Abstract
Diabetes and Alzheimer disease (AD)—two age-related diseases—are both increasing in prevalence, and numerous studies have demonstrated that patients with diabetes have an increased risk of developing AD compared with healthy individuals. The underlying biological mechanisms that link the development of diabetes with AD are not fully understood. Abnormal protein processing, abnormalities in insulin signaling, dysregulated glucose metabolism, oxidative stress, the formation of advanced glycation end products, and the activation of inflammatory pathways are features common to both diseases. Hypercholesterolemia is another factor that has received attention, owing to its potential association with diabetes and AD. This Review summarizes the mechanistic pathways that might link diabetes and AD. An understanding of this complex interaction is necessary for the development of novel drug therapies and lifestyle guidelines aimed at the treatment and/or prevention of these diseases.
Introduction
The population of the developed world is aging, and the incidence of age-related metabolic and neurodegenerative diseases is increasing. In the US, diabetes and Alzheimer disease (AD)—two high-morbidity age-related diseases—affect ≈23.6 and ≈5.3 million people, respectively. These figures are projected to rise considerably. The Centers for Disease Control and Prevention predict that >29 million people in the US will be affected by diabetes by 2050, while the Alzheimer’s Association forecasts that by this date, 11–16 million Americans will have AD. Numerous studies report that patients with diabetes have an increased risk of developing AD compared with healthy individuals.1,2 In fact, a study of the Mayo Clinic Alzheimer Disease Patient Registry revealed that 80% of patients with AD exhibited either impairments in glucose tolerance or frank diabetes.3
Diabetes is a complex metabolic disorder characterized by hyperglycemia and associated with microvascular and macrovascular complications, including retinopathy, nephropathy, neuropathy and cardiovascular disease.4 An association between diabetes and these complications is well established, although the impact of diabetes on the CNS—particularly in relation to cognitive dysfunction—is not understood in detail. This interaction has, however, received increasing attention over the past decade. 5–10% of patients with diabetes in the US have type 1 diabetes, which is associated with hyperglycemia and insulin deficiency. The severity of cognitive dysfunction experienced by individuals with this type of diabetes is affected by age of onset of diabetes, degree of glycemic control, and duration of diabetes.1 Type 2 diabetes is the most common form of this disease, accounting for ≈90–95% of cases of diabetes in the US, and is characterized by hyperinsulinemia and insulin resistance. Obesity, hypertension, hypercholesterolemia and hyperlipidemia are all associated with type 2 diabetes. Early cognitive changes in learning and memory, mental flexibility and mental speed are also associated with this form of the disease (Box 1).5,6
Box 1. Cognitive processes affected by diabetes.
Type 1 diabetes
Information processing
Psychomotor efficiency
Attention
Visuoconstruction
Memory
Visual-motor skills
Visual-spatial skills
Motor speed
Vocabulary
General intelligence
Motor strength
Type 2 diabetes
Psychomotor speed
Frontal lobe and executive function
Attention
Verbal fluency
Memory
Processing speed
Complex psychomotor function
AD accounts for 50–70% of all dementia cases and is characterized by cognitive deficits7 as well as several neuropathological markers, which include extracellular senile plaques and intracellular neurofibrillary tangles (NFTs). Familial AD is a rare form of dementia and is caused by autosomal dominant mutations in one or more of the genes encoding the amyloid precursor protein (APP), presenilin 1 or presenilin 2 (the latter two proteins form the catalytic core of γ-secretase).8 By contrast, late-onset AD might be caused by environmental and/or lifestyle factors.9 Interestingly, late-onset AD is characterized not only by the neuropathological markers mentioned above, but also by vascular lesions, and hyperglycemia, hyperinsulinemia, insulin resistance, glucose intolerance, adiposity, atherosclerosis and hypertension are all independent risk factors for AD.10
Owing to the profound socioeconomic impact of diabetes and AD, an understanding of the mechanisms that might link these two diseases together is imperative. Oxidative stress, the formation of advanced glycation end products (AGEs), and impairments in CNS insulin signaling11–14 are all associated with diabetes and AD. Furthermore, dysregulation of cellular processes—such as glucose metabolism, apolipoprotein E (ApoE) processing, cholesterol metabolism, mitochondrial activity, calcium homeostasis, and second messenger signaling—is thought to contribute to both diseases.3,14–16 This Review discusses the potential biological mechanisms that could underlie how diabetes might accelerate the progression of AD, and highlights possible points of intervention that future therapies could exploit to prevent or delay the progression of AD in patients with this metabolic condition.
Epidemiological studies
Multiple population-based studies have shown that patients with diabetes exhibit an increased risk of developing AD compared with nondiabetic individuals matched for age and sex;1–3 however, other studies have failed to find a link between these two conditions.17–19 The reason why some studies but not others have identified such a link might reflect differences in study participants (in terms of age, ethnicity and sex) and/or study designs between investigations. In studies that indicated a positive association between the two diseases, obesity, dyslipidemia, and high blood pressure were all identified as potential risk factors for both diabetes and AD.3,15 An analysis of nine high-quality studies demonstrated that individuals with probable type 2 diabetes have nearly a twofold higher risk of AD than individuals without diabetes (Table 1).2
Table 1.
Diabetes increases the risk of developing Alzheimer disease
| Reference | Patients (patients with diabetes/total number of patients) | Relative risk* |
|---|---|---|
| Ott et al. (1999)138 | 692/6,370 | 1.9 (95% CI 1.2–3.1) |
| Brayne et al. (1998)139 | 25/376‡ | OR 1.4 (95% CI 1.1–17.0) |
| Yoshitake et al. (1995)140 | 70/828 | 2.2 (95% CI 1.0–4.9) |
| Peila et al. (2002)98 | 900/2,574‡ | 1.7 (95% CI 1.0–2.8) |
| MacKnight et al. (2002)19 | 503/5,574‡ | 1.2 (95% CI 0.8–1.8) |
| Xu et al. (2004)141 | 114/1,301 | HR 1.3 (95% CI 0.8–1.9) |
| Leibson et al. (1997)142 | 1455/75,000‡ | SMR 1.6 (95% CI 1.3–2.0) |
| Luchsinger et al. (2005)143 | 231/1,138‡ | HR 2.4 (95% CI 1.8–3.2) |
| Arvanitakis et al. (2004)144 | 27/824‡ | HR 1.7 (95% CI 1.1–2.5) |
Patients with probable type 2 diabetes have nearly a twofold higher risk of AD than individuals without diabetes.
