involved in cardioprotection induced by addition of opioids and they further showed that GSK-3β inhibitors were protective even when added just before reperfusion (89).
What are the targets of GSK-3β and how might it mediate protection?
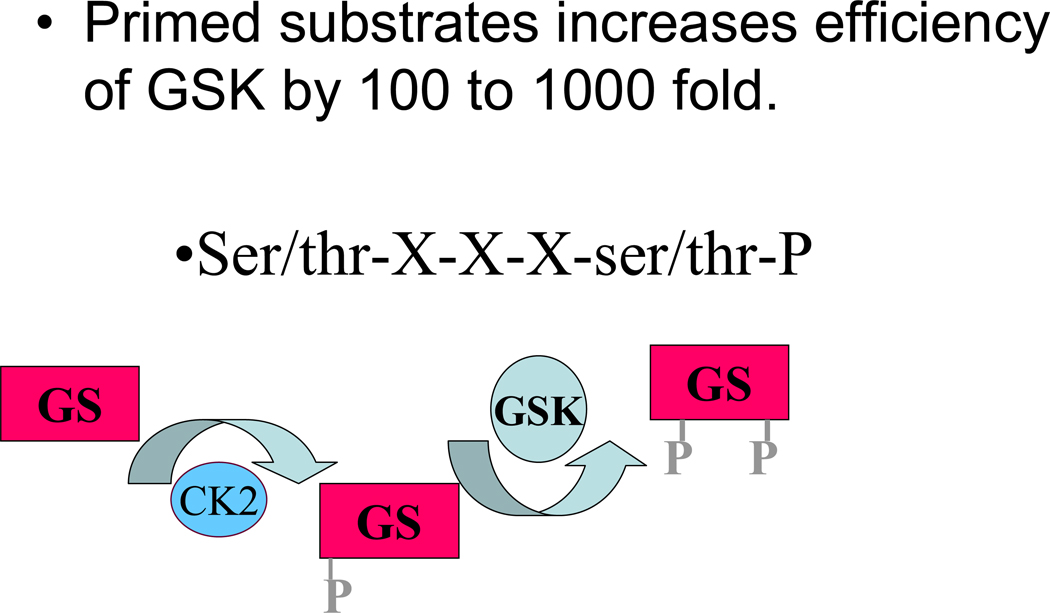
A large number of transcription factors, kinases and other enzymes are reported to be substrates for GSK-3β (175). GSK-3β has a strong preference for substrates that have been primed by previous phosphorylation by another kinase (e.g. primed substrates, see figure 9). This preference for primed substrates provides a means to integrate multiple signaling pathways. Glycogen synthetase was the first substrate describe for GSK-3β and it is from this substrate that GSK 3β takes its name. Phosphorylation and inhibition of GSK-3β results in reduced phosphorylation and thereby greater activity of glycogen synthetase leading to increased glycogen synthesis. Although PC has been reported to reduce glycogen breakdown, this is likely regulated by glycogen phosphorylase, which is not regulated by GSK-3β. However there is coordinate regulation of glycogen synthesis and breakdown. β-catenin is another established substrate for GSK3β. Addition of an adenoviral vector containing a constitutively active β-catenin decreased infarct size measured 7 days after a coronary occlusion (91). PC was also reported to increase β-catenin accumulation along with an increase in β-catenin mediated transcriptional activity and an increase in capillary density measured at 4 days post-myocardial infarction (125). Thus the beneficial effects of β-catenin appear to be primarily mediated by altered gene expression and therefore may have limited impact on acute PC. Juhaszova et al showed that inhibition of GSK-3β delayed the time to opening of MPT initiated by ROS. The mechanism by which inhibition of GSK-3β reduces MPT is unclear but it is plausible that GSK-3β alters MPT by altering phosphorylation of target substrates (175). GSK-3β is reported to phosphorylate the pro-apoptotic protein, BAX, and target BAX to the mitochondria; thus inhibition of GSK-3β would reduce phosphorylation of BAX. GSK-3β phosphorylates a number of enzymes involved in metabolism, such as acetyl CoA carboxylase, ATP citrate lyase, and pyruvate dehydrogenase. Obviously, with so many substrates, it will be challenging to define the mechanisms involved in the cardioprotection afforded by inhibition of GSK-3β. Because inhibition of GSK-3β has been shown to reduce MPT, a focus on mitochondrial targets of GSK-3β may be useful. However, the link between GSK-3β and MTP may be indirect, and may involve cytosolic intermediates.
Figure 9.
GSK-3β has a preference for primed substrates.
6) Endothelial nitric oxide synthase (eNOS)/Nitric oxide(NO)
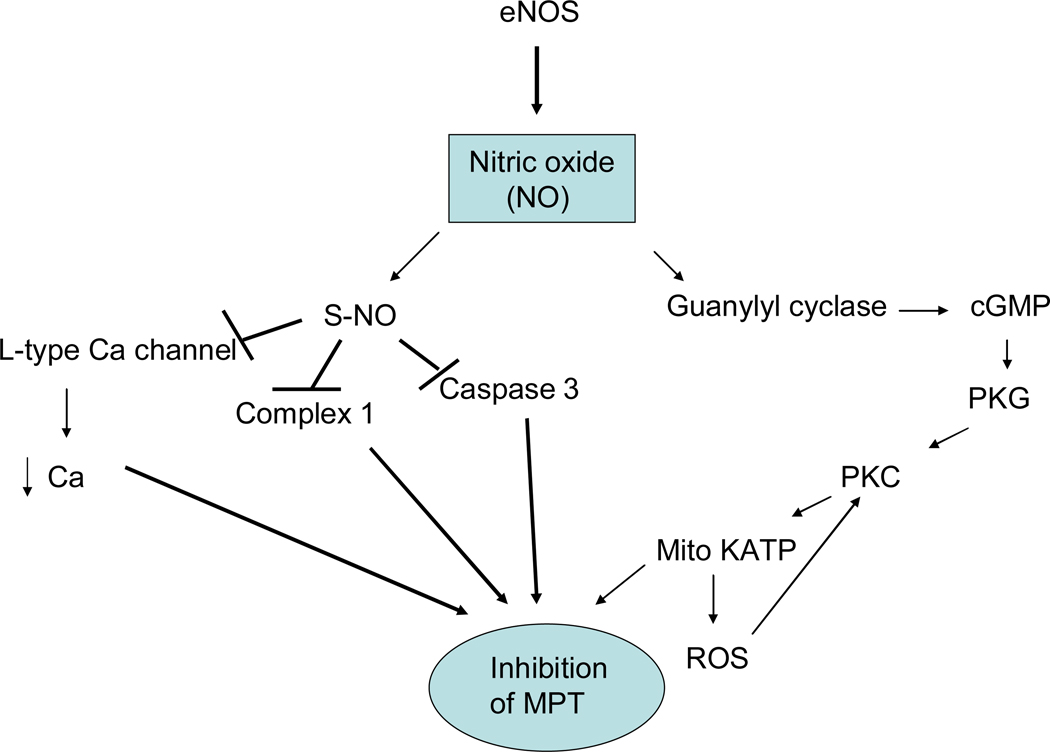
Nitric oxide (NO) has been shown to have an important role in PC and cardioprotection (28, 121). NO can be generated by eNOS (see figure 10), which is phosphorylated and activated by Akt. In addition, during ischemia with low intracellular pH and oxygen, NO may also be generated from nitrite by deoxymyoglobin or xanthine oxidoreductase (108, 145, 230). Nitrite has been shown to reduce ischemia-reperfusion injury (66).
Figure 10.
Nitric oxide signaling in cardioprotection. Nitric oxide (NO) is generated by nitric oxide synthases (NOS). NO can activate guanyly cyclase resulting in activation of protein kinase G. Alternatively NO can result in s-nitrosation of a number of proteins.
What are the targets of eNOS and how might it mediate protection?
eNOS generates nitric oxide (NO) and it is thought that the action of eNOS (as well as nNOS and iNOS) is mediated by NO. Early studies suggested that NO increased activity of the mitoKATP channel (214). Recent studies suggest that the effects of NO on mitoKATP channel are mediated via protein kinase G and PKCε (see figure 10). eNOS generates NO, which results in activation of guanylyl cyclase, which via protein kinase G is reported to activate a mitochondrial PKCε, which results in opening of the mitoKATP channel (48). As shown in figure 10, NO can also result in post-translational modification of proteins such as S-nitrosation (also know as S-nitrosylation). S-nitrosation is a reversible modification that can protect thiol groups from further oxidation. S-nitrosation can also alter the activity of enzymes and transporters. NO has also been shown to result in S-nitrosation of mitochondrial complex I (35). Although it seems counterintuitive, there are data suggesting that in the setting of ischemia-reperfusion, inhibition of complex I can reduce ischemic injury (39, 70). Inhibition of complex I has been suggested to reduce activation of the MPT (70). However long term inhibition of complex I has been shown to be detrimental. NO has also been shown to lead to S-nitrosation and inhibition of the cardiac L-type Ca2+ channel (243). Inhibition of the L-type Ca2+ channel would reduce Ca2+ loading during ischemia. NO has also been reported to modulate cell metabolism (156, 200). The anti-apoptotic effects of thioredoxin are enhanced by S-nitrosylation of thioredoxin at Cys-69 (249).
