Abstract
Background
Low 25 hydroxyvitamin D (25[OH]D) levels are common among patients with non-dialysis-dependent chronic kidney disease (CKD). The associations between low 25(OH)D levels and mortality among non-dialysis dependent CKD patients are unclear.
Study Design
Retrospective cohort study.
Setting & Participants
Patients with stages 3–4 CKD (estimated glomerular filtration rate of 15–59 ml/min/1.73m2) (n = 12,673) who had 25(OH)D levels measured after the confirmation of CKD in Cleveland Clinic Health System.
Predictor
Levels of 25(OH)D categorized into 3 groups: 25(OH)D <15 ng/ml, 15–29 ng/ml. and ≥ 30 ng/ml
Outcomes
We examined factors associated with low 25(OH)D levels and associations between low 25(OH)D levels and all-cause mortality (ascertained using the Social Security Death Index and our electronic medical record) using logistic regression, Cox-proportional hazard models and Kaplan-Meier survival curves.
Measurements
25(OH)D was measured using chemiluminescence immunoassay.
Results
Out of 12,763 CKD patients, 15% (n=1970) had 25(OH)D <15 ng/ml while 45% (n=5749) had 25(OH)D 15–29 ng/ml. Male gender, African American race, diabetes, coronary artery disease and lower eGFR were significantly associated with 25(OH)D <30 ng/ml. A graded increase in the risk for 25(OH)D <30 ng/ml was evident across increasing BMI levels. Patients who had 25(OH)D levels measured in Fall through Spring had higher odds for 25(OH)D <30 ng/ml. After covariate adjustment, CKD patients with 25(OH)D <15 ng/ml had a 33% increased risk for mortality (95% CI, 1.07–1.65). The 25(OH)D 15–29 ng/ml group did not exhibit a significantly increased risk for mortality (HR, 1.03; 95% CI, 0.86–1.22) compared to patients with 25(OH)D ≥30 ng/ml.
Limitations
Single center observational study, lack of data on albuminuria and other markers of bone and mineral disorders, and attrition bias
Conclusions
25(OH)D <15 ng/ml was independently associated with all-cause mortality in non-dialysis dependent CKD.
Keywords: Chronic kidney disease, vitamin D deficiency, mortality, obesity
Low 25 hydroxyvitamin D (25[OH]D) levels are common in patients with chronic kidney disease (CKD) and the prevalence of this condition increases as kidney function becomes more severely reduced1, 2. With a growing CKD population, the scope of this public health problem is expected to escalate3. Several factors such as increasing age, African American race, and comorbid conditions like diabetes and hypertension have been consistently associated with low 25(OH)D levels in individuals with both dialysis dependent and non-dialysis dependent CKD4, 5. Analysis from NHANES III (the Third National Health and Nutrition Examination Survey) showed an inverse association between BMI and 25(OH)D levels in persons with CKD6 while a longitudinal study found no such associations7. While seasonal variations in 25(OH)D levels have been reported in the general population and in dialysis patients, they have not been studied systematically in non-dialysis dependent CKD8,9.
In the general population, low 25(OH)D levels are associated with cardiovascular risk factors, cardiovascular disease and all-cause mortality10, 11. Recently, a cross-sectional study with stages 4 and 5 CKD reported a negative association between serum levels of 25(OH)D and vascular calcification12. Evidence linking low 25(OH)D levels and cardiovascular risk factors in non-dialysis dependent CKD has been accumulating in the recent years13. In the NHANES analyses, a positive association was reported between low 25(OH)D levels and mortality among persons with CKD defined as either estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 or the presence of microalbuminuria6. Similarly, in a single-center study, Ravani et al reported independent associations between low 25(OH)D levels and all-cause mortality7. But, another community-based study did not identify an association between 25(OH)D levels and cardiovascular mortality among community dwelling adults14.
Thus, in the present study, we investigated the factors associated with low 25(OH)D levels and the association of low 25(OH)D levels and all-cause mortality among stage 3 and stage 4 CKD patients (eGFR of 15–59 ml/min/1.73 m2) followed in our health care system.
Methods
Electronic Health Record-based CKD registry
We conducted an analysis using our electronic health record-based CKD registry and followed STROBE guidelines in the conduct of this retrospective analyses. The development and validation of an electronic health record-based CKD registry at Cleveland Clinic has been described in detail elsewhere15; we provide a brief summary of relevance to this study.
Patients who met the following inclusion criteria as of January 1, 2005 were included in this CKD registry: patients who had at least one in-person encounter as an outpatient with a Cleveland Clinic health care provider and a) had two estimated glomerular filtration rate (eGFR) values <60 ml/min/1.73 m2 (using either the 4-variable Modification of Diet in Renal Disease [MDRD] Study equation or the CKD Epidemiology Collaboration [CKD-EPI] equation) more than 90 days apart and b) patients with International Classification of Diseases (ICD-9) diagnosis codes (used at least twice in an outpatient encounter) for CKD, polycystic kidney disease, glomerulonephritis, diabetic nephropathy, hypertensive nephrosclerosis and renovascular disease16. Patients aged <18 years old, and those who were already diagnosed with end stage renal disease (ESRD) needing renal replacement therapy were excluded. Patients who met the inclusion/exclusion criteria until March 2010 were included in this analysis.
