Abstract
Cystic fibrosis (CF) is caused by a misfunctional CFTR protein, which is believed to contributes to the regulation of the airway surface liquid (ASL) pH. This study investigated acid and base secretion in freshly excised human nasal tissues from CF patients homozygous for the ΔF508 mutation. Human nasal mucosa was collected during sinus surgery and investigated in Ussing chambers. Mucosal equilibrium pH values and rate of acid and base secretion were determined using the pH-stat technique. The equilibrium pH of nasal epithelia from ΔF508 CF patients with chronic rhinosinusitis (CRS) was pH = 7.08 ± 0.09 and significantly lower compared to nasal epithelia from CRS without CF (pH = 7.33 ± 0.06) and normal subjects (pH = 7.34 ± 0.08, n = 6). The rate of base secretion in CF nasal tissues was 11.8 ± 2.4 nmol·min−1·cm−2, which was significantly lower than normal (57.2 ± 9.2 nmol·min−1·cm−2). HCO3−secretory rate was further increased by forskolin by 16.1% in normal, but not in CF tissues. Our data suggests that CF patients exhibited significantly lower base secretion by the nasal airway epithelium. It is possible that improper regulation of ASL pH in CF may negatively impact the innate host defense system.
Keywords: proton secretion, pH stat, Ussing chamber, cystic fibrosis, sinusitis, airway surface liquid (ASL), ΔF508 mutation
Introduction
The mucosa of the airway surface epithelium is covered with a thin layer of fluid called airway surface liquid (ASL) (1). Both ASL composition and volume are considered critical for innate defense of the airways against inhaled noxious and infectious agents (2). pH-dependent changes of ion conductance in ASL could alter salt and water movement and the concentrations of all components (2–4). The wide variation of ASL pH in various airway diseases supports the notion that the activity of epithelial acid and base transporters is differentially affected in each disease (5–7). The relationship between ASL pH, epithelial integrity and function, and antibacterial activity have not been worked out in detail (8).
Cystic fibrosis (CF) is a lethal hereditary disease caused by lack of functional expression of the CF transmembrane conductance regulator (CFTR) in the apical (superficial) membrane of airway epithelium in all regions, where it normally serves as a cAMP-regulated Cl channel that is also permeable for HCO3 and other anions (2, 9–11). Chronic inflammation of sinuses and nasal mucosa is found in 74 – 100% patients suffering from CF and nasal polyposis is found in 6–44% patients (12–14). Abnormal pH regulation of ASL may contribute to CF pathogenesis because biologic processes on airway surfaces are pH-sensitive (11). Coakley et al. showed that in cultured human CF airway epithelial cells, the alkalinization was missing rendering the pH of the ASL more acidic and limiting the response to acidic challenges to airway surfaces (2). However, little is known about the link between abnormal ion transport and airway disease in CF patients. The purpose of this study was to determine the effects of the ΔF508 CFTR mutation on sinonasal airway pH by measuring the pH and the rates of acid and base secretion in freshly excised human nasal tissues from normal subjects and CF patients homozygous for the ΔF508 mutation.
Methods
Study Subjects
This study was approved by the institutional review boards at Stanford University and Children’s Hospital Oakland. Normal nasal mucosa (septum or turbinate) was obtained intraoperatively from patients undergoing endoscopic sinus surgery (ESS) for pituitary tumor, benign sinonasal tumor, or lacrimal obstruction. Diseased nasal mucosa (septum or turbinate) was also taken from medically refractory chronic rhinosinusitis (CRS) patients with or without cystic fibrosis (CF) during ESS. All patients fulfilled diagnostic criteria for CRS developed by the Task Force for Defining Adult Chronic Rhinosinusitis and endorsed by the American Academy of Otolaryngology, and had failed medical management (15). CF nasal tissue (septum or turbinates) was obtained in nasal mucosa from patients that had been diagnosed as homozygous ΔF508 mutation by genetic analysis. The two clinical parameters (computed tomography (CT) scores, and sino-nasal outcomes test-22 (SNOT-22) were assessed in CRS patients with or without CF.