7) Receptor recycling and endosomal signaling
Activation of GPCRs has been shown to result in receptor internalization which was originally described as a mechanism to desensitize receptor signaling, but which has recently been shown to initiate novel endosomal signaling pathways (142). Recent studies suggest that homologous desensitization of GPCRs that is triggered by G-protein receptor kinase 2 (GRK2) mediated phosphorylation and β-arrestin binding, targets receptors to endosomes through an internalization process. β-arrestin has recently been shown to be a scaffolding protein that brings other signaling molecules in contact with GPCRs in the endosome during receptor recycling. There is increasing evidence that in addition to leading to receptor desensitization, β-arrestin mediated interaction can lead to activation of kinases such as ERK (22, 142, 226, 252). Inhibition of endosomal trafficking, and receptor recycling has also been shown to block the protective effects of preconditioning (256). Preconditioning was blocked in hearts treated with bafilomycin or monodansylcadaverine or when receptor recycling was blocked by sequestration of Gβγ (which inhibits receptor phosphorylation by GRK2) (256). In addition to elimination of protection, inhibition of endosomal/receptor recycling also inhibited PC mediated activation of ERK (256), consistent with a role for GPCR mediated endosomal signaling in PC. Interestingly, inhibition of microtubules with nocodazole or colchicine also blocked the protection afforded by PC, but did not increase infarct size in non-preconditioned hearts (115, 181). Arrestins have recently been shown to bind to microtubules (96).
PC has been reported to result in translocation of a number of important signaling molecules to specific intracellular locations, particularly the mitochondria. Connexin 43 (26), hexokinase (292), and Akt (3) have been suggested to translocate to the mitochondria during preconditioning. The molecular mechanisms regulating this translocation are not well understood. In general, translocation is thought to be regulated by alterations in post-translational modifications such as phosphorylation, which by altering conformation exposes sites that bind to scaffolding or anchoring proteins on specific organelles. However, it is likely that signaling molecules are also localized to the mitochondria by the cytoskeleton or other localizing mechanisms. It is tempting to speculate that perhaps endosomal signaling might be involved in targeting some of these kinases and other signaling molecules to the mitochondria or other intracellular locations.
8) Protein kinase Cε (PKCε)
Activation of PKCε has been shown in numerous studies to have an important role in cardioprotection. PKCε translocation to the membrane fraction has been used as a measure of activation. PC mediated translocation is blocked by WM suggesting it is downstream of PI3K (256). Ping et al showed that NO donors caused translocation of PKCε suggesting that NO (or eNOS) was upstream of PKCε (194). eNOS can be phosphorylated and activated by Akt, suggesting a mechanism that would place PKCε downstream of PI3K (254). Recently it has been shown that NO activates GC which in turn activates mitochondrial PKCε (48). PKCε also appears to be activated by the ROS generated during the brief preconditioning cycles of ischemia and reperfusion (15).
What are the targets of PKCε and how might it mediate protection?
PKCε has been shown using immunoblotting and immunofluorscence to form a mitochondrial localized signaling complex with mitogen-activate protein kinases (MAPKs) (20). Increased phosphorylation of mitochondrial ERK was only observed in mice expressing active PKCε, but not inactive PKCε. PKCε has also been reported to coimmunoprecipitate with VDAC, adenine nucleotide translocator (ANT) and hexokinase II (19). In vitro studies showed that PKCε can phosphorylate VDAC (19), although the functional effect of this phosphorylation was not explored. PKC activation has also been shown to activate the mito KATP channel (150). PKCε and the mitochondrial KATP channel co-purify, suggesting they associate in the mitochondria as a signaling complex (7). When this purified fraction was reconstituted into liposomes, a PKC activator produced a mito KATP dependent K flux; this effect was reversed by addition of the protein phosphatase 2A (7).
9) ERK
Preconditioning has been shown to increase phosphorylation of ERK (74, 195). ERK can be phosphorylated by PKC and Baines et al showed that mitochondrial ERK phosphorylation occurred in mice expressing active PKCε (20). Receptor mediated endosomal signaling appears to be involved in PC mediated activation of ERK since ERK phosphorylation was blocked by inhibitors of endosomal recycling (256). MitoKATP channel openers have also been reported to activate ERK, an effect that was blocked by the antioxidant N-acetyl cysteine (210). Most groups report that treatment with the MEK-1 inhibitor, PD 98059 blocks the protective effects of PC (53, 75, 195, 201, 240, 256). However, a few groups reported that PD 98059 did not block PC (134, 165). Mocanu et al (165) reported that although PD 98059 infused during PC, blocked PC mediated phosphorylation of ERK, it did not block PC mediated reduction in infarct size. However, as discussed later, this same group found that PD 98059 addition during reperfusion blocked the protection afforded by PC(100). Taken together ERK activation during ischemia and reperfusion appear to be important in mediating PC.
What are the targets of ERK and how might it mediate protection?
Activated ERK is known to modulate transcription factor activity; this would be important for delayed PC, but it is less likely to play a major role in acute PC, which appears to be mediated by post-translational modification of proteins.. Activation of ERK, particularly on reperfusion (195), has been shown to result in inhibition of the MPT. ERK has been shown to phosphorylate the pro-apoptotic protein BAD; phosphorylated BAD is sequestered by 14-3-3 and is therefore unable to bind and inhibit anti-apoptotic protein Bcl-2. ERK has also been shown to be involved in endosomal signaling pathways, which have been shown to be activated by PC. It is interesting to speculate that perhaps this endosomal signaling targets ERK to the proper compartment. This is particularly intriguing because PC has been shown to cause ERK translocation to different compartments.
10) p38 MAPK
p38 MAPK is a serine-threonine kinase that is activated by stress stimuli (241). The role of p38 MAPK in PC and cardioprotection is controversial (11, 182, 219, 233, 270). There are considerable data suggesting that inhibition of p38 MAPK is cardioprotective (152, 153, 219). There also are data in the literature suggesting that inhibition of p38MAPK blocks the protective effects of preconditioning (182, 270) and other data suggesting that inhibition of p38MAPK has no effect on PC (219). The reasons for this discrepancy are unclear and have been discussed in detail elsewhere. Briefly the discrepancy might be due to timing of activation of p38MAPK, activation of different isoforms (216), or differential localization (233).
What are the targets of p38MAPK and how might it mediate protection?
Activated p38 MAPK phosphorylates and activates ATF1 and 2, cAMP response element binding protein (CREB), and MAPK-activated protein (AP) kinase 2, which in turn phosphorylates substrates, including heart shock protein 27 (HSP27). Armstrong et al did not find a PC mediated increase in phosphorylation of HSP27, although they did find a PC induced increase in phosphorylation of p38MAPK (11). p38 MAPK is also reported to enhance uptake of the glucose analog 2-deoxyglucose into cells (253). Inhibition of p38 MAPK kinase has also been shown to increase contractility (219). Consistent with this finding, p38 MAPK has recently been reported to depress sarcomeric tension development and ATPase activity (262)
11) JNK
JNK is another member of the MAPK family that has been shown to be activated by stress. Early studies suggested that activation of JNK was pro-apoptotic (275). However, it is clear the effect of JNK on cell survival is very complex. Most studies report that PC results in activation of JNK; however there is disagreement regarding the role of JNK in PC. Sato et al (215), but not Iliodromitis et al (111) find that inhibition of JNK blocks the protective effects of PC. Milano et al (160) recently reported that addition of a new JNK inhibitor reduced ischemia-reperfusion injury, suggesting a detrimental role for JNK. Three different mouse models with reduced JNK activity in the heart were all found to have less ischemia-reperfusion injury and less apoptosis (126). However, the same study examined the effect of sustained JNK activation by generating mice with increase MKK7 (the kinase that phosphorylates JNK) in heart; hearts from these mice were also protected from ischemia-reperfusion injury (126). Thus, the effects of JNK in the heart in the setting of ischemia-reperfusion appear to be complex and likely depend on localization, timing, and which isoform is activated.
What are the targets of JNK and how might it mediate protection?
JNK has been reported to form a complex with the apoptosome and delay activation of caspase 9 (258). JNK is also reported to mediate re-activation of AKT (225). JNK has also been shown to phosphorylate transcription factors such as c-jun (for which it is named) and AP-1.
12) Mitochondrial Targets of PC
Mitochondrial KATP channels
Early PC studies showed that inhibitors of KATP channels such as glibenclamide blocked the protection afforded by PC and that activators of KATP channels mimicked PC. Studies reviewed elsewhere suggested that it was a mitochondrial rather than a sarcolemmal KATP channel that mediated protection (80, 189). The mitochondrial KATP channel (mitoKATP) was described by Garlid as a mitochondrial channel involved in regulating mitochondrial matrix volume; potassium influx results in matrix swelling (see (80, 81)). Pharmacological drugs such as diazoxide open mitoKATP and 5-hydroxydecanoic acid has been used as an inhibitor of mitoKATP (189). In PC, the mitoKATP channel has been shown to be activated by NO and PKCε; as mentioned it has been suggested that NO activates guanylyl cyclase which leads to activation of PKCε which in turn (by an unknown mechanism) activates mitoKATP (see figure 3). Activation of a mitochondrial Ca activated K channel has also been shown to lead to cardioprotection (276).
In spite of intense effort, the molecular structure of the mitoKATP channel is ill defined. It has been reported to be composed of succinate dehydrogenase, phosphate carrier, ANT, ATP synthase, and the ATP binding cassette protein 1 (10). Activation of mitoKATP has been measured in myocytes by monitoring an increase in mitochondrial flavoprotein fluorescence (189). In isolated mitochondria, activation of mitoKATP is frequently measured by monitoring an increase in mitochondrial volume using absorbance (80, 81). In many studies in intact heart, a role for mitoKATP is defined by the ability of 5-hydroxydecanoic acid to block the protection. Questions have been raised about the specificity of 5-hydroxydecanoic acid and diazoxide (147).
How does activation of mitoKATP mediate protection?