Demographic information, comorbid conditions, prescribed medications, laboratory data, imaging, and clinical measures of CKD patients are included in the registry. Previously, we validated the ICD-9 codes used to identify the registry cohort and the comorbid conditions such as diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, congestive heart failure, and hyperlipidemia using pre-specified validation criteria. Additionally, the CKD registry has been linked with the Social Security Death Index to obtain details about mortality rates for the study cohort.
Definitions and outcome measures
Kidney function
For this study, we applied the CKD-EPI equation to patients who had two serum creatinine levels measured 90 days apart as of January 2005 and included patients who had two eGFR values between 15–59 ml/min/1.73 m2 more than 90 days apart corresponding to CKD stage 3 or 4 according to the current classification system. All creatinine measurements were performed by the modified kinetic Jaffe reaction, using a Hitachi 747–200 Chemistry Analyzer (1996 to 2001) or a Hitachi D 2400 Modular Chemistry Analyzer thereafter (Roche Diagnostics, Indianapolis, IN) in our laboratory.
25 hydroxyvitamin D
25(OH)D is measured by chemiluminescence immunoassay using Liaison automated platform (Diasorin Inc. Stillwatwer, MN) in our laboratory. Within- and between-run imprecision of 25(OH)D varied between 4–7% and 6–10% respectively. Only outpatient laboratory measures were included in this analysis. The first measured 25(OH)D values after the patient was included in the registry based on the eGFR criteria described above were used for this analysis.
Demographics, Comorbid conditions and laboratory parameters
Demographics, such as age, sex, race/ethnicity, were extracted from the electronic health record. Diabetes mellitus, hypertension, coronary artery disease and other comorbid conditions were defined using prespecified criteria and previously validated15. 25(OH)D levels and other relevant outpatient laboratory details (hemoglobin, serum phosphorus, albumin, and calcium levels) were obtained electronically from our laboratory records.
Mortality
The primary outcome of interest was all-cause mortality which was ascertained from linkage of our registry with the Social Security Death Index. Patients were followed from their date of inclusion in the registry until March 2010.
Statistical analysis
We first compared baseline characteristics between CKD patients with measured 25(OH)D levels and patients without measured 25(OH)D levels using Chi-square and t-tests for categorical and continuous variables respectively. CKD patients with a measured outpatient 25(OH)D were further classified into three groups by 25(OH)D level: <15 ng/ml, 15–29 ng/ml, and ≥30 ng/ml. Associations of the baseline characteristics in these three groups were assessed using Mantel-Haenszel Chi-square and ANOVA tests for categorical and continuous variables respectively. A logistic regression analysis model was conducted to assess the association between different BMI categories and the presence of 25(OH)D <30 ng/ml. We selected the variables that have shown prior associations with low vitamin D levels in non-dialysis dependent CKD for adjustment and tested for collinearity. As such, we adjusted for age, sex, race, BMI, eGFR, diabetes, hypertension, hyperlipidemia, malignancy, congestive heart failure, cerebrovascular disease, coronary artery disease, season of 25(OH)D testing, serum albumin and hemoglobin and year of entry into our registry. For the 12,427 patients with mortality data, data were missing for the following variables: hemoglobin (n=744; 6.0%), albumin (n=341; 2.7%), and BMI at CKD confirmation (n=404; 3.3%). We did not impute any data for these missing values.
To evaluate whether survival among persons with CKD was associated with 25(OH)D levels, we used Kaplan-Meier plots and log-rank tests. The inception time for these plots was the date when 25(OH)D was measured. We also used Cox proportional hazards models to assess the association between the 25(OH)D level and mortality while adjusting for the same covariates used in the logistic regression analysis. We generated the Cox models in two different ways, testing log 25(OH)D as a continuous variable, and classifying it into <15 ng/ml, 15–29 ng/ml and ≥30 ng/ml based on previously utilized clinical criteria. We tested all 2-way interactions between 25(OH)D level and each of the covariates included in the Cox proportional hazards model.
All data analyses were conducted using Unix SAS version 9.2 (SAS Institute, Cary, NC), and graphs were created using R 2.11.1 (The R Foundation for Statistical Computing, Vienna, Austria). The CKD registry and this study were approved by the Cleveland Clinic Institutional Review Board.