Specimen Processing
After surgical removal, tissue specimens were stored immediately in ice cold LHC-9 media (Invitrogen, USA) and transferred to the laboratory for investigation. Acid and base secretion was measured using the pH stat titration method in an Easy Mount Ussing chamber (Physiologic Instruments Inc. San Diego, CA) as described in our previous study (7). A thin layer of epithelial tissue was dissected from the surgical specimen and mounted on sliders with apertures of 0.040 to 0.71cm2 and inserted between Ussing-type hemichambers. Tissues were bathed serosally with HEPES or HCO3−-buffered solution and mucosally with buffer-free solution (5 ml each). The bathing solutions were circulated by a gas-lift system at 37°C. The buffer-free solution on the mucosal (luminal) side contained (in mM) 140 NaCl, 2 KCl, 15 glucose, 2 CaCl2, and 1 MgCl2 and was gassed with nitrogen to prevent air CO2 from entering the solution. To detect acid secretion, the HEPES-buffered solution on the serosal side contained (in mM) 140 NaCl, 2 KCl, 5 glucose, 10 HEPES, 2 CaCl2, 1 MgCl2, and gassed with oxygen. To detect base secretion, the HCO3− -buffered solution on the serosal side contained (in mM) 120 NaCl, 25 NaHCO3, 5 KCl, 1.375 NaH2PO4, 5.6 glucose, 2 CaCl2, 1.2 MgCl2, and gassed with 5% CO2/air. The pH of the mucosal solution was initially allowed to equilibrate for ~15 min and the final pH was recorded as the equilibrium pH. Then the mucosal pH was continuously titrated to a target pH of 8.0 (to measure acid secretion) or 6.0 (to measure base secretion), respectively, by a pH stat titration apparatus (TitraLab 856, Radiometer Analytical SAS, Lyon, France) using 1 mM NaOH or 1 mM HCl as titrant, respectively. A mucosal pH of 8 was used to increase driving forces for H+ secretion. In separate experiments, base secretion was measured using a mucosal pH of 6 to inhibit H+ channels and allow for measurement of base secretion. From the amount of base or acid added at constant pH, the acid or base secretion, respectively, of tissues was determined and is given in nmol·min−1·cm−2. Forskolin (a cAMP elevating agonist) was used to activate HCO3− secretion.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 15.0) and p<0.05 was considered significant. One-way ANOVA followed by the Dunn multiple comparison tests, paired sample t-test, linear regression analysis were used as appropriate. Normality of data were tested using Shapiro-Wilk testing. Data are given as individual measurements or as mean ± SE.
Results
Patient Factors
Mean ages were 38.8 ± 3.0 years in normal group (n = 6), 29.7 ± 2.3 years in CRS patient without CF (n = 7), and 30.7 ± 2.8 in CRS patients with CF (n = 7; one-way ANOVA, p = 0.062). In the normal group, there were 3 male and 3 female subjects; in both diseased groups (CRS without CF and CRS with CF) there were 4 female and 3 males subjects. CRS with homozygous ΔF508 mutation had worse CT scores than CRS patients without CF (p = 0.001, Table 1). There was no statistical difference in SNOT-22 between the two groups.
TABLE I.
Computed Tomography (CT) score and Sino-Nasal Outcomes Test 22 (SNOT-22) in Study Patients (mean ± SE)
| CRS without CF (N=7) | CRS with homozygous ΔF508 mutation (N=7) |
P value | |
|---|---|---|---|
| CT score | 9.14 ± 1.55 | 18.71 ± 1.44 | p = 0.001 |
| SNOT-20 | 1.56 ± 0.35 | 1.49 ± 0.75 | p = 0.87 |
N = number of study patients
Equilibrium pH
Individual and average values of the equilibrium pH are plotted in Figure 1. Equilibrium pH values were 7.34 ± 0.08 for normal nasal epithelia (n = 6); pH 7.33 ± 0.06 for nasal epithelia from CRS without CF (n = 5); and pH 7.08 ± 0.09 for nasal epithelia from CRS with homozygous ΔF508 mutation (n = 7). The equilibrium pH of nasal epithelia from CRS with homozygous ΔF508 mutation was significantly lower compared to nasal epithelia from normal and CRS without CF (one-way ANOVA, post hoc Duncan analysis, p = 0.041).
Figure 1. Distribution of equilibrium pH of freshly excised sinonasal epithelia.