It has been shown that activation of the mitoKATP channel by diazoxide will reduce ischemia-reperfusion injury (189). It has been suggested that activation of the mitoKATP channel will slightly reduce the mitochondrial membrane potential which would reduce mitochondrial Ca uptake during ischemia (170). Activation of mitoKATP has been suggested to result in an increase in mitochondrial volume, which has been suggested to alter the Km for ADP entry into the mitochondria, perhaps by altering the conformation of VDAC (59). A decrease in ATP/ADP entry into the mitochondria during ischemia has been suggested to occur with overexpression of Bcl-2 and has been suggested to reduce the consumption of glycolytically generated ATP, which is used to maintain the mitochondrial Δψ (113); thus inhibition of ATP entry into the mitochondria during ischemia would reduce the Δψ and thereby reduce the uptake of Ca into the mitochondria (see Figure 1). In addition to acting as an “end-effector”, activation of the mitoKATP channel has been suggested to result in release of small signaling levels of ROS (71, 191) which may further enhance activation of PKCε and amplify the cardioprotective signaling. Clearly further studies will be needed to better define the molecular structure of the mitoKATP channel and how it mediates cardioprotection.
Connexin-43
Mice heterozygous for connexin 43 (Cx-43) are not protected by PC (221). The role for Cx-43 in preconditioning does not involve gap junction formation between cells, because the reduction in Cx-43 blocks protection in isolated myocytes, a model in which there are no cell-cell connections and therefore no gap junctions (146). It has been shown that PC results in translocation of Cx-43 to the mitochondria and that mitochondrial Cx-43 is important for the protection afforded by PC (204). Interestingly the diazoxide mediated increase in ROS which seems to be involved in signaling cardioprotection, does not occur in Cx-43 deficient mice (104).
Inhibition of MPT
There are considerable data suggesting that inhibitors of MPT, a large conductance channel, reduce ischemia-reperfusion injury (86, 92, 117, 224). The MPT has been suggested to be a multiprotein complex comprised of the ANT and VDAC, and which is modulated by cyclophilin (24, 246, 274). However, it appears that neither VDAC nor ANT are required components of MPT (17, 137). Cyclosporin which binds to and inhibits cyclophilin has been shown to inhibit the MPT (49, 72). Cyclosporin reduced injury in perfused hearts during ischemia-reperfusion and cardiomyocytes subjected to anoxia (86, 185). The effects of ANT inhibitors are complex. Bongkrekic acid and carboxyatractylase both inhibit ANT; however bongkrekic acid inhibits ANT in c-state, in which the binding pocket faces the cytosol and carboxyatractylase inhibits ANT in m-state in which the binding pocket faces the matrix (136). Bongkrekic acid prevents dissociation of ATP/ADP from ANT and causes mitochondrial contraction similar to that observed with addition of adenine nucleotides, whereas carboxyatractylase reverses the ADP/ATP induced contraction of mitochondria. Interestingly bongkrekic acid inhibits whereas carboxyatractylase activates MPT (140, 180). This would be consistent with inhibition of MPT by adenine nucleotides. Mice with cardiac specific loss of cyclophilin were shown to be less sensitive to calcium activation of MPT and were also shown to have significantly smaller infarcts following ischemia-reperfusion (16).
The MPT opens under conditions of high Ca2+, ROS, high NADH, depletion of adenine nucleotides and loss of Δψ (103, 109, 110); conditions that occur during ischemia-reperfusion (24, 65). Low pH, as occurs during ischemia, inhibits MPT. Intracellular pH is rapidly restored on reperfusion, thus allowing activation of the MPT. Furthermore, the reduced Δψ during ischemia would limit uptake of mitochondrial Ca2+, but the re-introduction of oxygen on reperfusion would reconstitute Δψ (stimulating Ca2+ uptake into the mitochondria) and the introduction of oxygen will also allow generation of ROS. Thus the conditions that exist right at the start of reperfusion are ideal to stimulate opening of the MPT. This may explain why strategies that inhibit MTP during the first few seconds of reperfusion can reduce infarct size. Opening of the MPT causes rapid loss of Δψ and robust mitochondrial swelling which can be followed by changes in absorbance in isolated mitochondria. The extensive mitochondrial swelling results in rupture of the outer mitochondrial membrane with loss of cytochrome c which can initiate apoptosis, although with full opening of MPT, it is likely cell death will occur before the apoptotic program is completed. Thus sustained activation of the MPT is incompatible with viability due to loss of mitochondrial function and resultant loss of ATP. It is interesting that many cardioprotective strategies would reduce opening of MPT. Protocols that reduce calcium loading of the cell reduce MPT and reduce infarct size. A reduction in cytosolic Ca2+ would reduce Ca2+ uptake into the mitochondria during ischemia and early reperfusion and thus reduce MPT. A reduction in ROS during ischemia and reperfusion would also reduce opening of MPT. Inhibition of complex I, which has been reported to be cardioprotective (39), has also been reported to reduce opening of MPT (70).
Thus reducing Ca2+ and ROS during ischemia and reperfusion and maintaining acid pH during the very start of reperfusion would all inhibit MPT. Although a large decrease in Δψ is a trigger for MPT, Δψ is also the driving force for Ca2+ uptake into the mitochondria and a high Δψ enhances generation of ROS. Thus a moderate reduction in Δψ, particularly during ischemia has been suggested to be protective (39, 170). Activation of the reperfusion injury salvage kinases (RISK) at the start of reperfusion has been reported to reduce MPT (100). It is less clear how the MPT is modulated by PC or activation of the RISK pathways (100, 101); several potential mechanisms are summarized in figure 3. One hypothesis is that the activation of cardioprotective kinases results in activation of the mito KATP channel which reduces Δψ, which as discussed would reduce the uptake of mitochondrial Ca2+ (170) and the generation of ROS. A number of kinases have also been reported to result in phosphorylation of mitochondrial proteins that might be involved in the MPT. PKCε has been reported to phosphorylate VDAC (19), a purported component of the MPT. The functional effects of PKCε phosphorylation of VDAC have not been delineated. It is likely that the kinases activated by cardioprotection have a number of undiscovered mitochondrial targets by which they mediate their protection. This is an area for future study.
B. Reperfusion Injury Survival Kinases - RISK Pathways
The RISK pathway was originally used to describe activation of survival kinases by addition of agonists such as adenosine and insulin at the start of reperfusion (102). It was shown that addition of these agonists at the start of reperfusion caused a rapid activation of PI3K and ERK resulting in cardioprotection. The activation of these survival kinases at the start of reperfusion is thought to mediate protection by inhibition of the MPT. Yellon and coworkers further demonstrated that preconditioning results in activation of ERK and Akt at the start of reperfusion and that inhibition of ERK and PI3K at the start of reperfusion blocks the protective effects of PC (101). These data suggest that at least part of the protection afforded by PC is mediated by activation of survival kinases at the start of reperfusion (100). There are data suggesting that activation of some kinase pathways during ischemia may initiate activation of survival kinases on reperfusion. For example, addition of erythropoietin (EPO) before ischemia is cardioprotective. Hanlon et al found that addition of PKC inhibitors, but not PI3K inhibitors, during ischemia, blocked the protective effects of EPO (95). However, addition of PI3K inhibitors at the start of reperfusion block the protection provided by EPO added prior to and during ischemia.
C. Postconditioning
In 2003 Zhao et al. reported that intermittent reperfusion, after ischemia, can also reduce infarct size (287). The precise postconditioning protocol (the number episodes and the duration of intermittent ischemia and reperfusion) required for protection appears to vary depending on the species. Yang also reported that if postconditioning was begun after 10 minutes of reperfusion, it was not protective (279). Most (32, 45, 105, 192, 193, 259, 278, 279, 287), but not all (61, 222) investigators report that postconditioning reduces infarct size. It has also been suggested that cardioprotection by postconditioning is limited to coronary occlusion durations less than 45 minutes (248). Interestingly, it has been shown that the protection afforded by postconditioning occur via activation of many of the same signaling kinases that are involved in PC mediated protection (101). Furthermore the protection afforded by PC and postconditioning are not additive (259). As shown in figure 3b, postconditioning has been suggested to involve activation at the time of reperfusion of PI3K, Akt, eNOS, PKG, PKCe, ERK and mitoKATP.
1) Adenosine Receptors
A number of groups have reported that adenosine receptor antagonists will block the protective effects of postconditioning. Morrison et al (169) reported that the protection afforded by postconditioning was lost in hearts from mice with deletion of the A2A adenosine receptor. The phosphorylation of ERK and Akt that occur during reperfusion in postconditioned heart was also blocked in A2A adenosine receptor knock-out mice. Yang et al (278) reported that treatment of rabbit heart with the adenosine receptor inhibitor (8-p (sulfophenyl) theophylline (SPT)) blocked the protection afforded by postconditioning. Philipp et al (193) reported that an adenosine A2b antagonist MRS 1754 blocked the postconditioning mediated reduction in infarct size. Philipp et al (193) further showed that infusion 5 minutes before reperfusion of an adenosine A2b agonist ((N-ethylcarboxamido)adenosine; NECA) was as protective as postconditioning.
2) PI3K
Tsang et al (259) demonstrated in rat heart that postconditioning activates Akt, eNOS, and p70S6K, and that activation is blocked by inhibitors of PI3K. Tsang et al also showed that postconditioning mediated reduction in infarct size is blocked by addition of inhibitors of PI3K administered during the first 15 minutes of reperfusion. Consistent with a role for PI3K in postconditioning, Yang et al (278) showed that treatment of rabbit hearts with inhibitors of PI3K added for 20 minutes starting 5 minutes before reperfusion blocked the infarct reduction associated with postconditioning. Bopassa et al (32) also found that addition of PI3K inhibitors added at the start of reperfusion blocked postconditioning mediated improvement in both postischemic function measured by rate pressure product and enzyme release. PI3K inhibition was also shown to block postconditioning in a remodeled myocardium (289). However, Darling et al (54) reported that addition of a PI3K inhibitor during reperfusion did not block postconditioning in the perfused rabbit heart. Schwartz and Lagranha (222) find that postconditioning leads to activation of Akt and ERK, but does not result in a reduction in infarct size in pigs.