Results
Patient characteristics
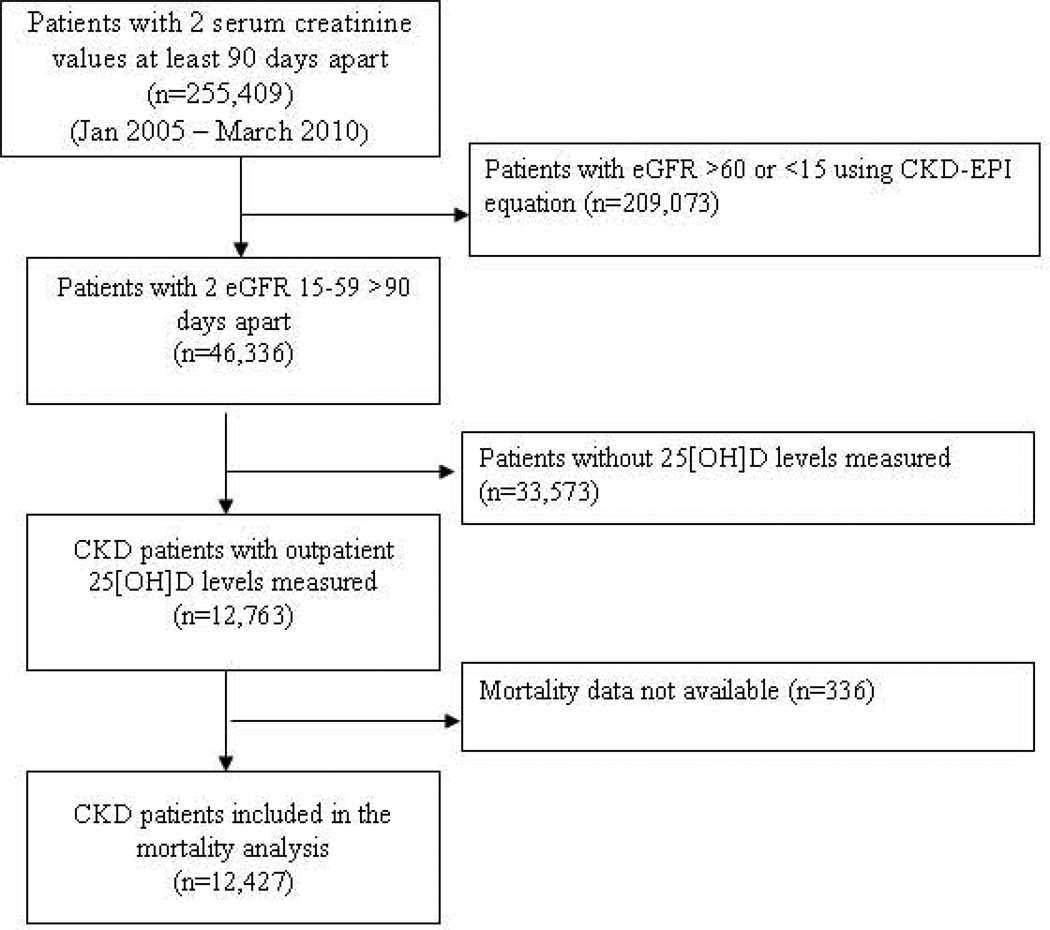
We identified patients from our registry with stage 3 and 4 CKD using the CKD-EPI equation (n=46,336). Of those, 12,763 (28%) had 25(OH)D levels measured after entry into the registry and constituted the study population (Figure 1). The mean age of the study cohort was 71.5 ± 11.7 years with 67% being females and 13% African Americans (Table 1). Patients who had 25(OH)D levels measured had more outpatient clinic visits, were more likely to be female, and have diabetes, hypertension, or hyperlipidemia compared to patients whose 25(OH)D levels were not measured (p<0.001).
Figure 1.
Flow chart of patients selection for analysis.
Table 1.
Characteristics of CKD patients by 25(OH)D measurement status
| Variable | 25(OH)D Not measured |
25(OH)D ≥30 ng/ml |
25(OH)D 15–29 ng/ml |
25(OH)D <15 ng/ml |
|---|---|---|---|---|
| No. | 33573 | 5044 | 5749 | 1970 |
| Age (y) | 72.9 +/− 11.8** | 72.3 +/− 11.1*** | 71.6 +/− 11.7*** | 69.3 +/− 12.9*** |
| eGFR (mL/min/1.73 m2) | 46.6 +/− 10.2** | 48.1 +/− 9.2*** | 47.5 +/− 9.8*** | 45.2 +/− 10.7*** |
| CKD stage | ||||
| 3a (eGFR = 45–59 ml/min/1.73 m2) | 21189 (63)* | 3547 (70)*** | 3953 (69)*** | 1159 (59)*** |
| 3b (eGFR = 30–44 ml/min/1.73 m2) | 9264 (28)* | 1220 (24)*** | 1359 (24)*** | 584 (30)*** |
| 4 (eGFR = 15–29 ml/min/1.73 m2) | 3120 (9)* | 277 (5)*** | 437 (8)*** | 227 (12)*** |
| No. of encounters in EHR | 160 +/− 127** | 233 +/− 161*** | 244 +/− 164*** | 267 +/− 182*** |
| Male gender, | 16726 (50)* | 1470 (29)*** | 2112 (37)*** | 660 (34)*** |
| African Americans, | 3867 (12)* | 415 (8)*** | 670 (12)*** | 556 (28)*** |
| Diabetes, | 6435 (19)* | 986 (20)*** | 1626 (28)*** | 777 (39)*** |
| Hypertension, | 26428 (79)* | 4569 (91)*** | 5270 (92)*** | 1844 (94)*** |
| Coronary artery disease, | 6833 (20)* | 877 (17)*** | 1190 (20)*** | 398 (20)*** |
| Cerebrovascular disease, | 2765 (8)* | 463 (9) | 534 (9) | 221 (11) |
| Congestive heart failure, | 2802 (8)* | 283 (6)*** | 338 (6)*** | 171 (8)*** |
| Malignancy, | 7835 (23)* | 989 (20) | 1098 (19) | 314 (16) |
| Hyperlipidemia, | 24447 (73)* | 4146 (82) | 4752 (83) | 1594 (81) |
| BMI Category | ||||
| <18.5 kg/m2 | 423 (1)* | 75 (1)*** | 42 (1)*** | 28 (1)*** |
| 18.5–24.9 kg/m2 | 7627 (23)* | 1495 (30)*** | 1152 (20)*** | 314 (16)*** |
| 25–29.9 kg/m2 | 11021 (33)* | 1875 (37)*** | 2026 (35)*** | 569 (29)*** |
| 30–34.9 kg/m2 | 6603 (20)* | 873 (17)*** | 1349 (23)*** | 453 (23)*** |