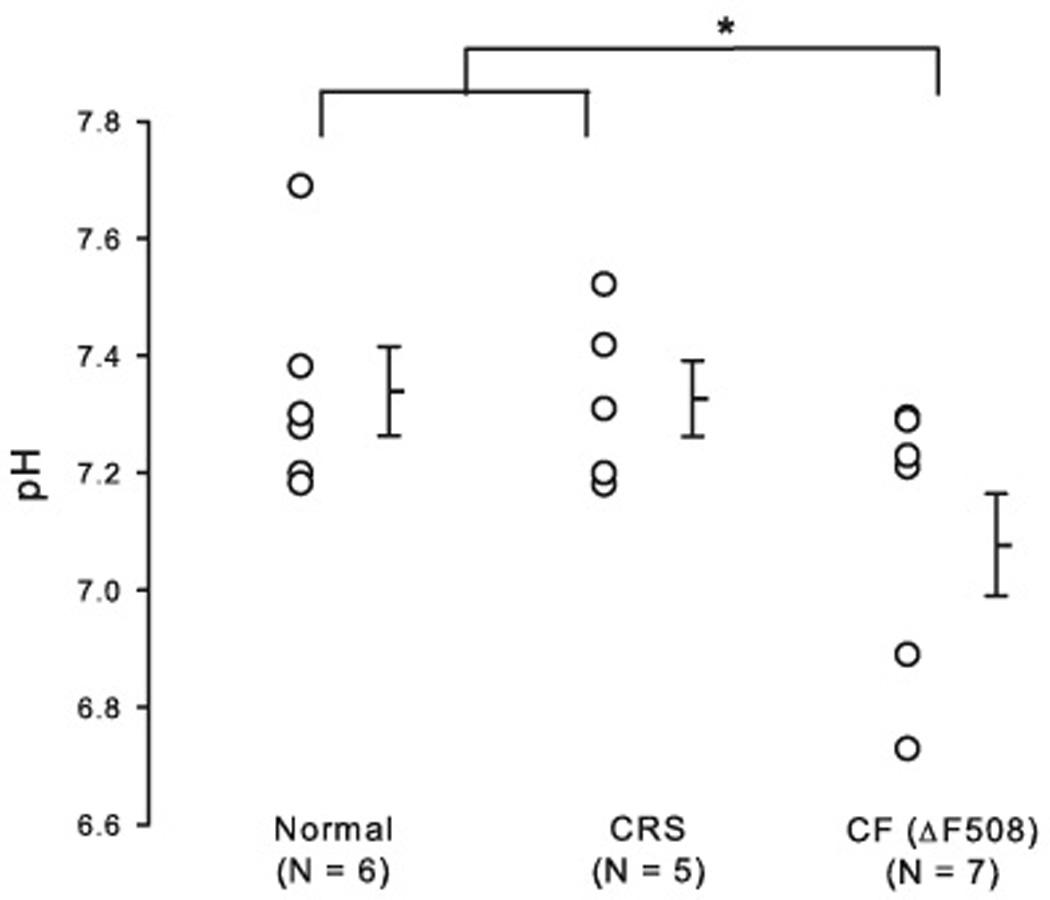
Individual equilibrium pH values are plotted and average values are shown as horizontal bars. Nasal epithelia from homozygous ΔF508 CF individual had significantly lower equilibrium pH (pH = 7.08 ± 0.09), compared to nasal epithelia from normal and CRS without asthma (p = 0.041). N refers to the number of tissue from different individuals. Error bars represent SEM (standard error of the mean); * denotes a significant difference (p < 0.05).
Proton secretion across freshly excised human nasal epithelia
At a mucosal pH of 8.0, all tested epithelia acidified the mucosal medium (Figure 2A). The rate of acid secretion was 79.8 ± 15.1 nmol·min−1·cm−2 in normal nasal epithelia (n = 6) and 51.4 ± 10.5 nmol·min−1·cm−2 in nasal epithelia from CRS without CF (n = 7). Nasal epithelia from CRS with homozygous ΔF508 mutation (n = 6) secreted protons at an average rate of 102.7 ± 46.3 nmol·min−1·cm−2 (not significant, one-way ANOVA, p = 0.423).
Figure 2. Acid secretion across freshly excised nasal epithelia.