3) PKC
Philipp et al (193) showed that the PKC inhibitor, chelerythrine blocked the postconditioning mediated reduction in infarct size. Philipp et al also reported that infusion 5 minutes before reperfusion of a PKC activator PMA was as protective as postconditioning. Furthermore, the protection afforded by PMA addition on reperfusion was blocked by either MRS 1754 or wortmannin, a PI3K inhibitor (193). These data suggest that protection afforded by PKC activation on reperfusion is mediated by activation of adenosine receptors and PI3K. Interestingly, this same group (139) has suggested that the activation of PKC that occurs in PC increased the sensitivity of the adenosine A(2b) receptor to adenosine. Zatta et al (286) also showed that the postconditioning mediated reduction in infarct size is blocked by inhibition of PKCε. Zatta et al further showed that phosphorylated PKCε was higher in postconditioned hearts and that translocation of phosphorylated PKCδ to the mitochondria was less in postconditioned hearts.
4) MAPK
Yang et al (279) reported that infusion, beginning 5 minutes before reperfusion, of PD98059, an inhibitor of ERK activation, abrogated the postconditioning mediated protection. Darling et al(54) reported that postconditioning resulted in an increase in phosphorylation of ERK and was blocked by PD98059. Sun et al (242) reported that hypoxiareoxygenation results in activation of JNK and p38MAPK; postconditioning reduces apoptosis in cardiomyocytes and also reduced activation of JNK and p38MAPK. Furthermore, addition of anisomycin, a JNK/p38MAPK activator, eliminated the inhibition of apoptosis by postconditioning.
5) PKG/NO
Yang et al (278{Yang, 2004 #320, 279) reported that infusion beginning 5 minutes before reperfusion of an inhibitor of NOS or inhibitors of guanylyl cyclase (ODQ) block the protection afforded by postconditioning. L-NAME, an inhibitor of NOS, was also shown to block postconditioning (279).
6) Mitochondria
Inhibition of mitoKATP channels has been reported to block the protection afforded by postconditioning (279). Interestingly, although loss of connexin 43 blocks preconditioning, it does not block the ability of postconditioning to reduce infarct size (105, 220). Hearts from mice lacking cyclophilin D, a component of the MPT, exhibit smaller infarcts than wild type mice and show no additional protection by postconditioning; thus the effect of loss of cyclophilin D and postconditioning are not additive, suggesting a role for the inhibition of MPT in postconditioning (148). Also consistent with a role for postconditioning mediated inhibition of MPT are recent data that suggest that postconditioning maintains acidosis during the start of reperfusion, which inhibits MPT (45, 76). Reperfusion with pH 7.7 buffer blocked protection induced by postconditioning (45).
V. Summary
Figure 11 summarizes the working hypothesis as to why cells die and how we might stop them. Data suggest that during ischemia, there is a rise in [Ca2+]i (due to increased [Na+]i and reverse mode NCX and Ca2+ entry via L-type Ca2+ channels and reduced Ca2+ efflux via Ca2+ ATPase). There is also ATP depletion, but this fall in ATP and the increase in [Ca2+]i during ischemia do not appear to immediately result in rupture of the plasma membrane and irreversible injury, at least not during moderate durations of ischemia. As much as 50% of the ATP consumed during ischemia is via reversal of the F1F0ATPase, energy which is used to generate mitochondrial membrane potential (Δψ). Maintaining Δψ will allow uptake of Ca2+ during ischemia and this Ca2+ when present at the start of reperfusion (when pH is restored) will initiate activation of the MPT. Furthermore, on reperfusion, oxygen is returned to the cell resulting in generation of ROS, regeneration of Δψ with uptake of potentially large amounts of Ca2+ into the mitochondria. This increase in matrix Ca2+, coupled with increased ROS and the restoration of a normal pH (acid pH inhibits MPT) results in activation of the MPT, which in turn totally depletes the cell of ATP, activates the mitochondrial apoptotic pathways including caspases. The activation of these proteolytic pathways coupled with the total loss of ATP and ROS mediated damage synergize, leading to rapid loss of plasma membrane integrity. Cardioprotective pathways appear to work primarily by inhibition of the MPT directly or by preventing the conditions that promote MPT opening. Lowering [Ca2+]i inhibits MPT. Inhibiting complex I inhibits MPT. Reducing ROS inhibits MPT. Lowering Δψ reduces mitochondrial Ca2+ uptake which would reduce MPT. As shown in figure 3, PC initiates a number of signaling pathways some of which reduce injury during ischemia and some of which activate survival kinases at reperfusion that protect primarily by inhibition of the MPT. Pre- and postconditioning both share activation of survival kinases. Postconditioning also slows recovery of extracellular pH, thus providing a lower intracellular pH that will also inhibit MPT. It is likely that postconditioning mediated maintenance of an acidic pH at the start of reperfusion keeps the MPT inhibited thus allowing time for the activation of the of the survival kinases to inhibit MPT.
Figure 11.
Pathways Leading to Cell Death in Ischemia-Reperfusion.
The signaling cascades initiated by pre- and postconditioning also appear to inhibit MPT directly, likely by post-translational modification of mitochondrial proteins. There are data suggesting that activation of PI3K results in phosphorylation and inactivation of GSK-3β which by some ill defined mechanism reduces MPT. ERK has also been shown to increase phosphorylation of BAD which would reduce apoptosis. PKC which is activated by the survival kinases has also been shown to phosphorylate VDAC. Unfortunately our understanding of the MPT is very limited. The precise molecular make-up of the MPT is poorly understood. The role of the MPT in normal cell physiology (if there is one) is not understood. Clearly a better understanding of MPT is imperative if we are to better learn how to inhibit it to reduce ischemic death.
The similarity in protection between pre- and postconditioning in many models of ischemia (particularly at relatively short times of ischemia) suggest that under these conditions activation of survival kinase at reperfusion is sufficient to inhibit MPT. However, with longer periods of ischemia, preconditioning can modify conditions during ischemia which inhibit the MPT and this added protection may allow PC to protect against injury with longer durations of ischemia. However, the ability to elicit protection at the start of reperfusion is a much more relevant clinical application.
The protection afforded by postconditioning suggests that treatment at the start of reperfusion can be cardioprotective. How do we reconcile this with the large number of clinical trials that failed to show cardioprotection. Reasons for this discrepancy have been discussed in detail elsewhere (30). However, many of the failed clinical trials included patients in which reperfusion had already started. Thus the drugs were not administered before or at the very start of reperfusion. Indeed in the Guardian trial, administration of NHE inhibitors was protective in the group receiving coronary artery bypass surgery. It appears that it is important to apply cardioprotective drugs no later than then start of reperfusion. Furthermore, as discussed elsewhere (30), it is important that basic studies (prior to clinical trials) demonstrate protection in clinical relevant models using clinically relevant outcomes. It is also important to demonstrate protection using clinically relevant durations of ischemia.
Where do we go in cardioprotection?
Many drugs and protocols protected when added at the start of reperfusion. However given the history of previous failed clinical trials in cardioprotection one needs to be cautious. It is important to assess protection after longer, more clinically relevant periods of ischemia. It is also imperative that treatments be initiated before, or immediately at the start of reperfusion, for example with cardiac surgery or angioplasty. It is worth considering a cocktail (Na-H exchange inhibitors, antioxidant, NO donors, erythropoietin, caspase inhibitors and MPT inhibitors) that blocks multiple mechanisms in cell death. Perhaps this cocktail can be taken, similar to aspirin at the onset of a heart attack, while the patient is taken to the hospital.
Acknowledgments
We thank Drs. Mark Gladwin, Michael Sack, Anne Deschamps, Junhui Sun, Renee Wong and Claudia Lagranha for their helpful comments and discussions. "This research was support in part by the Intramural Program of the NIH.