| 35–39.9 kg/m2 | 2787 (8)* | 348 (7)*** | 606 (11)*** | 280 (14)*** |
| ≥40 kg/m2 | 1866 (6)* | 193 (4)*** | 389 (7)*** | 265 (13)*** |
| Missing | 3246 (10)* | 185 (4)*** | 185 (3)*** | 61 (3)*** |
| Albumin (g/dl) | 4.21 +/− 0.36*** | 4.16 +/− 0.38*** | 4.05 +/− 0.47*** | |
| Hemoglobin (g/dl) | 13.0 +/− 1.5*** | 12.9 +/− 1.7*** | 12.4 +/− 1.7*** | |
| Phosphorus (mg/dl) | 3.58 +/− 0.68*** | 3.60 +/− 0.73*** | 3.71 +/− 0.91*** |
Note: 25(OH)D measurement status based on tests performed after CKD was confirmed. Continuous variables given as mean +/− SD; categorical variables as number (percentage). Conversion factors for units: albumin and hemoglobin in g/dL to g/L, ×10; phosphorus in mg/dL to mmol/L, ×0.3229.
Chi-square P≤0.01
t-test P≤0.01 comparing patients with 25(OH)D measured vs all those not measured.
P<0.05 for differences among 3 measured 25(OH)D groups with. Mantel-Haenszel Chi-square used to assess binary and ordered categorical variables against 3 ordered levels of vitamin D and ANOVA used for continuous variable.
Abbreviations: BMI, body mass index; CKD: chronic kidney disease, 25(OH)D: 25-hydroxy vitamin D, EHR, electronic health record; eGFR: estimated glomerular filtration rate
There were significant differences in demographics and comorbid conditions between patients with 25(OH)D levels ≥30 ng/ml, 15–29 ng/ml, and <15 ng/ml (Table 1). Patients with lower 25(OH)D levels were younger, more likely to be African American, have diabetes, and have a higher BMI than those with normal levels.
Factors associated with low 25(OH)D levels
A logistic regression model was conducted to evaluate independent factors associated with low 25(OH)D levels. In the multivariable analysis, males had 32% higher likelihood than females (95% CI, 1.22–1.44), African Americans had an 81% higher likelihood than Caucasians (95% CI, 1.60–2.05) and diabetics had 43% higher likelihood than non-diabetics (95% CI, 1.30–1.57) to have 25(OH)D <30 ng/ml (Table 2). Higher eGFR and the presence of hyperlipidemia were associated with lower odds of having 25(OH)D <30 ng/ml. Being tested in fall, winter or spring (compared to summer) was associated with higher odds of 25(OH)D <30 ng/ml. A graded increase in the risk of 25(OH)D <30 ng/ml with increasing BMI levels was noted.
Table 2.
Independent associations between low 25(OH)D level and clinically relevant covariates
| Variable | 25(OH)D <30 ng/ml |
|---|---|
| Age per 10-year increase | 0.98 (0.95–1.02) |