Relative risk unless otherwise stated.
Data represents number of patients at follow-up, all other data represent patient numbers at baseline.
Abbreviations: HR, hazard ratio; OR, odds ratio; SMR, standard morbidity ratio.
Diabetes and progression of AD
Protein processing
Many neurodegenerative disorders are characterized by abnormal protein processing, and two prominent pathological features of AD—both of which result from abnormal protein processing—are amyloid-β (Aβ) plaques and NFTs. Aβ is a 39–42 amino acid peptide generated by the proteolytic cleavage of APP by β-secretase and γ-secretase.20 Compared with shorter variants of Aβ, the 42 amino acid form of this peptide—Aβ42—has an increased propensity to aggregate, and accumulates as extracellular amyloid deposits; that is, senile plaques.21,22 As mentioned above, genetic analyses have shown that mutations in one or more of the genes encoding APP, presenilin 1 or presenilin 2 cause some cases of familial AD; however, the familial form of this disease is rare, and ≈90% of known AD cases are sporadic.9,10 The amyloid cascade hypothesis states that the neurodegeneration associated with AD is caused by the processing of APP via the amyloidogenic pathway,20,22 and now the production of senile plaques is widely considered to affect neuronal activity by impairment of synaptic function and induction of cell death.23,24 However, impaired synaptic function might precede the formation of plaques.25 The accumulation of Aβ-derived diffusible ligands (ADDL), which are soluble Aβ oligomers, has been shown to lead to functional deficits before plaque formation.26 As impaired insulin signaling, which is associated with type 2 diabetes is known to affect the expression and metabolism of Aβ,4 diabetes might, therefore, exacerbate the production of Aβ, synaptic impairment and neuronal cell death in patients with AD.
A major component of NFTs is hyperphosphorylated tau.27 Tau is a soluble microtubule-associated protein that is expressed in mature neurons and is localized within axons, and maintains the stability of neuronal microtubules. In AD, tau is abnormally phosphorylated and aggregates in cell bodies and proximal dendrites.28 Cleaved tau is detected in the brains of patients with AD as well as in neurons treated in vitro with Aβ.29–31 Cleaved tau has been shown to induce apoptosis of cortical neurons in vitro,30 and when expressed in transgenic animals, the cleaved form of this protein is associated with a reduction in spatial memory.32 Several proteases, including caspases (proteases associated with the proteasome) and calpains, are implicated in tau cleavage.33
In mouse models of diabetes, increased tau phosphorylation is evident in animals with either type 1 or type 2 diabetes compared with control wild-type mice.34 Tau phosphorylation and cleavage is considerably more pronounced in mice with type 2 diabetes than in mice with the type 1 form of this disease,34,35 although the change in phosphorylation status in the latter positively correlates with impairments in learning and memory.34,36–38 Considering that an increase in tau phosphorylation is observed in postmortem brain samples from patients with type 2 diabetes, patients with this form of the disease might also experience cognitive deficits as a result of dysfunctional glucose metabolism.39
Glycosylation of O-linked β-N-acetylglucosamine (GlcNAcylation) is a normally occurring post-translational protein modification. Studies show a reduction in GlcNAcylation under diabetic conditions, indicating that GlcNAcylation might attenuate abnormal tau phosphorylation and decrease tau-induced neuronal death.40
Insulin and glycogen synthase kinase
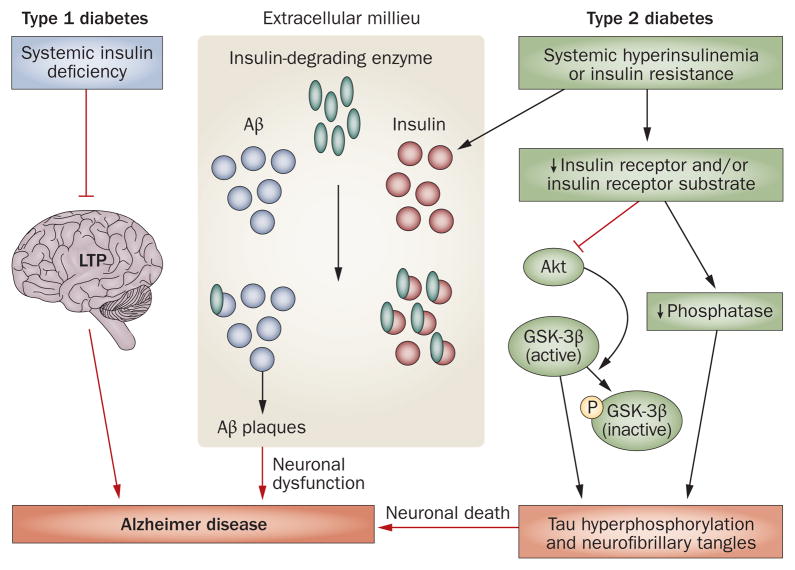
Insulin deficiency associated with type 1 diabetes contributes to the cognitive deficits observed in patients with this form of the disease.1 Furthermore, both spatial learning and hippocampal long-term potentiation (LTP) are attenuated following induction of experimental type 1 diabetes in rats with a single intravenous injection of streptozotocin.1,41 These changes in spatial learning and LTP can be prevented by insulin treatment (Figure 1).41
Figure 1.
Altered insulin signaling in diabetes might contribute to Alzheimer disease pathophysiology. In type 1 diabetes, insulin deficiency attenuates LTP and might lead to deficits in spatial learning and memory. In type 2 diabetes, insulin resistance leads to both Aβ plaque formation and tau hyperphosphorylation. During hyperinsulinemia, insulin and Aβ compete for insulin-degrading enzyme, leading to Aβ accumulation and plaque formation. A decrease in insulin receptor signaling leads to inhibition of Akt and dephosphorylation (activation) of GSK-3β, and results in tau hyperphosphorylation. Abbreviations: Aβ, amyloid-β; GSK-3β, glycogen synthase kinase 3β; LTP, long-term potentiation; P, phosphate.