References
- 1.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 2.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. Faseb J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ala-Rami A, Ylitalo KV, Hassinen IE. Ischaemic preconditioning and a mitochondrial KATP channel opener both produce cardioprotection accompanied by F1F0-ATPase inhibition in early ischaemia. Basic Res Cardiol. 2003;98:250–258. doi: 10.1007/s00395-003-0413-z. [DOI] [PubMed] [Google Scholar]
- 5.Ali SM, Sabatini DM. Structure of S6 kinase 1 determines whether raptor-mTOR or rictor-mTOR phosphorylates its hydrophobic motif site. J Biol Chem. 2005;280:19445–19448. doi: 10.1074/jbc.C500125200. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosio G, Becker LC, Hutchins GM, Weisman HF, Weisfeldt ML. Reduction in experimental infarct size by recombinant human superoxide dismutase: insights into the pathophysiology of reperfusion injury. Circulation. 1986;74:1424–1433. doi: 10.1161/01.cir.74.6.1424. [DOI] [PubMed] [Google Scholar]
- 7.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–H2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 8.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93 Suppl 3:8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 9.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 10.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong SC, Delacey M, Ganote CE. Phosphorylation state of hsp27 and p38 MAPK during preconditioning and protein phosphatase inhibitor protection of rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31:555–567. doi: 10.1006/jmcc.1998.0891. [DOI] [PubMed] [Google Scholar]
- 12.Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE. Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res. 2006;99:706–714. doi: 10.1161/01.RES.0000243995.74395.f8. [DOI] [PubMed] [Google Scholar]
- 13.Arroyo CM, Kramer JH, Dickens BF, Weglicki WB. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987;221:101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- 14.Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem. 2006;281:22943–22952. doi: 10.1074/jbc.M601025200. [DOI] [PubMed] [Google Scholar]
- 15.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 16.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 17.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 21.Barandon L, Dufourcq P, Costet P, Moreau C, Allieres C, Daret D, Dos Santos P, Daniel Lamaziere JM, Couffinhal T, Duplaa C. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res. 2005;96:1299–1306. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 24.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. Febs J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 25.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 26.Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Bognar Z, Kalai T, Palfi A, Hanto K, Bognar B, Mark L, Szabo Z, Tapodi A, Radnai B, Sarszegi Z, Szanto A, Gallyas F, Jr, Hideg K, Sumegi B, Varbiro G. A novel SOD-mimetic permeability transition inhibitor agent protects ischemic heart by inhibiting both apoptotic and necrotic cell death. Free Radic Biol Med. 2006;41:835–848. doi: 10.1016/j.freeradbiomed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 29.Bolli R. Causative role of oxyradicals in myocardial stunning: a proven hypothesis. A brief review of the evidence demonstrating a major role of reactive oxygen species in several forms of postischemic dysfunction. Basic Res Cardiol. 1998;93:156–162. doi: 10.1007/s003950050079. [DOI] [PubMed] [Google Scholar]
- 30.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 31.Bolli R, Patel BS, Jeroudi MO, Lai EK, McCay PB. Demonstration of free radical generation in "stunned" myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988;82:476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res. 2006;69:178–185. doi: 10.1016/j.cardiores.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Budas GR, Sukhodub A, Alessi DR, Jovanovic A. 3'Phosphoinositide-dependent kinase-1 is essential for ischemic preconditioning of the myocardium. Faseb J. 2006;20:2556–2558. doi: 10.1096/fj.06-6252fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- 37.Cascio WE, Yan GX, Kleber AG. Early changes in extracellular potassium in ischemic rabbit myocardium. The role of extracellular carbon dioxide accumulation and diffusion. Circ Res. 1992;70:409–422. doi: 10.1161/01.res.70.2.409. [DOI] [PubMed] [Google Scholar]
- 38.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–429. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 43.Chi LG, Tamura Y, Hoff PT, Macha M, Gallagher KP, Schork MA, Lucchesi BR. Effect of superoxide dismutase on myocardial infarct size in the canine heart after 6 hours of regional ischemia and reperfusion: a demonstration of myocardial salvage. Circ Res. 1989;64:665–675. doi: 10.1161/01.res.64.4.665. [DOI] [PubMed] [Google Scholar]
- 44.Cobbold PH, Bourne PK. Aequorin measurements of free calcium in single heart cells. Nature. 1984;312:444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- 45.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 46.Comelli M, Metelli G, Mavelli I. Downmodulation of mitochondrial F0F1 ATP synthase by diazoxide in cardiac myoblasts: a dual effect of the drug. Am J Physiol Heart Circ Physiol. 2007;292:H820–H829. doi: 10.1152/ajpheart.00366.2006. [DOI] [PubMed] [Google Scholar]
- 47.Contessi S, Metelli G, Mavelli I, Lippe G. Diazoxide affects the IF1 inhibitor protein binding to F1 sector of beef heart F0F1ATPsynthase. Biochem Pharmacol. 2004;67:1843–1851. doi: 10.1016/j.bcp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 49.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 50.Cross HR, Murphy E, Bolli R, Ping P, Steenbergen C. Expression of activated PKC epsilon (PKC epsilon) protects the ischemic heart, without attenuating ischemic H(+) production. J Mol Cell Cardiol. 2002;34:361–367. doi: 10.1006/jmcc.2001.1518. [DOI] [PubMed] [Google Scholar]
- 51.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 52.Czerski L, Nunez G. Apoptosome formation and caspase activation: is it different in the heart? J Mol Cell Cardiol. 2004;37:643–652. doi: 10.1016/j.yjmcc.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 53.da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Differential activation of mitogen-activated protein kinases in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100:59–69. doi: 10.1097/00000542-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005;289:H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 55.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, Hajjar RJ. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. The Journal of physiology. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Donoso P, Mill JG, O'Neill SC, Eisner DA. Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. The Journal of physiology. 1992;448:493–509. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP- sensitive K(+) channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2002;283:H284–H295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 60.Dosenko VE, Nagibin VS, Tumanovska LV, Moibenko AA. Protective effect of autophagy in anoxia-reoxygenation of isolated cardiomyocyte? Autophagy. 2006;2:305–306. doi: 10.4161/auto.2946. [DOI] [PubMed] [Google Scholar]
- 61.Dow J, Kloner RA. Postconditioning does not reduce myocardial infarct size in an in vivo regional ischemia rodent model. J Cardiovasc Pharmacol Ther. 2007;12:153–163. doi: 10.1177/1074248407300897. [DOI] [PubMed] [Google Scholar]
- 62.Downey JM, Omar B, Ooiwa H, McCord J. Superoxide dismutase therapy for myocardial ischemia. Free Radic Res Commun. 1991;12–13(Pt 2):703–720. doi: 10.3109/10715769109145850. [DOI] [PubMed] [Google Scholar]
- 63.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 64.du Toit EF, Opie LH. Inhibitors of Ca2+ ATPase pump of sarcoplasmic reticulum attenuate reperfusion stunning in isolated rat heart. J Cardiovasc Pharmacol. 1994;24:678–684. doi: 10.1097/00005344-199410000-00020. [DOI] [PubMed] [Google Scholar]
- 65.Duchen MR, McGuinness O, Brown LA, Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res. 1993;27:1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- 66.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eisner DA, Choi HS, Diaz ME, O'Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 68.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 69.Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N, et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- 70.Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation By electron flow through the respiratory chain complex i. J Biol Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- 71.Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 72.Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987;19:297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- 73.Fralix TA, Murphy E, London RE, Steenbergen C. Protective effects of adenosine in the perfused rat heart: changes in metabolism and intracellular ion homeostasis. Am J Physiol. 1993;264:C986–C994. doi: 10.1152/ajpcell.1993.264.4.C986. [DOI] [PubMed] [Google Scholar]
- 74.Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H1184–H1192. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- 75.Fryer RM, Pratt PF, Hsu AK, Gross GJ. Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther. 2001;296:642–649. [PubMed] [Google Scholar]
- 76.Fujita M, Asanuma H, Hirata A, Wakeno M, Takahama H, Sasaki H, Kim J, Takashima S, Tsukamoto O, Minamino T, Shinozaki Y, Tomoike H, Hori M, Kitakaze M. Prolonged transient acidosis during early reperfusion contributes to the cardioprotective effects of postconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H2004–H2008. doi: 10.1152/ajpheart.01051.2006. [DOI] [PubMed] [Google Scholar]
- 77.Gallagher KP, Buda AJ, Pace D, Gerren RA, Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986;73:1065–1076. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]
- 78.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 79.Garlick PB, Davies MJ, Hearse DJ, Slater TF. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987;61:757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- 80.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 81.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 82.Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Vasilcanu D, Girnita A, Lefkowitz RJ, Larsson O. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem. 2007;282:11329–11338. doi: 10.1074/jbc.M611526200. [DOI] [PubMed] [Google Scholar]
- 83.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Gottlieb R. ICE-ing the heart. Circ Res. 2005;96:1036–1038. doi: 10.1161/01.RES.0000169466.84402.c1. [DOI] [PubMed] [Google Scholar]
- 85.Green DW, Murray HN, Sleph PG, Wang FL, Baird AJ, Rogers WL, Grover GJ. Preconditioning in rat hearts is independent of mitochondrial F1F0 ATPase inhibition. Am J Physiol. 1998;274:H90–H97. doi: 10.1152/ajpheart.1998.274.1.H90. [DOI] [PubMed] [Google Scholar]
