Abstract
Background
Little is known about the psychological predictors of colorectal screening uptake in England and mediators of associations between uptake and socioeconomic status (SES). This study tested the hypotheses that while higher threat and efficacy beliefs, lower cancer fatalism, lower depression and better self-rated health would predict higher screening uptake, only efficacy beliefs, fatalism, depression and self-rated health would mediate associations between uptake and SES.
Methods
Data from 529 adults aged 60-69 who had completed a postal survey in 2005-6 were linked with data on fecal occult blood test (FOBt) uptake recorded at the screening ‘hub’ following its introduction in 2007, resulting in a prospective study.
Results
Screening uptake was 56% and was higher among people with higher SES , better self-rated health, higher self-efficacy beliefs and lower cancer fatalism in univariate analyses. Path analysis on participants with complete data (n=515) showed both better self-rated health and lower cancer fatalism were directly associated with higher uptake of FOBt screening and significantly mediated pathways from SES to uptake. Lower depression only had an indirect effect on uptake through better self-rated health. Efficacy beliefs did not mediate the relationship between SES and uptake.
Conclusions
SES differences in uptake of FOBt in England are partially explained by differences in cancer fatalism, self-rated health and depression.
Impact
This is one of only a few studies to examine mediators of the relationship between SES and screening uptake and future research could test the effectiveness of interventions to reduce fatalistic beliefs to increase equality of uptake.
Keywords: colorectal cancer screening, socioeconomic status, fatalism, self-reported health, health inequality
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in both the UK and worldwide (1), and shows clear differences in mortality by socioeconomic status (SES) (2). Fecal occult blood testing (FOBt) can reduce CRC mortality by 16% among people invited to participate, and by 25% among those who complete at least one FOB test (3). However, although national CRC screening programs using FOBt are currently offered in many countries (4, 5), uptake has rarely exceeded 60% (5, 6), and a consistent finding has been one of lower participation among lower SES groups (7-9). While lack of medical insurance may play a role in SES differences in uptake in some countries, similar inequalities are seen in contexts where health care is free at the point of delivery (7). Understanding the determinants of SES differences in screening uptake is therefore crucial to developing strategies to reduce SES disparities in CRC mortality.
The National Health Service in England began an organised CRC screening program (the National Bowel Cancer Screening Programme; NHS BCSP) in 2006, inviting adults aged 60 to 69 to participate on a biennial basis. Eligible adults are sent a FOBt kit and invited to complete and return it in a pre-paid reply envelope, with a reminder kit sent 28 days later if no response has been received. The overall uptake rate in the first round was 54% but uptake in the most deprived quintile of areas was close to half that of the least deprived quintile (using an area-based measure of material deprivation) (10). To date studies of participation in the English FOBt screening program have focused predominantly on demographic correlates (e.g. age, gender, ethnicity, SES), finding higher uptake among adults aged 65-69, women, members of the white majority and people with higher SES (7, 10). While qualitative analyses have identified factors such as perception of low risk and fear of cancer as potential deterrents to screening (11, 12), little work has quantitatively assessed individual psychosocial predictors of FOBt uptake in England or potential mediators of SES effects.
Potential mediators of the relation between SES and FOBt uptake
Cancer threat and efficacy beliefs and cancer fatalism
Threat and efficacy beliefs play a central role in a number of models of health motivation and behavior (e.g. the Health Belief Model (13, 14) Protection Motivation Theory (15, 16); the Extended Parallel Processing Model (EPPM) (17). Health motivation variables such as cancer worry/fear and perceived cancer risk are often associated with increased uptake of screening (18, 19), but worry and perceived risk tend to be higher in lower SES groups (20, 21) and are therefore unlikely to explain reduced uptake in lower SES groups. However higher efficacy beliefs are associated with greater screening uptake (22, 23), and efficacy beliefs are lower among people with lower levels of educational attainment (e.g. (24)) and could therefore mediate associations between SES and uptake.
Cancer fatalism has been defined as ‘the belief that death is inevitable when cancer is present’ and has emerged from empirical work into reasons for non-adherence to CRC screening among African-Americans (25). Higher cancer fatalism is associated with lower uptake of CRC screening, both in the UK and the USA (25, 26), and with lower SES (27) and hence could also mediate SES-uptake associations. According to Powe, there are four elements of fatalism - fear, predetermination, pessimism, and inevitability of death from cancer - however few studies have attempted to examine whether associations between cancer fatalism and screening uptake are independent of other related constructs such as cancer fear and depression (which would fuel pessimistic thinking).