Type 2 diabetes is also associated with cognitive impairment.42 As mentioned above, this form of diabetes is characterized by insulin resistance, hyperinsulinemia and impaired insulin signaling. Insulin receptors are expressed throughout the CNS; however, the function of these receptors in the brain is not fully understood. Insulin receptors might be involved in the regulation of synaptic activity and, hence, might affect cognitive processes.43 Neurodegeneration and cognitive impairment in type 2 diabetes and AD could be caused, in part, by impairments in insulin receptor signaling.44 In fact, decreases in the sensitivity of such receptors are known to affect the expression and metabolism of Aβ and tau,4 and impaired insulin receptor activity and hyperinsulinemia are observed in patients with AD and in animal models of this disease.45 In addition, dysfunction of insulin receptor signaling is associated with impairments in ADDL clearance.46
Insulin-degrading enzyme (IDE) is required for both insulin and Aβ degradation in neurons and microglia. Elevated insulin levels in type 2 diabetes induce Aβ accumulation through competition between insulin and Aβ for IDE.47 Insulin and insulin receptor densities are, however, decreased in patients with AD compared with age-matched healthy controls.12,45 Nevertheless, insulin sensitizers might increase insulin signaling and decrease the levels of insulin available to compete with Aβ for degradation by IDE. Treatment of mice with AD-like symptoms with an insulin sensitizer reduces Aβ42 levels and improves memory.48 Although a larger study is needed to confirm the findings, patients with early AD who were treated with the insulin sensitizer rosiglitazone failed to demonstrate a decline in Aβ42 levels, despite demonstrating better cognitive performances during treatment than before treatment.49
Insulin regulates tau phosphorylation in vitro50,51 and in vivo,52,53 and increases the rate of NFT development. In fact, insulin transiently increases tau phosphorylation in primary cortical neurons,51 and hyperinsulinemia results in tau hyperphosphorylation in rat brains.54 Furthermore, insulin receptor substrate 2 knockout mice demonstrate typical pathological signs of type 2 diabetes and have an increased number of NFTs in hippocampal neurons compared with control wild-type mice.53 Thus, impaired insulin signaling could increase tau phosphorylation and cleavage.34
Insulin receptor signaling leads to the activation of two major signaling pathways, the mitogen-activated protein kinase (MAPK) pathway and the Akt signaling pathway. MAPK signaling is a required component of cell differentiation, cell proliferation and cell death,55 whereas Akt signaling is involved in the regulation of cell growth, cell proliferation, protein synthesis (via the mammalian target of rapamycin signaling pathway) and cell survival (through the inhibition of several pro-apoptotic agents).56,57 Both the MAPK and Akt pathways are implicated in AD pathogenesis. MAPK expression is increased in the brains of patients with AD compared with healthy individuals. Moreover, MAPK immunoreactivity is greater in postmortem brain samples from patients with AD than in such samples from healthy controls.58 The expression of this protein kinase is positively associated with Aβ plaques and NFTs. Indeed, MAPK co-localizes with NFTs in hippocampal and cortical regions in AD brains.58,59 Studies indicate that MAPK signaling is involved in neuroinflammation, tau phosphorylation and synaptic plasticity.59 For example, in transgenic mice with hyperphosphorylated tau, aggregated tau co-localizes with MAPK.60
Akt signaling induces the inhibition of glycogen synthase kinase-3β (GSK-3β)44,61,62 (Figure 1), which phosphorylates and, hence, inactivates glycogen synthase, a key enzyme in glycogenesis. Thus, under normal conditions, insulin signaling via the insulin receptor leads to GSK-3β inactivation, whereas insulin resistance leads to GSK-3β dephosphorylation and activation.36,61 The regulation of GSK-3β in the hippocampus and cortex changes in response to changes in glucose and insulin concentrations,63 and in type 2 diabetes an increase in GSK-3β activity might lead to insulin resistance by reducing glucose clearance.62 Increased GSK-3β activation might also lead to an elevation in Aβ production (resulting from a GSK-3β-mediated increase in presenilin 1 activity)64 and an increase in tau phosphorylation associated with NFT formation (Figure 1).61 By contrast, inhibition of GSK-3β attenuates APP processing and inhibits hyperphosphorylated tau-associated neurodegeneration in cell-culture and animal models of AD.64,65
Abnormal glucose metabolism
Glucose metabolism and insulin signaling are important for normal brain function. Imaging studies have revealed that patients with AD and individuals at risk of developing this disease typically have reductions in glucose metabolism in temporal and parietal brain regions.66 Of note, reductions in glucose metabolism have been observed in the hippocampus of patients with AD.67 Moreover, compared with healthy individuals, patients with AD might also have increased fasting plasma insulin levels and/or a decreased cerebrospinal fluid (CSF)-to-plasma insulin ratio. Intravenous administration of insulin—while maintaining normal blood glucose levels—or glucose facilitates cognitive functioning in patients with AD and in healthy older adults.68 This finding indicates that normal glucose metabolism is required for the performance of cognitive functions, and that impairments in glucose metabolism might contribute to cognitive dysfunction. The negative effect of impaired glucose metabolism on cognitive functioning might be caused, in part, by the formation of AGEs, an increase in oxidative stress and, subsequently, an increase in local inflammation within the brain.