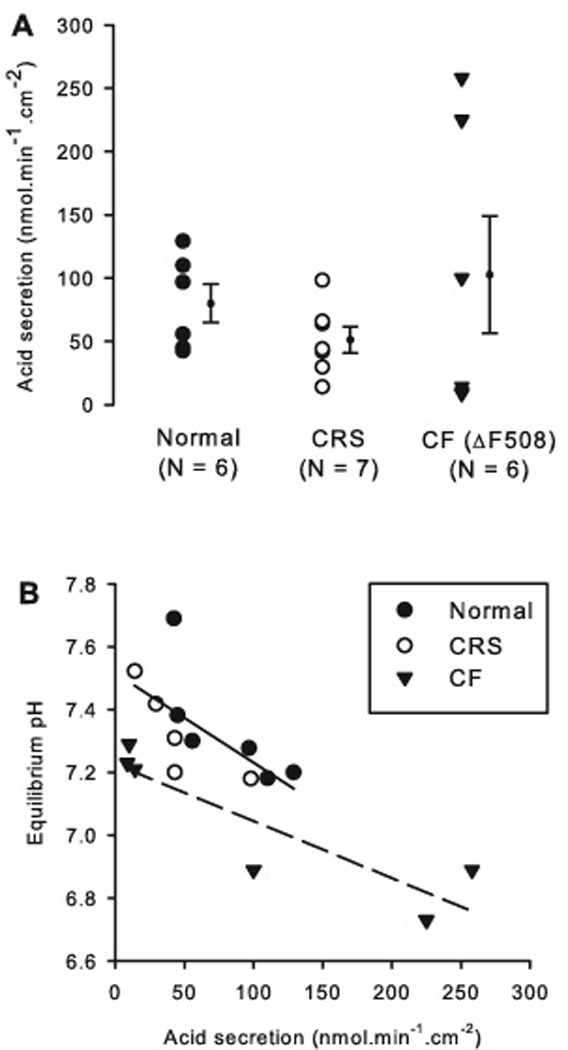
A: Rates of proton secretion; individual experimental results (symbols) and average ± SE are shown. N refers to the number of tissues from different individuals.
B: Correlation between acid secretion and equilibrium pH by group. There were significant correlations in the normal and the CRS group, which were not different from one another, resulting in a combined slope of −0.0029 ± 0.0011 pH/(nmole.h−1.cm−2) and an offset pH = 7.52 ± 0.08, p = 0.026, R2 = 0.44 (solid line). The CF group showed a significant correlation (dashed line, R2 = 0.79, p = 0.018) with a slope of −0.0018 ± 0.0005 pH/(nmol·min−1·cm−2) (not different from normal/CRS, p = 0.51) and a significantly lower offset of pH = 7.23 ± 0.07 (p = 0.028).
The CF group showed a wide spread of data, which included both the lowest and the highest values measured in this study (Fig. 2A). The spread of data was further analyzed by correlating the observed rate of acid secretion with its corresponding equilibrium pH values (Fig. 2B). In the CF group, there was a significant correlation between the equilibrium pH and the rate of acid secretion (p = 0.018; Fig. 2B), i.e., tissues with a high rate of acid secretion showed correspondingly low pH values. In the normal and CRS group there was also a significant correlation (p = 0.026), which was not different between the two. However, the correlation in the CF group was shifted by 0.29 pH units to more acidic values compared to normal and CRS tissues (Fig. 2B, compare solid and dashed regression lines). Thus, any rate of acid secretion resulted in more acidic pH values in CF nasal tissues compared to normal and CRS tissues.
Base secretion across freshly excised human nasal epithelia
The second part of this study compared base secretion by nasal tissues between normal and CRS with CF (all with homozygous ΔF508 mutations), i.e., the role of functional CFTR in bicarbonate secretion by nasal epithelium was investigated by using normal and CF nasal tissues. No tissues from CRS without CF were available in this part of the study (see Discussion). The rate of base secretion was 57.2 ± 9.2 nmol·min−1·cm−2 in normal nasal epithelia (n = 5) and was significantly reduced in nasal epithelia from CRS with CF (11.8 ± 2.4 nmol·min−1·cm−2, n = 4, p = 0.004, Fig. 3A). We further tested the effect of CFTR activation by forskolin on base secretion in normal and CF tissues. Exposure of the mucosal surface to 10 µM forskolin significantly increased base secretion in normal tissue (from 57.2 ± 9.2 nmol·min−1·cm−2 to 66.8 ± 11.0 nmol·min−1·cm−2, p = 0.009, Fig. 3B). In CF tissues, there was no significant effect on base secretion after the exposure of forskolin (11.8 ± 2.4 nmol·min−1·cm−2 vs. 9.1 ± 1.5 nmol·min−1·cm−2, p = 0.195, Fig. 3B), possibly owing to the absence of CFTR-dependent HCO3− secretion.