| Male gender | 1.32 (1.22–1.44) |
| African American race | 1.81 (1.60–2.05) |
| eGFR (per 10 ml/min/1.73 m2 increase) | 0.94 (0.90–0.97) |
| Diabetes | 1.43 (1.30–1.57) |
| Hypertension | 1.10 (0.96–1.25) |
| Hyperlipidemia | 0.89 (0.81–0.98) |
| Coronary artery disease | 1.13 (1.02–1.25) |
| Congestive heart failure | 0.92 (0.79–1.09) |
| Season of vitamin D testing | |
| Fall vs. summer | 1.15 (1.04–1.27) |
| Winter vs. summer | 1.24 (1.12–1.38) |
| Spring vs. summer | 1.39 (1.25–1.55) |
| BMI | |
| <18.5 kg/m2 vs. 18.5–24.9 kg/m2 | 0.96 (0.68–1.36) |
| 25–29.9 kg/m2 vs. 18.5–24.9 kg/m2 | 1.31 (1.19–1.44) |
| 30–34.9 kg/m2 vs. 18.5–24.9 kg/m2 | 1.90 (1.70–2.12) |
| 35–39.9 kg/m2 vs. 18.5–24.9 kg/m2 | 2.31 (1.99–2.68) |
| ≥40 kg/m2 vs. 18.5–24.9 kg/m2 | 2.99 (2.48–3.59) |
Note: Analysis pertains to associations after CKD confirmation. Values shown are odds ratio (95% confidence interval). Model was additionally adjusted for the year of inclusion in the registry;
Abbreviations: 25(OH)D: 25-hydroxy vitamin D, eGFR: estimated glomerular filtration rate, BMI: body mass index
25(OH)D levels and all-cause mortality
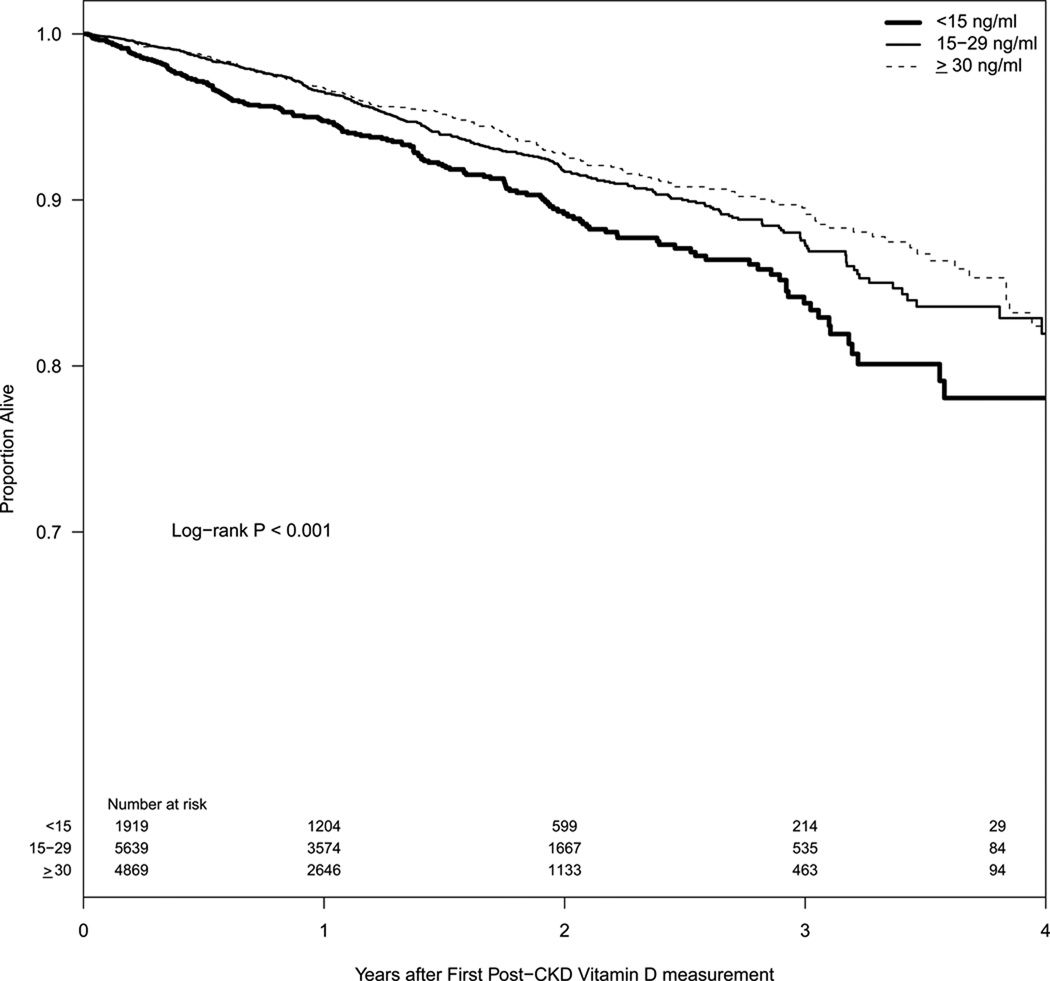
Among our study population, 97% (n=12,427) had mortality data and 767 of them died during an average follow-up of 1.4 years (median of 1.2 years). The Kaplan-Meier analysis showed significant differences in all-cause mortality for CKD patients in the different 25(OH)D groups (Log-rank p<0.001, Figure 2). Log-rank pair-wise comparisons indicated that the 25(OH)D <15 ng/ml group had significantly different mortality from the other 2 groups (p < 0.001 for both) after adjustment for multiple comparisons. There was no statistically significant difference between the group with 25(OH)D 15–29 ng/ml and that with 25(OH)D ≥30 ng/ml for all-cause mortality (p = 0.2). To examine the effect of unequal follow-up time for patients who entered the CKD registry earlier vs. later, we ran two different Kaplan-Meier plots that included only patients with at least 6 months and 1 year of follow-up, respectively. The proportion of the 3 groups of 25(OH)D did vary across year of entry into our registry (data not shown), but there was no meaningful difference between the Kaplan Meier survival curves for patients who had 6 and 12 month follow up and our original survival curve that included all patients (Figure 2) and the qualitative conclusions of the statistical analysis remained consistent.
Figure 2.