Advanced glycation end products
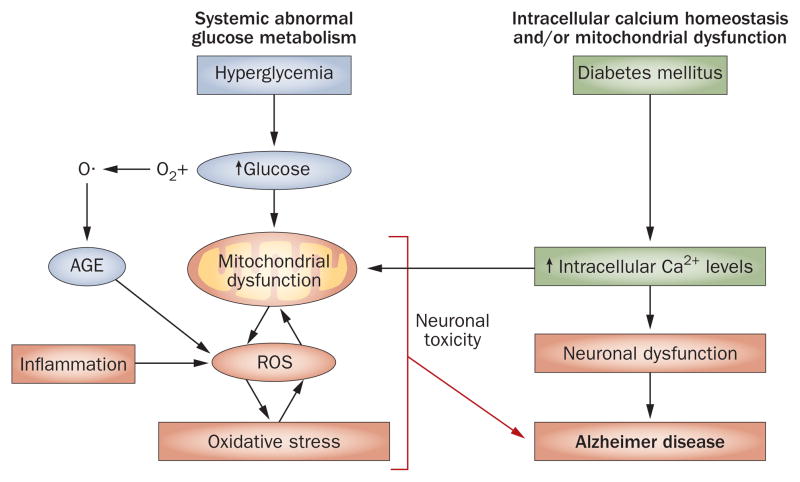
Hyperglycemia can lead to a number of pathophysiological processes, including oxidative stress and AGE formation that can cause brain damage.69 In fact, both abnormal glucose metabolism and oxidative stress contribute to the formation of AGEs. These substances comprise a heterogeneous group of molecules formed by irreversible, non-enzymatic reactions between sugars and the free amino groups of proteins, lipids and nucleic acids. Auto-oxidation of glucose leads to the formation of oxygen radicals, which are intermediates in the AGE pathway and the predominate source of endogenous AGEs (Figure 2).70 The formation and accumulation of AGEs occurs during normal aging; however, these processes are exacerbated in patients with diabetes,71,72 and the binding of AGE to its receptor (receptor for AGEs or RAGE) induces a series of biological processes that cause further diabetic complications.71
Figure 2.
Pathological mechanisms associated with diabetes might cause AD. Mitochondrial dysfunction, oxidative stress and dysregulated calcium homeostasis are all associated with diabetes and might be contributory factors to the development of AD. Glucose auto-oxidation can lead to AGE formation and, as a result, oxidative stress, which is associated with mitochondrial dysfunction. Oxidative stress combined with an increase in intracellular calcium result in a feedforward cycle of continued mitochondrial damage that can cause neuronal death and, hence contribute to AD pathology. Abbreviations: AD, Alzheimer disease; AGE, advanced glycation end product; ROS, reactive oxygen species.
AGE immunoreactivity is present in both Aβ plaques and NFTs in patients with AD.73 Furthermore, hippocampal neurons from patients with this neurodegenerative disease contain Aβ-positive, AGE-positive and RAGE-positive granules.73,74 Whether the modification of Aβ and tau by AGEs is a primary or secondary event in AD is a controversial topic. Nevertheless, AGEs are widely accepted to be active participants in the progression of AD, since AGE-induced glycation of Aβ and tau protein has been shown to cause Aβ aggregation and the formation of NFTs, respectively.75 Moreover, diabetic mice with cognitive impairments exhibit increased RAGE expression in neurons and glia compared with wild-type control mice,76 and in one clinical study, AGE immunostaining was increased in postmortem brain slices from patients with AD and diabetes compared with nondiabetic patients with AD.77 Another study has failed, however, to detect a difference in AGE immunostaining in NFTs and senile plaques between patients with diabetes and age-matched control individuals.78
Oxidative stress
Abnormal glucose metabolism can increase the production of free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS). This overproduction of free radicals can exhaust a cell’s antioxidant capacity and lead to a condition known as oxidative stress, which is a hallmark of both type 1 and type 2 diabetes and a contributing factor to diabetic neuropathy.79,80 ROS-induced and RNS-induced protein and/or lipid peroxidation result in cell damage that can lead to cell death, and are increased in patients with diabetes or AD compared with healthy controls.81,82 Brain and CSF levels of lipid peroxidation biomarkers—including malondialdehyde and 4-hydroxynonenal—are higher in individuals with AD than in healthy people.11 Furthermore, levels of oxidized proteins are increased in the frontal and parietal lobes and in the hippocampus of patients with mild cognitive impairment compared with healthy controls, indicating that oxidative damage might occur early in the development of AD.83
Oxidative stress and lipid peroxidation seem to be able to induce Aβ accumulation: studies in a mouse model of AD have demonstrated that brain lipid peroxidation increases before Aβ levels increase,84 and that the onset of Aβ deposition is associated with an increase in the level of RNS.85 Further evidence supporting this hypothesis has been obtained from studies of a mouse model of AD in which mutations in the genes encoding APP and pre-senilin 1 cause an elevation in Aβ42 production. In these animals, lipid and protein peroxidation are evident at disease onset.86 In another transgenic animal model of AD, in which mice develop Aβ plaques, NFTs and cognitive defects, a decrease in antioxidant capacity and an increase in lipid peroxidation were noted before the development of AD pathology.87 Oxidative stress seems to affect APP either directly, by increasing APP levels, or indirectly, by modulating APP processing, and both mechanisms could increase levels of Aβ. Studies in transgenic mice and postmortem brain tissue from patients with AD also suggest that an increase in Aβ production leads to a rise in the production of ROS. Evidence indicates that insertion of Aβ into the mitochondrial membrane can disrupt the mitochondrial electron transport chain,88 markedly attenuating cellular energy production and increasing the formation of ROS and, thus, driving a feedforward process involving ROS and Aβ. This feedforward process might also result from microglia activation by Aβ protofibrils: these fibrils can induce a robust inflammatory response and the local release of neurotoxins or neurotoxic cytokines from activated microglia.89
Oxidative stress increases both the activation of inflammatory pathways (Figure 2)11 and the release of inflammatory mediators such as C-reactive protein and interleukin 6 into the circulation.90 Local inflammation initiated by activated microglia and reactive astrocytes surrounding extracellular Aβ plaques can lead to activation of the complement cascade and neuronal cell damage.91 Cytokines and chemokines, including interleukins, tumor necrosis factor and macrophage inflammatory protein 1α, are all upregulated in patients with AD.91 Furthermore, transgenic mice exhibiting Aβ plaques and cognitive defects have higher levels of cytokines and chemokines than have wild-type control animals.92 Observational epidemiological studies have reported that anti-inflammatory drugs are associated with a decreased risk of AD;93 however, experimental trials in which patients with AD were treated with anti-inflammatory drugs failed to demonstrate any beneficial effects of taking these agents.94,95
Cholesterol, APOE and metabolic syndrome
Dyslipidemia and hypercholesterolemia both contribute to the pathology of diabetes and are also independent risk factors for AD. Apolipoprotein E (APOE), which is expressed predominantly in the liver and brain, participates in the transport of cholesterol and lipoproteins within the circulatory system and can, therefore, markedly affect blood lipid levels. In fact, humans with an APOE deficiency exhibit an increase in the level of plasma cholesterol,96 and ApoE knockout mice have been shown to have elevated blood cholesterol levels compared with wild-type control mice, even when the knockout mice have been on a strict low-fat, low-cholesterol diet.97 Four alleles of APOE exist. In the general population, 77% of individuals have an APOE ε3 allele (the most common APOE variant), while 15% possess an ε4 variant. By contrast, 40% of patients with AD possess an ε4 allele, and individuals with one ε4 allele are 3–4-fold more likely to develop AD than individuals who are not ε4 carriers.