Figure 3. Base secretion across freshly excised human nasal epithelia.
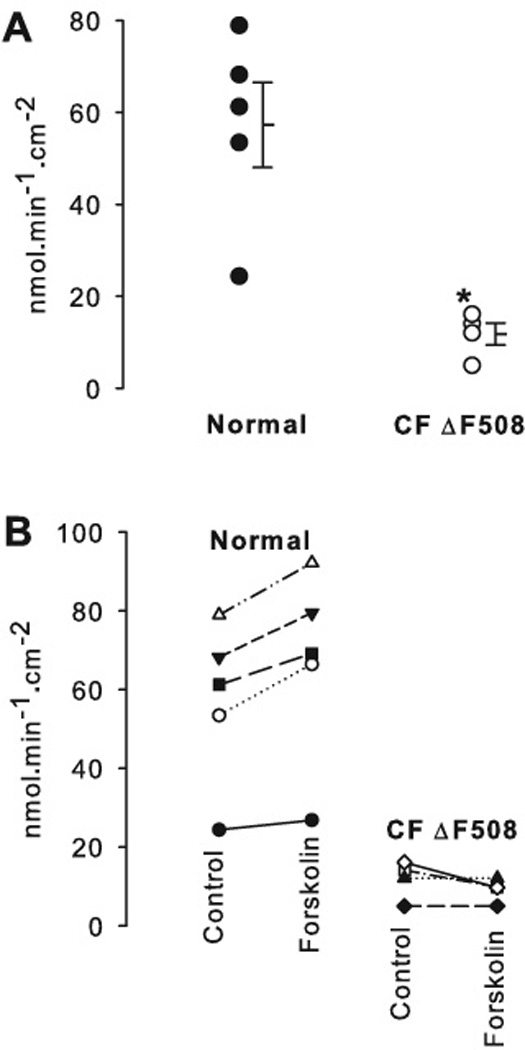
A: The rate of HCO3− secretion in CF (n = 4) was 11.8 ± 2.4 nmol·min−1·cm−2, which is significantly lower than normal (57.2 ± 9.2 nmol·min−1·cm−2, n = 5, t-test, p = 0.004). Error bars represent SEM (standard error of the mean). * denotes a significant difference (p < 0.05).
B: Rates of base secretions plotted for individual experiments. Exposure of the mucosal surface to forskolin significantly increased base secretion in normal tissue (from 57.2 ± 9.2 nmol·min−1·cm−2 to. 66.8 ± 11.0 nmol·min−1·cm−2, p = 0.009). In CF tissue, there was no statistically significant difference in base secretion after the exposure of forskolin (11.8 ± 2.4 nmol·min−1·cm−2 vs. 9.1 ± 1.5 nmol·min−1·cm−2, p = 0.195). *: denotes a significant difference (p < 0.05).
Discussion
This study aimed to investigate the equilibrium pH and the rates of acid and base secretion using freshly excised nasal epithelial tissue from CF patients homozygous for the ΔF508 mutation. As others have previously reported, subjects with CF had significantly worse baseline CT scores compared to control subjects with CRS (16). It is interesting to note that there was no statistical significance in SNOT-22 between the two groups. It seems that CF patients present with extensive sinonasal inflammation without worsening sinonasal symptoms compared to control subjects with CRS. However, the relatively small sample size in this study may have obscured a possible difference between the two groups.