Kaplan-Meier survival curve based on 25(OH)D levels among CKD patients
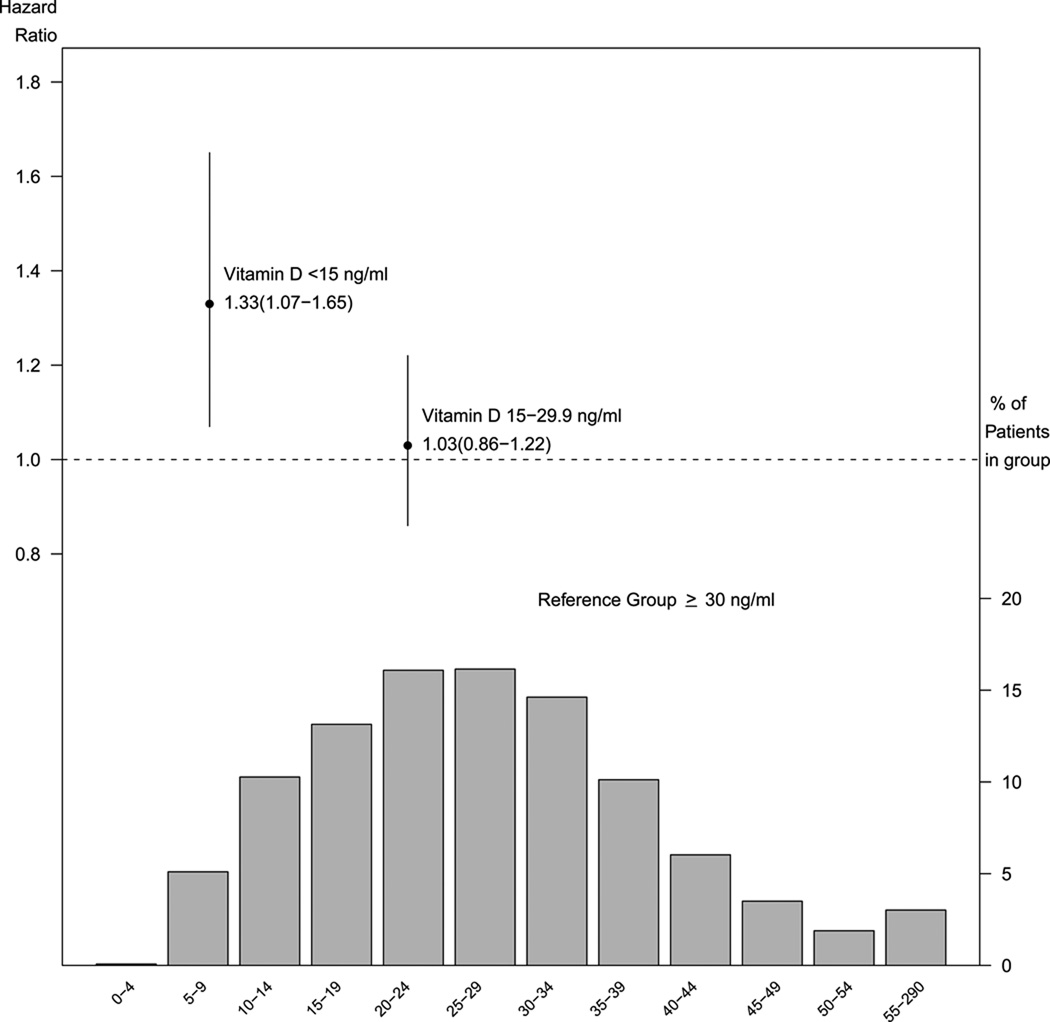
In the unadjusted Cox proportional hazards model, there was a 53% increased risk for mortality with 25(OH)D <15 ng/ml compared to 25(OH)D ≥30 ng/ml while the 25(OH)D 15–29 ng/ml group was not significantly different from 25(OH)D ≥30 ng/ml. When adjusted for age, sex, race, BMI, eGFR, diabetes, hypertension, hyperlipidemia, malignancy, congestive heart failure, cerebrovascular disease, coronary artery disease, season of 25(OH)D testing, serum albumin and hemoglobin, the hazard ratio for all-cause mortality for 25(OH)D < 15 ng/ml vs. 25(OH)D ≥30 ng/ml was 1.33 (95% CI, 1.07–1.65)(Figure 3). The 25(OH)D 15–29 ng/ml group did not have a significantly different hazard for mortality from the 25(OH)D ≥30 ng/ml group (HR, 1.03; 95% CI, 0.86–1.22).
Figure 3.
Proportion of patients with 25(OH)D levels ≥ 30 ng/ml, 15–29 ng/ml and <15 ng/ml and the hazard ratios for mortality for these subgroups.
None of the 2-way interactions with 25(OH)D were significant, except for the interaction between 25(OH)D and hemoglobin (p = 0.05). This interaction indicated that the protective effect of hemoglobin was evident among patients with 25(OH)D 15 ng/ml and above but no longer significant for the 25(OH)D <15 ng/ml group. In both the unadjusted and adjusted Cox models using continuous log 25(OH)D, each unit increase in 25(OH)D levels was associated with reduced risk for mortality (HR, 0.82; 95% CI, 0.70–0.96). None of the 2-way interactions with log 25(OH)D included in the model were significant.