The risk of AD associated with the APOE ε4 allele might be exacerbated by diabetes, as patients with diabetes who are ε4 carriers are twofold more likely to develop AD than individuals who harbor the ε4 allele but are not diabetic.98 Results from one study have indicated that diabetes only increases the risk of AD in individuals who have the ε4 allele;18 however, other investigations have failed to demonstrate a correlation between APOE ε4 status, diabetes and AD.99,100
How APOE4 carrier status might confer an increased risk of developing AD is unclear. APOE has, however, been implicated in the clearance of Aβ,101 and APOE ε4 and Aβ aggregates have been shown to synergistically induce neurodegeneration in the brains of mice with AD-like symptoms.102 Evidence indicates that APOE ε4 also affects the processing of tau: for example, overexpression of APOE ε4 in transgenic mice seems to promote neuronal tau phosphorylation.103 APOE receptors, specifically the low-density lipoprotein receptor (LDLR)-related protein, are known to interact with APP, increasing its endocytic trafficking and amyloidogenic processing.104
As highlighted above, hypercholesterolemia is associated with an increased risk of type 2 diabetes, and patients with this form of the disease have an increased risk of cognitive decline compared with healthy individuals.105 In fact, hypercholesterolemia is present in 70% of patients diagnosed with diabetes and 77% of patients with this condition who go undiagnosed.106 In an animal model of diabetes an accumulation of cholesterol within pancreatic β-cells has been shown to lead to a decrease in insulin secretion, β-cell mass, and a deterioration of diabetic symptoms.107 Hypercholesterolemia could increase Aβ production by increasing the expression of β-secretase and RAGE, which transports Aβ from the circulation to the brain, and by decreasing the expression of IDE and LDLR-related protein, both of which are involved in the clearance of Aβ from the brain to the circulation.108 Furthermore, cholesterol, cholesterol oxidase, and APOE have all been shown to co-localize with Aβ in fibrillar plaques in transgenic mouse models of AD,109 and cholesterol and oxidized cholesterol (oxysterols) have also been shown to accumulate in the dense core of Aβ plaques,110 indicating that cholesterol might be involved in the formation of senile plaques. In the brain, cholesterol is oxidized to 24-hydroxycholesterol (24-HC) by 24-hydroxylase, and 24-HC levels in plasma and CSF reflect neuronal cholesterol synthesis. In addition, 27-HC is one of the main oxysterols found in the circulation, and the ratio of 24-HC and 27-HC is altered in AD.111 In fact, the level of 27-HC in the brain increases in this neurodegenerative disease111 and this rise might, subsequently, elevate the production of Aβ.112 Thus, statins that cross the blood–brain barrier could reduce the risk of AD,113 owing to their ability to reduce the levels of neuronal cholesterol, tau phosphorylation and amyloid formation.114,115
Mitochondrial dysfunction
Diabetes and AD are associated with deficits in mitochondrial activity.16 Mitochondria are essential for ATP synthesis and for maintaining calcium homeostasis, which is required for normal neuronal function.116 The calcium hypothesis states that dysregulation of calcium homeostasis is a central process in normal aging of the brain and in age-related diseases.117,118 Excessive calcium uptake by mitochondria leads to an increase in ROS production, inhibition of ATP synthesis, release of cytochrome c, and a sudden increase in inner membrane permeability termed mitochondrial permeability transition.119 Mitochondrial dysfunction triggers neuronal degeneration and cell death, and is thought to contribute to AD pathophysiology. The role mitochondrial dysfunction has in AD is not fully understood, but APP is known to be associated with the outer mitochondrial membrane,120 and β-secretase and Aβ, which inhibits cytochrome oxidase in the presence of copper, are present in mitochondria, indicating that Aβ could negatively affect mitochondrial electron transport.121 Indeed, dysfunction of mitochondrial electron transport proteins and a decrease in cytochrome oxidase activity are both associated with AD.122 Furthermore, neurons affected by AD pathology exhibit an overall decrease in mitochondrial mass, an increase in cytoplasmic mitochondrial DNA, and an increase in cytochrome oxidase in lipofuscin-containing vacuoles.123
Dysregulation of calcium homeostasis seems to occur as a result of a decrease in mitochondrial function. Brain tissue from patients with AD shows an increase in the concentration of calcium, and intracellular calcium levels are higher in neurons containing NFTs than in neurons from healthy control patients.124 Furthermore, levels of calcium-dependent proteases are increased in neurons containing NFTs compared with neurons without NFTs,125 and neurons that are vulnerable to degeneration exhibit an increase in the levels of calcium–calmodulin-dependent protein kinase II.126 Transglutaminase, a calcium-activated enzyme that induces crosslinking of tau molecules, is also increased in patients with AD compared with controls,127 and cells exposed to agents that induce calcium influx show elevations in Aβ production.128 Thus, an increase in levels of intracellular calcium might be involved in enhancement of APP processing and Aβ production.