Physical and emotional wellbeing
Other factors that could explain SES differences in CRC screening uptake are physical and emotional wellbeing. Explanations of the relationship between SES and health outcomes in general have focused on the role of negative emotions (28, 29), which contribute to poorer health outcomes both directly, through biological processes (e.g. (30)) and indirectly, through poorer health behaviors (e.g. (31)). Rates of negative affect are higher in lower SES groups but the role of emotions such as depression has attracted limited attention in cancer screening research.
Depression is associated with poorer health practices (e.g. smoking (31)) as well as lower adherence to treatment recommendations (e.g. (32)), and several studies have found direct effects with lower uptake of mammography among women with depression (33-35). However in two studies of CRC screening, one found no association between receipt of colorectal screening in the last five years and the presence of depressive disorder among women (33), while the other found no association between history of depression/anxiety and FOBt uptake (8), but in both of these studies depression was treated as a categorical variable (i.e. the presence or absence of major depressive disorder) rather than as a continuum. In addition negative affect may also have indirect effects on screening uptake because expectations can be influenced by negative mood states and such effects remain unexplored. For example, higher depression might be associated with both lower perceived efficacy and more fatalistic beliefs about cancer due to a greater tendency towards negative or pessimistic thinking.
Another important variable is current health status. Poorer self-rated health is strongly associated with lower SES (36), and people who take up offers of screening typically report better health (23). Self-rated health might therefore mediate the association between SES and screening uptake, either directly due to an inability to carry out screening, or indirectly through its influence on health expectations (37) and hence perceptions of the benefits of screening.
The aim of the present study was to examine i) predictors of uptake of FOBt screening, and ii) mediators of the association between SES and screening uptake. On the basis of the existing literature, we predicted that higher perceived cancer threat, higher cancer fear, higher cancer efficacy beliefs and lower cancer fatalism would be associated with higher screening uptake, but only efficacy beliefs and cancer fatalism would mediate associations between SES and uptake. We also hypothesized that both lower depression and better self-rated health would have direct and indirect effects on promoting uptake (through higher efficacy and lower fatalism) and would mediate the relationship between SES and uptake.
Method
Sample
Participants were a community sample of older adults who had previously taken part in a postal questionnaire survey on attitudes towards health and cancer worry (38). The questionnaire was sent to people aged 50-70, identified from the lists of three General Practitioner (GP) Surgeries in Camden and Islington, London, UK between August 2005 and January 2006, at which time national screening for colorectal cancer was not available. FOBt screening was subsequently introduced in the boroughs of Camden and Islington in April 2007. Survey completion rates were 50.1% (38). 1018 adults aged 60-69 (the age at which people are invited for FOBt screening) completed the survey. Linking the survey data to information on FOBt uptake recorded by the London Bowel Cancer Screening ‘Hub’ allowed us to examine prospective predictors of FOBt screening. Ethical approval was given for the study by Camden and Islington Community Research Ethics Committee. The ethics committee permitted us to contact people who had completed the survey if a) they had expressed willingness to be approached in the future (n=751) and b) the list of participants was checked first by the person’s GP. Participants were excluded by GPs (n=172) if they were deceased, had been diagnosed with bowel cancer, their GP felt they would be distressed by receiving the study information, or they were no longer registered at the practice. The remaining 579 were written to and asked if they had any objection to us contacting the screening service to find out if they had returned a FOBt kit. Twenty-two (>4%) opted out, so information on 557 were given to a data administrator at the London Bowel Cancer Screening Hub, who successfully matched 95% of all cases (N = 529).
Measures
Screening uptake
Uptake relates to people’s participation in their first round of screening. For the purpose of this study, uptake was defined as successful completion and return of a FOBt kit (yes/no). At present, England only offers FOBt screening as part of the NHS BCSP so no other form of screening would have been routinely available to participants. Average kit return time was 5 weeks and 99% of participants returned the kit within 18 weeks. Non-responders were people who did not return a kit or returned a kit but there was no interpretable result associated with it and it was unclear whether the kit had been returned blank (there were only 4 such participants).