Other than 25(OH)D <15 ng/ml, older age, male gender, BMI <18.5 kg/m2 (vs. normal), malignancy, congestive heart failure, and coronary artery disease were significantly associated with a higher risk for mortality; In contrast, higher BMI levels (vs. normal range), higher eGFR, hyperlipidemia, higher albumin and higher hemoglobin were associated with a lower risk for mortality (data not shown). Race, diabetes, hypertension, cerebrovascular disease and season of vitamin D testing were not significantly associated with increased mortality. In our study population, 41% (n=5,122) also had serum phosphorus levels measured. In this sub sample, serum phosphorus was not associated with mortality in either the unadjusted (HR, 0.97; 95% CI, 0.85–1.10; p = 0.6) or adjusted models (HR, 0.98; 95% CI, 0.87–1.10; p = 0.7).
Discussion
Among stage 3 and 4 CKD patients, our study found a positive association between male gender, African American race, diabetes, coronary artery disease, higher BMI levels (>25 kg/m2) and the presence of 25(OH)D <30 ng/ml. Patients who had 25(OH)D levels measured from fall through spring had a higher risk for lower 25(OH)D levels when compared to those who had it measured in summer. Increasing age and kidney function were negatively associated with 25(OH)D <30 ng/ml. 25(OH)D <15 ng/ml was associated with a 34% higher risk for all-cause mortality after adjusting for demographic factors, comorbid conditions, season of 25(OH)D measurement, kidney function and other potential confounding laboratory parameters. 25(OH)D 15–29 ng/ml was not independently associated with mortality.
Associations between race, diabetes and hypertension and 25(OH)D deficiency have been previously documented in CKD4, 5, 17. Obesity is associated with low 25(OH)D levels in the general population even after adjusting for factors such as low physical activity and low vitamin D intake18. We noted an association between increasing BMI (>25 kg/m2) and low 25(OH)D levels. Sequestration of vitamin D in adipocytes and increased conversion of 25(OH)D to 1,25 dihydroxyvitamin D were proposed to account for the higher 25(OH)D deficiency seen in obese non-CKD patients19, 20. While the former might be applicable to the CKD population and might explain our results, the latter would be of less relevance in CKD. More importantly, for clinical practice, these findings imply that CKD patients with higher BMI might require a higher dose for a longer period of time to correct 25(OH)D deficiency21. Further prospective studies are needed to address this question.
Seasonal variations of 25(OH)D levels have been reported in both general population and dialysis patients8, 9, 22. In general, low 25(OH)D levels have been reported in winter months in previous studies and most studies have classified these into summer and winter months. In vivo and in vitro studies have shown that the lack of or reduced sun exposure in winter months result in impaired Vitamin D synthesis23. We classified patients who had 25(OH)D levels measured into 4 different seasons and noted that patients who had it measured during the spring had the highest risk for vitamin D deficiency. This may be due to the fact that spring measurement of 25(OH)D levels might reflect their lack of sun exposure in winter months.
25(OH)D deficiency is associated with mortality in CKD and non-CKD populations. Various mechanistic links have been proposed to explain these associations24–26. These include suppression of the renin-angiotensin-aldosterone system, cardiac myocyte hypertrophy, vascular calcification, and anti-inflammatory actions with vitamin D supplementation. An analysis using NHANES III data that included both patients with low eGFR and albuminuria, of which 70% of their study participants had stage 1 and stage 2 CKD, revealed a similar increased risk for all-cause mortality with 25(OH)D <15 ng/ml6. Another single center study reported a higher risk for progression of kidney disease and death with low 25(OH)D levels in patients with stage 2–5 CKD7.
In the NHANES analysis, the risk for death was higher in some subgroups compared to the other (for instance, females had higher risk than males and hypertensives had higher risk than non-hypertensives). However, we did not find any significant interaction in our analysis that would suggest potential differences between various sub-groups. Similar to the NHANES analysis, our study did not find an association between all-cause mortality and 25(OH)D 15–29 ng/ml6. A recent study reported a lack of association between 25(OH)D levels and cardiovascular mortality14. However, we could not examine this because data relating to exact cause of death (cardiovascular deaths vs. others) were not available.
Our study has significant strengths. This analysis has a large number of stage 3 and 4 CKD patients, compared to the prior studies, followed in a large health care system with a significant proportion of African American patients. Further, we were able to adjust for relevant confounding variables such as the season of measurement which were not included in the previous studies. Strengths of our registry also include the prior validation of the included comorbid conditions using standard definitions in the literature.
Apart from the inherent bias of an observational study, there are important limitations to point out. We included patients with eGFR <60 ml/min/1.73 m2; however, patients with an earlier stage of CKD (eGFR ≥60 ml/min/1.73 m2 with albuminuria and other structural abnormalities) were not included. Thus, our findings may not be applicable to patients with less severe forms of kidney disease. This analysis also suffers from attrition bias as we included only patients who had 25(OH)D levels measured. We adjusted for several potential confounding variables in this analysis but did not have consistently reliable data relating to albuminuria and mineral and bone disorder parameters, independent predictors of mortality in this population, and for which we could have seen different results. The overarching caveat that should be applied to our retrospective study is that despite the strong associations between mortality and low 25(OH)D levels in this cohort of CKD patients, no causal relationship was demonstrated. Demonstration of causality will require prospective interventions with 25(OH)D supplementation to show an effect on mortality27. However, given the consistent significant and strong association noted in this and prior studies suggest the urgent need for such clinical studies.