ASL pH reflects a balance between acid and base secretion by the epithelium (2). In previous studies, measurement of ASL pH in CF primary cell cultures resulted in acidic values of ~6.0, while nasal pH recordings in CF patients showed no increased acidity compared to normal (2, 6). In our study, the mean equilibrium ASL pH in freshly excised human nasal epithelia from ΔF508 homozygous CF individuals was 7.08, which was significantly lower than those from normal and CRS individuals. Since the CT was significantly different in the CF group, the observed difference in equilibrium pH may be also related to disease severity. However, there is strong evidence from studies in the upper airway epithelium that (i) CFTR is a bicarbonate conductor, (ii) CF airways are more acidic, and (iii) CF airways show reduced bicarbonate secretion (10, 17, 18). Thus, our observations in nasal tissues are consistent with these previous reports supporting the notion that the lack of CFTR function in CF nasal tissues resulted in acidic pH values in our study.
In addition we found that nasal acid secretion is well correlated with the equilibrium pH suggesting that the amount of acid secretion determines the mucosal pH. At all rates of acid secretion, CF tissues showed lower pH values, which is an indication that the mucosa of CF tissues is less well pH buffered. Since bicarbonate is a major pH buffer in the ASL, and CF tissues show reduced bicarbonate secretion (Fig. 3), these data indicate that the excessive acidity of CF nasal epithelia is largely based on the reduced bicarbonate secretion (8). The wide spread of acid secretion (Fig. 2A) and corresponding equilibrium pH (Fig. 1) in CF nasal tissues may reflect a heterogeneity of tissues selected for analysis, differing metabolic states, or local tissue differences (i.e., septum vs. turbinate). Owing to the small sample size in this study, this was not further investigated.
Airway epithelial cells express at least three different cellular mechanisms to secrete protons across the apical membrane into the ASL (8). There are two ATP-driven mechanisms (the H+/K+-ATPase and the H+-ATPase), which secrete H+ against a gradient into an acidic ASL, and H+ channels allow for passive, gradient-driven H+ release (1, 2, 19). Coakley et al. suggested that H+/K+-ATPase activity is similar in CF and normal cultures and, thus, not the basis of abnormal ASL pH on CF airway epithelia (2). On the other hand, gradient-driven secretion is expected during intracellular acidification, such as during NADPH oxidation (8, 20). The airway NADPH oxidases Duox1 and Duox2 have been shown to be upregulated by the inflammatory cytokines IL-4, IL-13, and IFN-γ, suggesting that the resulting, inflammation-induced ASL acidification of CF airway epithelia is based on this mechanism (20, 21). A future avenue of investigation could be to determine whether the degree of ASL acidification in CF responds differently to medical and/or surgical interventions.
As others have previously noted, we were also able to detect a greatly reduced base secretion in CF tissues supporting the hypothesis that HCO3− secretion is mediated by CFTR (2, 18, 22). In addition, the lack of an effect of forskolin stimulation on HCO3− secretion on CF tissues is readily explained by the absence of functional CFTR channels. However, our study of base secretion in nasal tissue does not allow to distinguish the effects of CRS by itself on base secretion from effects caused by CF. Nevertheless, given the evidence outlined above that CF interfered with epithelial bicarbonate secretion in a number of other studies, our data are supportive of the notion that CFTR function governs base secretion also in nasal tissue, however, this needs to be investigated further. Lack of proper secretion of bicarbonate by the nasal epithelium and the resulting abnormal regulation of ASL pH might contribute to CF pathogenesis (2). Failure to compensate for acidification may heighten the inflammatory response in the airway by interfering with bactericidal activity, promoting secretion of cytotoxic chemical mediators from immune cells to the airway, and reducing the efficacy of antibiotics against such pathogens as Pseudomonas aeruginosa (2, 23–26).
Conclusions
Our data suggest that freshly excised nasal tissues from homozygous ΔF508 CF individuals exhibited significantly stronger ASL acidification, and the airway epithelial alkalinization function was significantly reduced, likely due to a lack of CFTR-mediated HCO3−secretory transport. This is expected to render the ASL pH more acidic and limit any response to acidic challenges to airway surfaces. Abnormal ASL pH regulation in response to mucosal acid challenges may be an important contributor to impairment of innate airway defenses.
Acknowledgements
This work was supported by R01 HL086323, R21 HL089196, and CFF FISCHE07G0. We thank all patients who donated tissue for this research.
Footnotes
Presented at the American Rhinologic Society’s Spring Meeting Program, Las Vegas, NV, April 29, 2010
The authors had no conflicts of interest or conflicting financial interests to disclose.
The study protocol has been approved by the Institutional Review Boards of Stanford University and Children's Hospital Oakland.