- 86.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 87.Griffiths EJ, Ocampo CJ, Savage JS, Rutter GA, Hansford RG, Stern MD, Silverman HS. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovasc Res. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 88.Griffiths EJ, Ocampo CJ, Savage JS, Stern MD, Silverman HS. Protective effects of low and high doses of cyclosporin A against reoxygenation injury in isolated rat cardiomyocytes are associated with differential effects on mitochondrial calcium levels. Cell Calcium. 2000;27:87–95. doi: 10.1054/ceca.1999.0094. [DOI] [PubMed] [Google Scholar]
- 89.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 90.Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, Green DW. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am J Physiol Heart Circ Physiol. 2004;287:H1747–H1755. doi: 10.1152/ajpheart.01019.2003. [DOI] [PubMed] [Google Scholar]
- 91.Hahn JY, Cho HJ, Bae JW, Yuk HS, Kim KI, Park KW, Koo BK, Chae IH, Shin CS, Oh BH, Choi YS, Park YB, Kim HS. Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. J Biol Chem. 2006;281:30979–30989. doi: 10.1074/jbc.M603916200. [DOI] [PubMed] [Google Scholar]
- 92.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 93.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 94.Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006;20:445–462. doi: 10.1007/s10557-006-0583-7. [DOI] [PubMed] [Google Scholar]
- 95.Hanlon PR, Fu P, Wright GL, Steenbergen C, Arcasoy MO, Murphy E. Mechanisms of erythropoietin-mediated cardioprotection during ischemia-reperfusion injury: role of protein kinase C and phosphatidylinositol 3-kinase signaling. Faseb J. 2005;19:1323–1325. doi: 10.1096/fj.04-3545fje. [DOI] [PubMed] [Google Scholar]
- 96.Hanson SM, Cleghorn WM, Francis DJ, Vishnivetskiy SA, Raman D, Song X, Nair KS, Slepak VZ, Klug CS, Gurevich VV. Arrestin mobilizes signaling proteins to the cytoskeleton and redirects their activity. J Mol Biol. 2007;368:375–387. doi: 10.1016/j.jmb.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci U S A. 2001;98:9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 99.Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 100.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 101.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 103.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 104.Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, Garcia-Dorado D, Di Lisa F, Schulz R, Heusch G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res. 2005;97:583–586. doi: 10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
- 105.Heusch G, Buchert A, Feldhaus S, Schulz R. No loss of cardioprotection by postconditioning in connexin 43-deficient mice. Basic Res Cardiol. 2006;101:354–356. doi: 10.1007/s00395-006-0589-0. [DOI] [PubMed] [Google Scholar]
- 106.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 107.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 108.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 110.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 111.Iliodromitis EK, Gaitanaki C, Lazou A, Bofilis E, Karavolias GK, Beis I, Kremastinos DT. Dissociation of stress-activated protein kinase (p38-MAPK and JNKs) phosphorylation from the protective effect of preconditioning in vivo. J Mol Cell Cardiol. 2002;34:1019–1028. doi: 10.1006/jmcc.2002.2039. [DOI] [PubMed] [Google Scholar]
- 112.Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E. Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res. 2005;97:916–921. doi: 10.1161/01.RES.0000187456.06162.cb. [DOI] [PubMed] [Google Scholar]
- 113.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 114.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 115.Ismaeil MS, Tkachenko I, Hickey RF, Cason BA. Colchicine inhibits isoflurane-induced preconditioning. Anesthesiology. 1999;91:1816–1822. doi: 10.1097/00000542-199912000-00036. [DOI] [PubMed] [Google Scholar]
- 116.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 117.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jennings RB, Reimer KA, Steenbergen C. Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J Mol Cell Cardiol. 1991;23:1383–1395. doi: 10.1016/0022-2828(91)90185-o. [DOI] [PubMed] [Google Scholar]
- 119.Jonassen AK, Mjos OD, Sack MN. p70s6 kinase is a functional target of insulin activated Akt cell-survival signaling. Biochem Biophys Res Commun. 2004;315:160–165. doi: 10.1016/j.bbrc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 120.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 121.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 122.Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H277–H282. doi: 10.1152/ajpheart.00236.2002. [DOI] [PubMed] [Google Scholar]
- 123.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jung DW, Apel LM, Brierley GP. Transmembrane gradients of free Na+ in isolated heart mitochondria estimated using a fluorescent probe. Am J Physiol. 1992;262:C1047–C1055. doi: 10.1152/ajpcell.1992.262.4.C1047. [DOI] [PubMed] [Google Scholar]
- 125.Kaga S, Zhan L, Altaf E, Maulik N. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol. 2006;40:138–147. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 126.Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 127.Kajstura J, Bolli R, Sonnenblick EH, Anversa P, Leri A. Cause of death: suicide. J Mol Cell Cardiol. 2006;40:425–437. doi: 10.1016/j.yjmcc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 128.Karmazyn M. Amiloride enhances postischemic ventricular recovery: possible role of Na+-H+ exchange. Am J Physiol. 1988;255:H608–H615. doi: 10.1152/ajpheart.1988.255.3.H608. [DOI] [PubMed] [Google Scholar]
- 129.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 131.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 132.Kilgore KS, Friedrichs GS, Johnson CR, Schasteen CS, Riley DP, Weiss RH, Ryan U, Lucchesi BR. Protective effects of the SOD-mimetic SC-52608 against ischemia/reperfusion damage in the rabbit isolated heart. J Mol Cell Cardiol. 1994;26:995–1006. doi: 10.1006/jmcc.1994.1120. [DOI] [PubMed] [Google Scholar]
- 133.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;290:H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 134.Kim SO, Baines CP, Critz SD, Pelech SL, Katz S, Downey JM, Cohen MV. Ischemia induced activation of heat shock protein 27 kinases and casein kinase 2 in the preconditioned rabbit heart. Biochem Cell Biol. 1999;77:559–567. [PubMed] [Google Scholar]
- 135.King LM, Opie LH. Does preconditioning act by glycogen depletion in the isolated rat heart? J Mol Cell Cardiol. 1996;28:2305–2321. doi: 10.1006/jmcc.1996.0224. [DOI] [PubMed] [Google Scholar]
- 136.Klingenberg M, Grebe K, Scherer B. Opposite effects of bongkrekic acid and atractyloside on the adenine nucleotides induced mitochondrial volume changes and on the efflux of adenine nucleotides. FEBS Lett. 1971;16:253–256. doi: 10.1016/0014-5793(71)80363-0. [DOI] [PubMed] [Google Scholar]
- 137.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koretsune Y, Marban E. Cell calcium in the pathophysiology of ventricular fibrillation and in the pathogenesis of postarrhythmic contractile dysfunction. Circulation. 1989;80:369–379. doi: 10.1161/01.cir.80.2.369. [DOI] [PubMed] [Google Scholar]
- 139.Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A(2b)-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007 doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Le Quoc K, Le Quoc D. Involvement of the ADP/ATP carrier in calcium-induced perturbations of the mitochondrial inner membrane permeability: importance of the orientation of the nucleotide binding site. Arch Biochem Biophys. 1988;265:249–257. doi: 10.1016/0003-9861(88)90125-7. [DOI] [PubMed] [Google Scholar]
- 141.Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H456–H463. doi: 10.1152/ajpheart.00777.2002. [DOI] [PubMed] [Google Scholar]
- 142.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 143.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 144.Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR. The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. The Journal of physiology. 1996;496(Pt 1):111–128. doi: 10.1113/jphysiol.1996.sp021669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 146.Li X, Heinzel FR, Boengler K, Schulz R, Heusch G. Role of connexin 43 in ischemic preconditioning does not involve intercellular communication through gap junctions. J Mol Cell Cardiol. 2004;36:161–163. doi: 10.1016/j.yjmcc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 147.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: The essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 150.Liu Y, Gao WD, O'Rourke B, Marban E. Synergistic modulation of ATP-sensitive K+ currents by protein kinase C and adenosine. Implications for ischemic preconditioning. Circ Res. 1996;78:443–454. doi: 10.1161/01.res.78.3.443. [DOI] [PubMed] [Google Scholar]
- 151.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 152.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 153.Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem. 1999;274:6272–6279. doi: 10.1074/jbc.274.10.6272. [DOI] [PubMed] [Google Scholar]
- 154.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 155.Marban E, Kitakaze M, Chacko VP, Pike MM. Ca2+ transients in perfused hearts revealed by gated 19F NMR spectroscopy. Circ Res. 1988;63:673–678. doi: 10.1161/01.res.63.3.673. [DOI] [PubMed] [Google Scholar]
- 156.Martin C, Schulz R, Post H, Gres P, Heusch G. Effect of NO synthase inhibition on myocardial metabolism during moderate ischemia. Am J Physiol Heart Circ Physiol. 2003;284:H2320–H2324. doi: 10.1152/ajpheart.01122.2002. [DOI] [PubMed] [Google Scholar]
- 157.Matsumoto-Ida M, Akao M, Takeda T, Kato M, Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation. 2006;114:1497–1503. doi: 10.1161/CIRCULATIONAHA.106.628834. [DOI] [PubMed] [Google Scholar]
- 158.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H1923–H1935. doi: 10.1152/ajpheart.00935.2003. [DOI] [PubMed] [Google Scholar]
- 159.Miki T, Cohen MV, Downey JM. Failure of N-2-mercaptopropionyl glycine to reduce myocardial infarction after 3 days of reperfusion in rabbits. Basic Res Cardiol. 1999;94:180–187. doi: 10.1007/s003950050141. [DOI] [PubMed] [Google Scholar]
- 160.Milano G, Morel S, Bonny C, Samaja M, von Segesser LK, Nicod P, Vassalli G. A peptide inhibitor of c-Jun NH2-terminal kinase reduces myocardial ischemia-reperfusion injury and infarct size in vivo. Am J Physiol Heart Circ Physiol. 2007;292:H1828–H1835. doi: 10.1152/ajpheart.01117.2006. [DOI] [PubMed] [Google Scholar]
- 161.Miura T, Goto M, Urabe K, Endoh A, Shimamoto K, Iimura O. Does myocardial stunning contribute to infarct size limitation by ischemic preconditioning? Circulation. 1991;84:2504–2512. doi: 10.1161/01.cir.84.6.2504. [DOI] [PubMed] [Google Scholar]
- 162.Miyamae M, Camacho SA, Weiner MW, Figueredo VM. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. Am J Physiol. 1996;271:H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- 163.Miyata H, Lakatta EG, Stern MD, Silverman HS. Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res. 1992;71:605–613. doi: 10.1161/01.res.71.3.605. [DOI] [PubMed] [Google Scholar]