We cannot exclude the reverse causation in this analyses i.e., individuals who are near death may develop lower 25(OH)D levels due to less sun exposure and poor nutrition. Furthermore, we did not have sufficient information on Vitamin D supplementation or repletion in this cohort. Females were overrepresented, suggesting a greater awareness of bone disease and 25(OH)D in this population, especially with a higher incidence of osteoporosis and thus greater utilization of 25(OH)D testing. Despite CKD and low 25(OH)D being widely prevalent, it is surprising that only 28% of CKD patients had their 25(OH)D levels measured and highlights the need for measuring 25(OH)D levels in stage 3 and 4 CKD patients. We did not have provider specific data to assess whether primary care physicians differed from nephrologists in ordering these tests.
In summary, various demographic factors, comorbid conditions and timing of 25(OH)D measurement are associated with 25(OH)D <30 ng/ml. 25(OH)D <15 ng/ml is independently associated with all-cause mortality among patients with CKD. Future studies should explore the mechanistic links that might explain the observed associations and whether vitamin D supplementation could reduce mortality rates among patients with stage 3 and stage 4 CKD.
Acknowledgements
Part of the work in this manuscript will be presented as a poster at the World Congress of Nephrology, Vancouer, Canada on April 10 2011.
The authors wish to thank Welf Saupe, and John Sharp of Cleveland Clinic who aided in data extraction during the development of the registry.
Support: Dr Navaneethan is supported by grant RR024990 from the National Institutes of Health (NIH) National Center for Research Resources Multidisciplinary Clinical Research Career Development program. Dr Jolly is supported by NIH Diversity Supplement 3U01HL064244-10S1. The creation of the registry was funded by an unrestricted grant from Amgen, Inc to the Department of Nephrology and Hypertension Research and Education fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(4):1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Ishimura E, Nishizawa Y, Inaba M, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999;55(3):1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 5.National Kidney Foundation KDOQI Clinical Practice Guidelines Committee. NKF/KDOQI clinical practice guidelines on bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4) Suppl 3:S1–S201. [PubMed]
- 6.Mehrotra R, Kermah DA, Salusky IB, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the united states. Kidney Int. 2009;76(9):977–983. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 8.Bhan I, Burnett-Bowie SA, Ye J, Tonelli M, Thadhani R. Clinical measures identify vitamin D deficiency in dialysis. Clin J Am Soc Nephrol. 2010;5(3):460–467. doi: 10.2215/CJN.06440909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolouian R, Rao DS, Goggins M, Bhat S, Gupta A. Seasonal variation of vitamin D in patients on hemodialysis. Clin Nephrol. 2010;74(1):19–24. doi: 10.5414/cnp74019. [DOI] [PubMed] [Google Scholar]
- 10.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsayed EF, Tighiouart H, Griffith J, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167(11):1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Canton C, Bosch E, Ramirez A, et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant. 2011 Jul;26(7):2250–2256. doi: 10.1093/ndt/gfq650. Epub 2010 Oct 18. [DOI] [PubMed] [Google Scholar]
- 13.Petchey WG, Howden EJ, Johnson DW, Hawley CM, Marwick T, Isbel NM. Cardiorespiratory fitness is independently associated with 25-hydroxyvitamin D in chronic kidney disease. Clin J Am Soc Nephrol. 2011 Mar;6(3):512–518. doi: 10.2215/CJN.06880810. Epub 2010 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jassal SK, Chonchol M, von Muhlen D, Smits G, Barrett-Connor E. Vitamin d, parathyroid hormone, and cardiovascular mortality in older adults: The rancho bernardo study. Am J Med. 2010;123(12):1114–1120. doi: 10.1016/j.amjmed.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navaneethan SD, Jolly SE, Schold JD, et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6(1):40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakova T, Gutierrez OM, Patel NM, Andress DL, Wolf M, Levin A. Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: Complex interactions. J Ren Nutr. 2011 Jul;21(4):295–302. doi: 10.1053/j.jrn.2010.07.002. Epub 210 Sep 3. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Rodriguez E, Navia B, Lopez-Sobaler AM, Ortega RM. Vitamin D in overweight/obese women and its relationship with dietetic and anthropometric variables. Obesity (Silver Spring) 2009;17(4):778–782. doi: 10.1038/oby.2008.649. [DOI] [PubMed] [Google Scholar]
- 19.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 20.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 21.Lee P, Greenfield JR, Seibel MJ, Eisman JA, Center JR. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med. 2009;122(11):1056–1060. doi: 10.1016/j.amjmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Hypponen E, Power C. Hypovitaminosis D in british adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 23.Thieden E, Philipsen PA, Heydenreich J, Wulf HC. Vitamin D level in summer and winter related to measured UVR exposure and behavior. Photochem Photobiol. 2009;85(6):1480–1484. doi: 10.1111/j.1751-1097.2009.00612.x. [DOI] [PubMed] [Google Scholar]
- 24.Zehnder D, Quinkler M, Eardley KS, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74(10):1343–1353. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 26.Chonchol M, Scragg R. 25-hydroxyvitamin D, insulin resistance, and kidney function in the third national health and nutrition examination survey. Kidney Int. 2007;71(2):134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 27.Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6(1):50–62. doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]