References
- 1.Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol. 2002;282(4):C736–C743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- 2.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A. 2003;100(26):16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J. Gen. Physiol. 2001;118(2):223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awayda MS, Boudreaux MJ, Reger RL, Hamm LL. Regulation of the epithelial Na+ channel by extracellular acidification. Am J Physiol Cell Physiol. 2000;279(6):C1896–C1905. doi: 10.1152/ajpcell.2000.279.6.C1896. [DOI] [PubMed] [Google Scholar]
- 5.Adler K, Wooten O, Philippoff W, Lerner E, Dulfano M. Physical properties of sputum. 3. Rheologic variability and intrinsic relationships. Am. Rev. Respir. Dis. 1972;106(1):86–96. doi: 10.1164/arrd.1972.106.1.86. [DOI] [PubMed] [Google Scholar]
- 6.McShane D, Davies JC, Davies MG, Bush A, Geddes DM, Alton EWFW. Airway surface pH in subjects with cystic fibrosis. Eur. Respir. J. 2003;21(1):37–42. doi: 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 7.Cho D-Y, Hajighasemi M, Hwang PH, Illek B, Fischer H. Proton secretion in freshly excised sinonasal mucosa from asthma and sinusitis patients. Am. J. Rhinol. Allergy. 2009;23:10–13. doi: 10.2500/ajra.2009.23.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211(3):139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. American Journal of Physiology Lung Cellular and Molecular Physiology. 1997;272:L752–L761. doi: 10.1152/ajplung.1997.272.4.L752. [DOI] [PubMed] [Google Scholar]
- 10.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA. 1994;91(12):5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, et al. Characterization of wild-type and ΔF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol. Biol. Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babinski D, Trawinska-Bartnicka M. Rhinosinusitis in cystic fibrosis: not a simple story. Int J Pediatr Otorhinolaryngol. 2008;72(5):619–624. doi: 10.1016/j.ijporl.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Drake-Lee AB, Morgan DW. Nasal polyps and sinusitis in children with cystic fibrosis. J Laryngol Otol. 1989;103(8):753–755. doi: 10.1017/s0022215100109983. [DOI] [PubMed] [Google Scholar]
- 14.Jorissen MB, De Boeck K, Cuppens H. Genotype-phenotype correlations for the paranasal sinuses in cystic fibrosis. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1412–1416. doi: 10.1164/ajrccm.159.5.9712056. [DOI] [PubMed] [Google Scholar]
- 15.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 16.Khalid AN, Mace J, Smith TL. Outcomes of sinus surgery in adults with cystic fibrosis. Otolaryngol Head Neck Surg. 2009;141(3):358–363. doi: 10.1016/j.otohns.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hug MJ, Tamada T, Bridges RJ. CFTR and Bicarbonate Secretion to Epithelial Cells. News Physiol Sci. 2003;18(1):38–42. doi: 10.1152/nips.01412.2002. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290(3):C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 19.Inglis SK, Wilson SM, Olver RE. Secretion of acid and base equivalents by intact distal airways. Am J Physiol Lung Cell Mol Physiol. 2003;284(5):L855–L862. doi: 10.1152/ajplung.00348.2002. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J. Biol. Chem. 2004;279(35):36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 21.Harper RW, Xu C, Eiserich J, Chen Y, Kao C-Y, Thai P, et al. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. Febs Letters. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. Journal of Clinical Investigation. 199;89(4):1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132(9):991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 24.Corkill JE, Sisson PR, Smyth A, Deveney J, Freeman R, Shears P, et al. Application of pyrolysis mass spectroscopy and SDS-PAGE in the study of the epidemiology of Pseudomonas cepacia in cystic fibrosis. J Med Microbiol. 1994;41(2):106–111. doi: 10.1099/00222615-41-2-106. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama K, Jia YX, Hirai H, Shinkawa M, Yamaya M, Sekizawa K, et al. Acid Stimulation Reduces Bactericidal Activity of Surface Liquid in Cultured Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2002;26(1):105–113. doi: 10.1165/ajrcmb.26.1.4425. [DOI] [PubMed] [Google Scholar]
- 26.Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F, et al. Extracellular Acidification Induces Human Neutrophil Activation. J Immunol. 1999;162(8):4849–4857. [PubMed] [Google Scholar]