Type 1 and type 2 diabetes are associated with an increase in intracellular calcium levels (Figure 2).129 Abnormal calcium homeostasis is common in patients with diabetes and has been shown to occur in animal models of this disease.129 Hormonal regulation of intracellular calcium might also be abnormal in diabetes. In type 2 diabetes, high levels of intracellular calcium in pancreatic β-cells and alterations in membrane cation pumps might contribute to impaired insulin secretion.130 Insulin deficiency is associated with calcium overload, which affects metabolic function by activating calcium-dependent protein kinases, phosphatases, proteases, phospholipases and lysosomal enzymes.131 In animal models of type 1 diabetes, neuronal mitochondria exhibit a decrease in antioxidant capacity owing to a low content of coenzyme Q9, which is a result of oxidative stress.132 Inherited defects in mitochondrial DNA are known to cause an insulin-deficient form of diabetes mellitus that resembles type 1 diabetes. In patients with type 2 diabetes, the activity of mitochondrial oxidative enzymes is lower than in age-matched controls; however, patients with this form of diabetes are obese, and obesity is associated with smaller mitochondria and reduced bioenergetic capacity than lean controls.133
Animal models of AD and diabetes
Several studies have demonstrated that the induction of diabetes in mouse models of AD leads to an acceleration of AD neuropathology. For example, the induction of type 1 diabetes—by means of an intraperitoneal injection of streptozotocin—in transgenic mice prone to tau pathology is associated with an increase in levels of hyperphosphorylated and insoluble tau.134 Furthermore, results from a study conducted by Jolivalt et al. indicate that hypoinsulinemia increases AD pathology in transgenic mice with AD and experimental type 1 diabetes. Rises in the level of Aβ42, the number of immunoreactive Aβ plaques, GSK-3β activity, and the degree of tau phosphorylation—all contributing factors to neurodegeneration and neuronal loss in AD—were evident in the transgenic mice after induction of experimental diabetes.135 By contrast, induction of type 2 diabetes did not increase Aβ levels in the brains of transgenic mice that develop AD pathology, although the development of diabetes did cause the early onset of cognitive dysfunction.136 Taken together, these results suggest that diabetes-associated cerebral amyloid angiopathy, but not Aβ accumulation, is responsible for cognitive dysfunction, at least in the early stages of the disease. Owing to the fact that the mice with experimental type 2 diabetes were leptin deficient, and that leptin is involved in synaptic function and affects cognition and behavior,137 the absence of leptin signaling could be responsible for the observed cognitive phenotype. More studies are needed to fully elucidate the link between diabetes and AD.
Conclusions
The work presented here highlights the overlap and the many of the points of intersection that exist between the molecular mechanisms underlying diabetes and AD, and indicates how diabetes could exacerbate AD pathology. Hyperglycemia and hypoglycemia in the CNS result in the dysregulation of multiple extracellular and intracellular signaling cascades, which in turn could lead to decreases in neuronal and synaptic function and, ultimately, to an increase in neuronal loss. An understanding of how each molecular pathway intersects and affects the others is essential for the development of future drug intervention strategies for AD.
Key points.
Alzheimer disease (AD) and diabetes are both associated with enormous and increasing socioeconomic effects
Diabetes affects the processing of amyloid-β and tau, and might increase the rate of formation of senile plaques and neurofibrillary tangles, the main neuropathological hallmarks of AD
Hyperinsulinemia is associated with amyloid-β accumulation and regulates tau phosphorylation
Oxidative stress activates inflammatory pathways and, hence, might exacerbate AD neuropathology
Mitochondrial dysfunction is associated with both diabetes and AD, and leads to intracellular calcium dysregulation and abnormal processing of the amyloid precursor protein
Induction of diabetes exacerbates AD neuropathology in mouse models of this neurodegenerative disease
Acknowledgments
The authors greatly appreciate the helpful discussion with Dr K. A. Sullivan (University of Michigan, Ann Arbor, MI, USA). This work was supported by an Aging Training Grant (NIA T32-AG000114), and grants from the Animal Models of Diabetic Complications Consortium (NIH U01-DK076160), the Taubman Institute and the Program for Neurology Research and Discovery.
Footnotes
Competing Interests
The authors declare no competing interests.
Author contributions
C. Sims-Robinson researched the data for the article, provided substantial contributions to discussions of the content, and contributed to the writing, reviewing and editing of the manuscript. B. Kim researched the data for the article and contributed to the writing, reviewing and editing of the manuscript. A. Rosko researched the data for the article and contributed to the writing of the manuscript. E. L. Feldman provided substantial contributions to discussions of the content and contributed to the reviewing and editing of the manuscript.
References
- 1.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 2.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 3.Janson J, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- 6.Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- 7.Gilman S. Alzheimer’s disease. Perspect Biol Med. 1997;40:230–245. doi: 10.1353/pbm.1997.0020. [DOI] [PubMed] [Google Scholar]
- 8.Gotz J, Schild A, Hoerndli F, Pennanen L. Amyloid-induced neurofibrillary tangle formation in Alzheimer’s disease: insight from transgenic mouse and tissue-culture models. Int J Dev Neurosci. 2004;22:453–465. doi: 10.1016/j.ijdevneu.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Rocchi A, Pellegrini S, Siciliano G, Murri L. Causative and susceptibility genes for Alzheimer’s disease: a review. Brain Res Bull. 2003;61:1–24. doi: 10.1016/s0361-9230(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 10.Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pract Neurol. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 11.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyer S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm. 1998;105:415–422. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 13.Nelson TJ, Alkon DL. Insulin and cholesterol pathways in neuronal function, memory and neurodegeneration. Biochem Soc Trans. 2005;33:1033–1036. doi: 10.1042/BST20051033. [DOI] [PubMed] [Google Scholar]
- 14.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Martins IJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 16.Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J Neurol Sci. 2007;257:206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Nielson KA, et al. Apolipoprotein-E genotyping of diabetic dementia patients: is diabetes rare in Alzheimer’s disease? J Am Geriatr Soc. 1996;44:897–904. doi: 10.1111/j.1532-5415.1996.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 18.Akomolafe A, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 19.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 20.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 21.Vetrivel KS, Thinakaran G. Amyloidogenic processing of β-amyloid precursor protein in intracellular compartments. Neurology. 2006;66:S69–S73. doi: 10.1212/01.wnl.0000192107.17175.39. [DOI] [PubMed] [Google Scholar]