- 164.Mocanu MM, Baxter GF, Yellon DM. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br J Pharmacol. 2000;130:197–200. doi: 10.1038/sj.bjp.0703336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol. 2002;34:661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- 166.Mocanu MM, Yellon DM. p53 down-regulation: a new molecular mechanism involved in ischaemic preconditioning. FEBS Lett. 2003;555:302–306. doi: 10.1016/s0014-5793(03)01260-2. [DOI] [PubMed] [Google Scholar]
- 167.Mocanu MM, Yellon DM. PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury? Br J Pharmacol. 2007;150:833–838. doi: 10.1038/sj.bjp.0707155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 169.Morrison RR, Tan XL, Ledent C, Mustafa SJ, Hofmann PA. Targeted Deletion of A2A Adenosine Receptors Attenuates the Protective Effects of Myocardial Postconditioning. AmJ Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00612.2007. [DOI] [PubMed] [Google Scholar]
- 170.Murata M, Akao M, O'Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 171.Murohara Y, Yui Y, Hattori R, Kawai C. Effects of superoxide dismutase on reperfusion arrhythmias and left ventricular function in patients undergoing thrombolysis for anterior wall acute myocardial infarction. Am J Cardiol. 1991;67:765–767. doi: 10.1016/0002-9149(91)90538-v. [DOI] [PubMed] [Google Scholar]
- 172.Murphy E, Cross H, Steenbergen C. Sodium regulation during ischemia versus reperfusion and its role in injury. Circ Res. 1999;84:1469–1470. doi: 10.1161/01.res.84.12.1469. [DOI] [PubMed] [Google Scholar]
- 173.Murphy E, Imahashi K, Steenbergen C. Bcl-2 regulation of mitochondrial energetics. Trends Cardiovasc Med. 2005;15:283–290. doi: 10.1016/j.tcm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 174.Murphy E, Perlman M, London RE, Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res. 1991;68:1250–1258. doi: 10.1161/01.res.68.5.1250. [DOI] [PubMed] [Google Scholar]
- 175.Murphy E, Steenbergen C. Inhibition of GSK-3beta as a target for cardioprotection: the importance of timing, location, duration and degree of inhibition. Expert Opin Ther Targets. 2005;9:447–456. doi: 10.1517/14728222.9.3.447. [DOI] [PubMed] [Google Scholar]
- 176.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 177.Murry CE, Richard VJ, Jennings RB, Reimer KA. Myocardial protection is lost before contractile function recovers from ischemic preconditioning. Am J Physiol. 1991;260:H796–H804. doi: 10.1152/ajpheart.1991.260.3.H796. [DOI] [PubMed] [Google Scholar]
- 178.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 179.Naga Prasad SV, Jayatilleke A, Madamanchi A, Rockman HA. Protein kinase activity of phosphoinositide 3-kinase regulates beta-adrenergic receptor endocytosis. Nat Cell Biol. 2005;7:785–796. doi: 10.1038/ncb1278. [DOI] [PubMed] [Google Scholar]
- 180.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 181.Nakamura Y, Miura T, Nakano A, Ichikawa Y, Yano T, Kobayashi H, Ikeda Y, Miki T, Shimamoto K. Role of microtubules in ischemic preconditioning against myocardial infarction. Cardiovasc Res. 2004;64:322–330. doi: 10.1016/j.cardiores.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 182.Nakano A, Baines CP, Kim SO, Pelech SL, Downey JM, Cohen MV, Critz SD. Ischemic preconditioning activates MAPKAPK2 in the isolated rabbit heart: evidence for involvement of p38 MAPK. Circ Res. 2000;86:144–151. doi: 10.1161/01.res.86.2.144. [DOI] [PubMed] [Google Scholar]
- 183.Namekata I, Shimada H, Kawanishi T, Tanaka H, Shigenobu K. Reduction by SEA0400 of myocardial ischemia-induced cytoplasmic and mitochondrial Ca2+ overload. Eur J Pharmacol. 2006;543:108–115. doi: 10.1016/j.ejphar.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 184.Naslund U, Haggmark S, Johansson G, Marklund SL, Reiz S, Oberg A. Superoxide dismutase and catalase reduce infarct size in a porcine myocardial occlusion-reperfusion model. J Mol Cell Cardiol. 1986;18:1077–1084. doi: 10.1016/s0022-2828(86)80294-2. [DOI] [PubMed] [Google Scholar]
- 185.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 186.Neely JR, Grotyohann LW. Role of glycolytic products in damage to ischemic myocardium. Dissociation of adenosine triphosphate levels and recovery of function of reperfused ischemic hearts. Circ Res. 1984;55:816–824. doi: 10.1161/01.res.55.6.816. [DOI] [PubMed] [Google Scholar]
- 187.Nejima J, Knight DR, Fallon JT, Uemura N, Manders WT, Canfield DR, Cohen MV, Vatner SF. Superoxide dismutase reduces reperfusion arrhythmias but fails to salvage regional function or myocardium at risk in conscious dogs. Circulation. 1989;79:143–153. doi: 10.1161/01.cir.79.1.143. [DOI] [PubMed] [Google Scholar]
- 188.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ooiwa H, Stanley A, Felaneous-Bylund AC, Wilborn W, Downey JM. Superoxide dismutase conjugated to polyethylene glycol fails to limit myocardial infarct size after 30 min ischemia followed by 72 h of reperfusion in the rabbit. J Mol Cell Cardiol. 1991;23:119–125. doi: 10.1016/0022-2828(91)90099-8. [DOI] [PubMed] [Google Scholar]
- 191.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 192.Philipp S, Critz SD, Cui L, Solodushko V, Cohen MV, Downey JM. Localizing extracellular signal-regulated kinase (ERK) in pharmacological preconditioning's trigger pathway. Basic Res Cardiol. 2006;101:159–167. doi: 10.1007/s00395-005-0566-z. [DOI] [PubMed] [Google Scholar]
- 193.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 194.Ping P, Takano H, Zhang J, Tang XL, Qiu Y, Li RC, Banerjee S, Dawn B, Balafonova Z, Bolli R. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res. 1999;84:587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- 195.Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, Qiu Y, Manchikalapudi S, Auchampach JA, Black RG, Bolli R. PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol. 1999;276:H1468–H1481. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- 196.Piper HM, Abdallah Y, Schafer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365–371. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 197.Piper HM, Kasseckert S, Abdallah Y. The sarcoplasmic reticulum as the primary target of reperfusion protection. Cardiovasc Res. 2006;70:170–173. doi: 10.1016/j.cardiores.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 198.Piper HM, Meuter K, Schafer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S644–S648. doi: 10.1016/s0003-4975(02)04686-6. [DOI] [PubMed] [Google Scholar]
- 199.Qin F, Yan C, Patel R, Liu W, Dong E. Vitamins C and E attenuate apoptosis, beta-adrenergic receptor desensitization, and sarcoplasmic reticular Ca2+ ATPase downregulation after myocardial infarction. Free Radic Biol Med. 2006;40:1827–1842. doi: 10.1016/j.freeradbiomed.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 200.Recchia FA, Osorio JC, Chandler MP, Xu X, Panchal AR, Lopaschuk GD, Hintze TH, Stanley WC. Reduced synthesis of NO causes marked alterations in myocardial substrate metabolism in conscious dogs. Am J Physiol Endocrinol Metab. 2002;282:E197–E206. doi: 10.1152/ajpendo.2002.282.1.E197. [DOI] [PubMed] [Google Scholar]
- 201.Reid EA, Kristo G, Yoshimura Y, Ballard-Croft C, Keith BJ, Mentzer RM, Jr., Lasley RD. In vivo adenosine receptor preconditioning reduces myocardial infarct size via subcellular ERK signaling. Am J Physiol Heart Circ Physiol. 2005;288:H2253–H2259. doi: 10.1152/ajpheart.01009.2004. [DOI] [PubMed] [Google Scholar]
- 202.Resjo S, Goransson O, Harndahl L, Zolnierowicz S, Manganiello V, Degerman E. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell Signal. 2002;14:231–238. doi: 10.1016/s0898-6568(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 203.Rizvi A, Tang XL, Qiu Y, Xuan YT, Takano H, Jadoon AK, Bolli R. Increased protein synthesis is necessary for the development of late preconditioning against myocardial stunning. Am J Physiol. 1999;277:H874–H884. doi: 10.1152/ajpheart.1999.277.3.H874. [DOI] [PubMed] [Google Scholar]
- 204.Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miro E, Totzeck A, Heusch G, Schulz R, Garcia-Dorado D. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ Res. 2006;99:93–101. doi: 10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- 205.Rouslin W. Protonic inhibition of the mitochondrial oligomycin-sensitive adenosine 5'-triphosphatase in ischemic and autolyzing cardiac muscle. Possible mechanism for the mitigation of ATP hydrolysis under nonenergizing conditions. J Biol Chem. 1983;258:9657–9661. [PubMed] [Google Scholar]
- 206.Rouslin W, Broge CW. IF1 function in situ in uncoupler-challenged ischemic rabbit, rat, and pigeon hearts. J Biol Chem. 1996;271:23638–23641. doi: 10.1074/jbc.271.39.23638. [DOI] [PubMed] [Google Scholar]
- 207.Rouslin W, Erickson JL, Solaro RJ. Effects of oligomycin and acidosis on rates of ATP depletion in ischemic heart muscle. Am J Physiol. 1986;250:H503–H508. doi: 10.1152/ajpheart.1986.250.3.H503. [DOI] [PubMed] [Google Scholar]
- 208.Ruiz-Meana M, Garcia-Dorado D, Miro-Casas E, Abellan A, Soler-Soler J. Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res. 2006;71:715–724. doi: 10.1016/j.cardiores.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 209.Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc Res. 2006;72:210–219. doi: 10.1016/j.cardiores.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 210.Samavati L, Monick MM, Sanlioglu S, Buettner GR, Oberley LW, Hunninghake GW. Mitochondrial K(ATP) channel openers activate the ERK kinase by an oxidant-dependent mechanism. Am J Physiol Cell Physiol. 2002;283:C273–C281. doi: 10.1152/ajpcell.00514.2001. [DOI] [PubMed] [Google Scholar]
- 211.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 212.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 213.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 214.Sasaki N, Sato T, Ohler A, O'Rourke B, Marban E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- 215.Sato M, Cordis GA, Maulik N, Das DK. SAPKs regulation of ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2000;279:H901–H907. doi: 10.1152/ajpheart.2000.279.3.H901. [DOI] [PubMed] [Google Scholar]
- 216.Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, Wang Y, Marber MS. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. Faseb J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 217.Schieke SM, Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387:1357–1361. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- 218.Schieke SM, Phillips D, McCoy JP, Jr., Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 219.Schneider S, Chen W, Hou J, Steenbergen C, Murphy E. Inhibition of p38 MAPK alpha/beta reduces ischemic injury and does not block protective effects of preconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H499–H508. doi: 10.1152/ajpheart.2001.280.2.H499. [DOI] [PubMed] [Google Scholar]
- 220.Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12:261–266. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- 221.Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740–H1742. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- 222.Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. Am J Physiol Heart Circ Physiol. 2006;290:H1011–H1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 223.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 224.Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005;289:H237–H242. doi: 10.1152/ajpheart.01192.2004. [DOI] [PubMed] [Google Scholar]