- 22.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 23.Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Aβ toxicity: from top to bottom. Nat Rev Neurosci. 2001;2:595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 24.Naslund J, et al. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 25.Hsia AY, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catalano SM, et al. The role of amyloid-β derived diffusible ligands (ADDLs) in Alzheimer’s disease. Curr Top Med Chem. 2006;6:597–608. doi: 10.2174/156802606776743066. [DOI] [PubMed] [Google Scholar]
- 27.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 28.Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 29.Rohn TT, et al. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11:341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 30.Chung CW, et al. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 31.Gamblin TC, Berry RW, Binder LI. Tau polymerization: role of the amino terminus. Biochemistry. 2003;42:2252–2257. doi: 10.1021/bi0272510. [DOI] [PubMed] [Google Scholar]
- 32.Hrnkova M, Zilka N, Minichova Z, Koson P, Novak M. Neurodegeneration caused by expression of human truncated tau leads to progressive neurobehavioural impairment in transgenic rats. Brain Res. 2007;1130:206–213. doi: 10.1016/j.brainres.2006.10.085. [DOI] [PubMed] [Google Scholar]
- 33.Chun W, Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci. 2007;12:733–756. doi: 10.2741/2097. [DOI] [PubMed] [Google Scholar]
- 34.Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- 36.Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Planel E, et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolivalt CG, et al. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res. 2008;86:3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer disease. J Neurochem. 2009;111:242–249. doi: 10.1111/j.1471-4159.2009.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol Biosyst. 2007;3:766–772. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- 41.Biessels GJ, et al. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 42.Stolk RP, et al. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care. 1997;20:792–795. doi: 10.2337/diacare.20.5.792. [DOI] [PubMed] [Google Scholar]
- 43.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 44.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 45.Frolich L, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 46.Zhao WQ, et al. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric Aβ. J Biol Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends Neurosci. 2003;26:404–406. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen WA, et al. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Watson GS, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 50.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 51.Lesort M, Johnson GV. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. doi: 10.1016/s0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- 52.Cheng CM, et al. Tau is hyperphosphorylated in the insulin-like growth factor-I null brain. Endocrinology. 2005;146:5086–5091. doi: 10.1210/en.2005-0063. [DOI] [PubMed] [Google Scholar]
- 53.Schubert M, et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freude S, et al. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes. 2005;54:3343–3348. doi: 10.2337/diabetes.54.12.3343. [DOI] [PubMed] [Google Scholar]
- 55.Pearson LL, Castle BE, Kehry MR. CD40-mediated signaling in monocytic cells: up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int Immunol. 2001;13:273–283. doi: 10.1093/intimm/13.3.273. [DOI] [PubMed] [Google Scholar]
- 56.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 57.Tremblay ML, Giguere V. Phosphatases at the heart of FoxO metabolic control. Cell Metab. 2008;7:101–103. doi: 10.1016/j.cmet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Hensley K, et al. p38 kinase is activated in the Alzheimer’s disease brain. J Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- 59.Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology. 2010;58:561–568. doi: 10.1016/j.neuropharm.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Kelleher I, Garwood C, Hanger DP, Anderton BH, Noble W. Kinase activities increase during the development of tauopathy in htau mice. J Neurochem. 2007;103:2256–2267. doi: 10.1111/j.1471-4159.2007.04930.x. [DOI] [PubMed] [Google Scholar]
- 61.Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3β and Alzheimer’s disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63:1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract. 2007;77 (Suppl 1):S49–S57. doi: 10.1016/j.diabres.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 63.Clodfelder-Miller B, De Sarno P, Zmijewska AA, Song L, Jope RS. Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. J Biol Chem. 2005;280:39723–39731. doi: 10.1074/jbc.M508824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 65.Noble W, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Small GW, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garrido GE, et al. Relation between medial temporal atrophy and functional brain activity during memory processing in Alzheimer’s disease: a combined MRI and SPECT study. J Neurol Neurosurg Psychiatry. 2002;73:508–516. doi: 10.1136/jnnp.73.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. Eur J Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 69.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441:1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- 70.Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988;256:205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 72.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 73.Sasaki N, et al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol. 1998;153:1149–1155. doi: 10.1016/S0002-9440(10)65659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sasaki N, et al. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res. 2001;888:256–262. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- 75.Ledesma MD, Bonay P, Colaco C, Avila J. Analysis of microtubule-associated protein tau glycation in paired helical filaments. J Biol Chem. 1994;269:21614–21619. [PubMed] [Google Scholar]
- 76.Toth C, et al. Diabetes, leukoencephalopathy and rage. Neurobiol Dis. 2006;23:445–461. doi: 10.1016/j.nbd.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 77.Girones X, et al. N epsilon-carboxymethyllysine in brain aging, diabetes mellitus, and Alzheimer’s disease. Free Radic Biol Med. 2004;36:1241–1247. doi: 10.1016/j.freeradbiomed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 78.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- 79.Russell JW, et al. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol Dis. 2008;30:420–429. doi: 10.1016/j.nbd.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 81.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 82.Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimers Dis. 2004;6:171–175. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- 83.Butterfield DA, et al. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007;1148:243–248. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Apelt J, Bigl M, Wunderlich P, Schliebs R. Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int J Dev Neurosci. 2004;22:475–484. doi: 10.1016/j.ijdevneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 86.Matsuoka Y, Picciano M, La Francois J, Duff K. Fibrillar β-amyloid evokes oxidative damage in a transgenic mouse model of Alzheimer’s disease. Neuroscience. 2001;104:609–613. doi: 10.1016/s0306-4522(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 87.Resende R, et al. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan J, et al. Microglial activation resulting from CD40-CD40L interaction after β-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 90.van de Ree MA, Huisman MV, Princen HM, Meinders AE, Kluft C. Strong decrease of high sensitivity C-reactive protein with high-dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis. 2003;166:129–135. doi: 10.1016/s0021-9150(02)00316-7. [DOI] [PubMed] [Google Scholar]