- 225.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao YM, Hou WM, Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006;98:111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 226.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 227.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 228.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 229.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 230.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 231.Shuter SL, Davies MJ, Garlick PB, Hearse DJ, Slater TF. Myocardial tissue preparation for ESR spectroscopy: some methods may cause artifactual generation of signals. Free Radic Res Commun. 1990;9:55–63. doi: 10.3109/10715769009148573. [DOI] [PubMed] [Google Scholar]
- 232.Soares PR, de Albuquerque CP, Chacko VP, Gerstenblith G, Weiss RG. Role of preischemic glycogen depletion in the improvement of postischemic metabolic and contractile recovery of ischemia-preconditioned rat hearts. Circulation. 1997;96:975–983. doi: 10.1161/01.cir.96.3.975. [DOI] [PubMed] [Google Scholar]
- 233.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- 234.Steenbergen C, Murphy E, Levy L, London RE. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987;60:700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- 235.Steenbergen C, Murphy E, Watts JA, London RE. Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circ Res. 1990;66:135–146. doi: 10.1161/01.res.66.1.135. [DOI] [PubMed] [Google Scholar]
- 236.Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res. 1993;72:112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- 237.Stein AB, Tang XL, Guo Y, Xuan YT, Dawn B, Bolli R. Delayed adaptation of the heart to stress: late preconditioning. Stroke. 2004;35:2676–2679. doi: 10.1161/01.STR.0000143220.21382.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stephanou A, Brar B, Liao Z, Scarabelli T, Knight RA, Latchman DS. Distinct initiator caspases are required for the induction of apoptosis in cardiac myocytes during ischaemia versus reperfusion injury. Cell Death Differ. 2001;8:434–435. doi: 10.1038/sj.cdd.4400846. [DOI] [PubMed] [Google Scholar]
- 239.Strohm C, Barancik M, von Bruehl M, Strniskova M, Ullmann C, Zimmermann R, Schaper W. Transcription inhibitor actinomycin-D abolishes the cardioprotective effect of ischemic reconditioning. Cardiovasc Res. 2002;55:602–618. doi: 10.1016/s0008-6363(02)00453-4. [DOI] [PubMed] [Google Scholar]
- 240.Strohm C, Barancik T, Bruhl ML, Kilian SA, Schaper W. Inhibition of the ER-kinase cascade by PD98059 and UO126 counteracts ischemic preconditioning in pig myocardium. J Cardiovasc Pharmacol. 2000;36:218–229. doi: 10.1097/00005344-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 241.Sugden PH, Clerk A. "Stress-responsive" mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res. 1998;83:345–352. doi: 10.1161/01.res.83.4.345. [DOI] [PubMed] [Google Scholar]
- 242.Sun HY, Wang NP, Halkos M, Kerendi F, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006;11:1583–1593. doi: 10.1007/s10495-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 243.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 244.Sun J, Steenbergen C, Murphy E. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 2006;8:1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(Pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330:206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- 247.Takagi H, Matsui Y, Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal. 2007;9:1373–1382. doi: 10.1089/ars.2007.1689. [DOI] [PubMed] [Google Scholar]
- 248.Tang XL, Sato H, Tiwari S, Dawn B, Bi Q, Li Q, Shirk G, Bolli R. Cardioprotection by postconditioning in conscious rats is limited to coronary occlusions <45 min. Am J Physiol Heart Circ Physiol. 2006;291:H2308–H2317. doi: 10.1152/ajpheart.00479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected] Proc Natl Acad Sci U S A. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Theroux P, Chaitman BR, Danchin N, Erhardt L, Meinertz T, Schroeder JS, Tognoni G, White HD, Willerson JT, Jessel A. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Guard during ischemia against necrosis (GUARDIAN) Investigators. Circulation. 2000;102:3032–3038. doi: 10.1161/01.cir.102.25.3032. [DOI] [PubMed] [Google Scholar]
- 251.Thornton J, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, Downey JM. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol. 1990;259:H1822–H1825. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- 252.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 253.Tong H, Chen W, London RE, Murphy E, Steenbergen C. Preconditioning enhanced glucose uptake is mediated by p38 MAP kinase not by phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:11981–11986. doi: 10.1074/jbc.275.16.11981. [DOI] [PubMed] [Google Scholar]
- 254.Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 255.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 256.Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res. 2004;94:1133–1141. doi: 10.1161/01.RES.0000126048.32383.6B. [DOI] [PubMed] [Google Scholar]
- 257.Toth A, Nickson P, Qin LL, Erhardt P. Differential regulation of cardiomyocyte survival and hypertrophy by MDM2, an E3 ubiquitin ligase. J Biol Chem. 2006;281:3679–3689. doi: 10.1074/jbc.M509630200. [DOI] [PubMed] [Google Scholar]
- 258.Tran TH, Andreka P, Rodrigues CO, Webster KA, Bishopric NH. Jun kinase delays caspase-9 activation by interaction with the apoptosome. J Biol Chem. 2007;282:20340–20350. doi: 10.1074/jbc.M702210200. [DOI] [PubMed] [Google Scholar]
- 259.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of "modified reperfusion" protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 260.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 261.Uraizee A, Reimer KA, Murry CE, Jennings RB. Failure of superoxide dismutase to limit size of myocardial infarction after 40 minutes of ischemia and 4 days of reperfusion in dogs. Circulation. 1987;75:1237–1248. doi: 10.1161/01.cir.75.6.1237. [DOI] [PubMed] [Google Scholar]
- 262.Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, Solaro RJ. p38-MAPK induced dephosphorylation of alpha-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res. 2007;100:408–415. doi: 10.1161/01.RES.0000258116.60404.ad. [DOI] [PubMed] [Google Scholar]
- 263.Vanden Hoek TL, Shao Z, Li C, Zak R, Schumacker PT, Becker LB. Reperfusion injury on cardiac myocytes after simulated ischemia. Am J Physiol. 1996;270:H1334–H1341. doi: 10.1152/ajpheart.1996.270.4.H1334. [DOI] [PubMed] [Google Scholar]
- 264.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;2006:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
- 265.Vander Heide RS, Delyani JA, Jennings RB, Reimer KA, Steenbergen C. Reducing lactate accumulation does not attenuate lethal ischemic injury in isolated perfused rat hearts. Am J Physiol. 1996;270:H38–H44. doi: 10.1152/ajpheart.1996.270.1.H38. [DOI] [PubMed] [Google Scholar]
- 266.Vander Heide RS, Hill ML, Reimer KA, Jennings RB. Effect of reversible ischemia on the activity of the mitochondrial ATPase: relationship to ischemic preconditioning. J Mol Cell Cardiol. 1996;28:103–112. doi: 10.1006/jmcc.1996.0010. [DOI] [PubMed] [Google Scholar]
- 267.Vanhaecke J, Van de Werf F, Ronaszeki A, Flameng W, Lesaffre E, De Geest H. Effect of superoxide dismutase on infarct size and postischemic recovery of myocardial contractility and metabolism in dogs. J Am Coll Cardiol. 1991;18:224–230. doi: 10.1016/s0735-1097(10)80243-8. [DOI] [PubMed] [Google Scholar]
- 268.Wain CM, Westwick J, Ward SG. Heterologous regulation of chemokine receptor signaling by the lipid phosphatase SHIP in lymphocytes. Cell Signal. 2005;17:1194–1202. doi: 10.1016/j.cellsig.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 269.Wang Y, Meyer JW, Ashraf M, Shull GE. Mice with a null mutation in the NHE1 Na+-H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ Res. 2003;93:776–782. doi: 10.1161/01.RES.0000094746.24774.DC. [DOI] [PubMed] [Google Scholar]
- 270.Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997;29:2383–2391. doi: 10.1006/jmcc.1997.0473. [DOI] [PubMed] [Google Scholar]
- 271.Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 2000;275:40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- 272.Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 273.Wolfe CL, Sievers RE, Visseren FL, Donnelly TJ. Loss of myocardial protection after preconditioning correlates with the time course of glycogen recovery within the preconditioned segment. Circulation. 1993;87:881–892. doi: 10.1161/01.cir.87.3.881. [DOI] [PubMed] [Google Scholar]
- 274.Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336(Pt 2):287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 276.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029-–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 277.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 279.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 280.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998;97:276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- 281.Yellon DM, Baxter GF. A "second window of protection" or delayed preconditioning phenomenon: future horizons for myocardial protection? J Mol Cell Cardiol. 1995;27:1023–1034. doi: 10.1016/0022-2828(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 282.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 283.Ylitalo KV, Ala-Rami A, Liimatta EV, Peuhkurinen KJ, Hassinen IE. Intracellular free calcium and mitochondrial membrane potential in ischemia/reperfusion and preconditioning. J Mol Cell Cardiol. 2000;32:1223–1238. doi: 10.1006/jmcc.2000.1157. [DOI] [PubMed] [Google Scholar]
- 284.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci U S A. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 286.Zatta AJ, Kin H, Lee G, Wang N, Jiang R, Lust R, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Vinten-Johansen J. Infarct-sparing effect of myocardial postconditioning is dependent on protein kinase C signalling. Cardiovasc Res. 2006;70:315–324. doi: 10.1016/j.cardiores.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 287.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 288.Zhu L, Yu Y, Chua BH, Ho YS, Kuo TH. Regulation of sodium-calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. J Mol Cell Cardiol. 2001;33:2135–2144. doi: 10.1006/jmcc.2001.1476. [DOI] [PubMed] [Google Scholar]
- 289.Zhu M, Feng J, Lucchinetti E, Fischer G, Xu L, Pedrazzini T, Schaub MC, Zaugg M. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 290.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 291.Zucchi R, Ronca F, Ronca-Testoni S. Modulation of sarcoplasmic reticulum function: a new strategy in cardioprotection? Pharmacol Ther. 2001;89:47–65. doi: 10.1016/s0163-7258(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 292.Zuurbier CJ, Eerbeek O, Meijer AJ. Ischemic preconditioning, insulin, and morphine all cause hexokinase redistribution. Am J Physiol Heart Circ Physiol. 2005;289:H496–H499. doi: 10.1152/ajpheart.01182.2004. [DOI] [PubMed] [Google Scholar]
- 293.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- 294.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]