- 91.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sly LM, et al. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res Bull. 2001;56:581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- 93.Szekely CA, et al. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: a systematic review. Neuroepidemiology. 2004;23:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 94.Aisen PS, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 95.Reines SA, et al. Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- 96.Schaefer EJ, et al. Familial apolipoprotein E deficiency. J Clin Invest. 1986;78:1206–1219. doi: 10.1172/JCI112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishibashi S, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu–Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 99.Kuusisto J, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Profenno LA, Faraone SV. Diabetes and overweight associate with non-APOE4 genotype in an Alzheimer’s disease population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:822–829. doi: 10.1002/ajmg.b.30694. [DOI] [PubMed] [Google Scholar]
- 101.LaDu MJ, et al. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 102.Belinson H, Lev D, Masliah E, Michaelson DM. Activation of the amyloid cascade in apolipoprotein E4 transgenic mice induces lysosomal activation and neurodegeneration resulting in marked cognitive deficits. J Neurosci. 2008;28:4690–4701. doi: 10.1523/JNEUROSCI.5633-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brecht WJ, et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ulery PG, et al. Modulation of β-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J Biol Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- 105.Yaffe K. Metabolic syndrome and cognitive decline. Curr Alzheimer Res. 2007;4:123–126. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- 106.Harris MI. Hypercholesterolemia in diabetes and glucose intolerance in the U.S population. Diabetes Care. 1991;14:366–374. doi: 10.2337/diacare.14.5.366. [DOI] [PubMed] [Google Scholar]
- 107.Ishikawa M, et al. Cholesterol accumulation and diabetes in pancreatic β-cell-specific SREBP-2 transgenic mice: a new model for lipotoxicity. J Lipid Res. 2008;49:2524–2534. doi: 10.1194/jlr.M800238-JLR200. [DOI] [PubMed] [Google Scholar]
- 108.Sharma S, Prasanthi RPJ, Schommer E, Feist G, Ghribi O. Hypercholesterolemia-induced Aβ accumulation in rabbit brain is associated with alteration in IGF-1 signaling. Neurobiol Dis. 2008;32:426–432. doi: 10.1016/j.nbd.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burns MP, et al. Co-localization of cholesterol, apolipoprotein E and fibrillar Aβ in amyloid plaques. Brain Res Mol Brain Res. 2003;110:119–125. doi: 10.1016/s0169-328x(02)00647-2. [DOI] [PubMed] [Google Scholar]
- 110.Mori T, et al. Cholesterol accumulates in senile plaques of Alzheimer disease patients and in transgenic APP(SW) mice. J Neuropathol Exp Neurol. 2001;60:778–785. doi: 10.1093/jnen/60.8.778. [DOI] [PubMed] [Google Scholar]
- 111.Bjorkhem I, Heverin M, Leoni V, Meaney S, Diczfalusy U. Oxysterols and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 112.Prasanthi JR, et al. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on β-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener. 2009;4:1. doi: 10.1186/1750-1326-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 114.Distl R, Meske V, Ohm TG. Tangle-bearing neurons contain more free cholesterol than adjacent tangle-free neurons. Acta Neuropathol. 2001;101:547–554. doi: 10.1007/s004010000314. [DOI] [PubMed] [Google Scholar]
- 115.Refolo LM, et al. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 116.Rizzuto R, et al. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- 117.Khachaturian ZS. Calcium hypothesis of Alzheimer’s disease and brain aging. Ann NY Acad Sci. 1994;747:1–11. doi: 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- 118.Kostyuk E, et al. Diabetes-induced changes in calcium homeostasis and the effects of calcium channel blockers in rat and mice nociceptive neurons. Diabetologia. 2001;44:1302–1309. doi: 10.1007/s001250100642. [DOI] [PubMed] [Google Scholar]
- 119.Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 120.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Crouch PJ, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-β1–42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blass JP, Gibson GE. The role of oxidative abnormalities in the pathophysiology of Alzheimer’s disease. Rev Neurol (Paris) 1991;147:513–525. [PubMed] [Google Scholar]
- 123.Hirai K, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murray FE, Landsberg JP, Williams RJ, Esiri MM, Watt F. Elemental analysis of neurofibrillary tangles in Alzheimer’s disease using proton-induced X-ray analysis. Ciba Found Symp. 1992;169:201–210. doi: 10.1002/9780470514306.ch12. [DOI] [PubMed] [Google Scholar]
- 125.Nixon RA, et al. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease. Ann NY Acad Sci. 1994;747:77–91. doi: 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- 126.McKee AC, Kosik KS, Kennedy MB, Kowall NW. Hippocampal neurons predisposed to neurofibrillary tangle formation are enriched in type II calcium/calmodulin-dependent protein kinase. J Neuropathol Exp Neurol. 1990;49:49–63. doi: 10.1097/00005072-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 127.Johnson GV, et al. Transglutaminase activity is increased in Alzheimer’s disease brain. Brain Res. 1997;751:323–329. doi: 10.1016/s0006-8993(96)01431-x. [DOI] [PubMed] [Google Scholar]
- 128.Querfurth HW, Selkoe DJ. Calcium ionophore increases amyloid beta peptide production by cultured cells. Biochemistry. 1994;33:4550–4561. doi: 10.1021/bi00181a016. [DOI] [PubMed] [Google Scholar]
- 129.Levy J, Gavin JR, 3rd, Sowers JR. Diabetes mellitus: a disease of abnormal cellular calcium metabolism? Am J Med. 1994;96:260–273. doi: 10.1016/0002-9343(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 130.Levy J, Zemel MB, Sowers JR. Role of cellular calcium metabolism in abnormal glucose metabolism and diabetic hypertension. Am J Med. 1989;87:7S–16S. doi: 10.1016/0002-9343(89)90489-0. [DOI] [PubMed] [Google Scholar]
- 131.Studer RK, Ganas L. Effect of diabetes on hormone-stimulated and basal hepatocyte calcium metabolism. Endocrinology. 1989;125:2421–2433. doi: 10.1210/endo-125-5-2421. [DOI] [PubMed] [Google Scholar]
- 132.Moreira PI, Santos MS, Sena C, Seica R, Oliveira CR. Insulin protects against amyloid β-peptide toxicity in brain mitochondria of diabetic rats. Neurobiol Dis. 2005;18:628–637. doi: 10.1016/j.nbd.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 133.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 134.Ke YD, Delerue F, Gladbach A, Gotz J, Ittner LM. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer’s disease. PLoS ONE. 2009;4:e7917. doi: 10.1371/journal.pone.0007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jolivalt CG, et al. Type 1 diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp Neurol. 2010;223:422–431. doi: 10.1016/j.expneurol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takeda S, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ott A, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 139.Brayne C, et al. Vascular risks and incident dementia: results from a cohort study of the very old. Dement Geriatr Cogn Disord. 1998;9:175–180. doi: 10.1159/000017043. [DOI] [PubMed] [Google Scholar]
- 140.Yoshitake T, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 141.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 142.Leibson CL, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 143.Luchsinger JA, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]