Demographic characteristics
Age and gender were both supplied as part of GP lists. The remaining measures were obtained via the postal survey.
Socio-economic status. Although the most common indicators of SES include occupation and income, both of these are problematic if used in older adults. Over a third of the current sample were either retired or too ill to work, and because reasons for leaving work can be health-related, any associations observed between occupation and health could be a result of reverse causation (poor health causing occupational status rather than the other way round). Income is strongly related to occupation and hence suffers from similar problems to the use of the latter as a marker of SES. Furthermore, accurate and detailed information regarding income is hard to obtain in older populations as they often have a number of income streams, some of which may not be paid directly to them. Composite measures of SES, combining an individual marker such as occupation or educational qualifications alongside a household measure of deprivation (e.g. car ownership), have been shown to be a more sensitive measure of SES among older adults than single items (39). Individual SES was therefore measured using educational qualifications, home ownership and car ownership. A point was given for educational qualifications (i.e. the individual had passed public examinations within school or college, yes vs. no), home ownership (yes vs. no) and car ownership (yes vs. no), giving a maximum score of 3 (denoting highest level of SES) and a minimum of 0 (for lowest level of SES). This measure has been used in previous studies examining mediators of the relationship between SES and screening (20).
Ethnicity was measured with the item ‘What is your ethnic group’ with seven response options: ‘White’, ‘mixed’, ‘Asian or Asian British’, ‘Black or Black British’, ‘Chinese’, ‘other’, ‘do not wish to answer’. 91% of the sample was White so this variable was recoded into White vs. non-white.
Health and emotional wellbeing
Self-rated health was assessed with the single item ‘Would you say that for someone of your age your own health in general is…’ ‘Poor’, ‘Fair’, ‘Good’, ‘Excellent’ on a scale of 1-4 with higher scores indicating better health (40).
Depression was measured using the 10 item version of the Center for Epidemiological Studies Depression scale (CES-D) (41) and assessed mood over the last three months using the ‘Yale’ response categories (four item response scale ‘rarely or none of the time’, ‘a little of the time’, ‘a moderate amount of time’, ‘most of the time’). Higher scores indicate higher depression on a scale of 0 to 30 (Cronbach’s alpha=0.85).
Perceived threat
Susceptibility to cancer was assessed using the relevant sub-scale of the Risk Behavior Diagnosis Scale (42) adapted to ask about cancer in general, e.g. ‘It is likely that I will get cancer’. Response options comprised a 5-point Likert scale from ‘strongly disagree’ to ‘strongly agree’ on a scale of 1 to 5 with higher scores indicating greater agreement (Cronbach’s alpha=0.78).
Cancer severity was assessing using the 8 item-scale from the FOBt pilot project questionnaire (43) e.g. ‘I am certain that if I were to develop cancer it would limit my social life’. Response options and range of scores were the same as for Susceptibility (Cronbach’s alpha=0.82).
Cancer fear was measured using a modified version of the Breast Cancer Fear Questionnaire developed by Champion et al. (44) with the wording altered to ask about cancer in general. Participants were asked: ‘How do you feel when you think about cancer?’ and requested to endorse eight statements (e.g. ‘The thought of cancer scares me’; ‘When I think about cancer, I get upset’). Response options and range of scores were the same as for Susceptibility (Cronbach’s alpha=0.91).
Efficacy beliefs
Response and self-efficacy items covered eight behaviors: maintaining a healthy body weight, not smoking/stopping smoking, eating five portions of fruit and vegetables a day, taking regular exercise, reducing alcohol intake, reducing sun exposure, checking their body for signs of cancer, going for cancer screening, and followed the phrasing used in the Risk Behavior Diagnosis Scale (42) (e.g. Response efficacy: ‘I believe maintaining a healthy body weight is effective in preventing some types of cancer’; Cronbach’s alpha=0.78) Self-efficacy: ‘Maintaining a healthy body weight in order to decrease my chances of getting some types of cancer is easy for me to do’; Cronbach’s alpha=0.64). Response options and range of scores were the same as for Susceptibility.
Fatalism
Cancer fatalism was measured using the 15 item Powe Fatalism Index (25). The wording was altered to ask about cancer in general, e.g. ‘If someone is meant to have cancer they will have cancer.’ Response options were Yes/No. A point was given for each ‘yes’ and scores were summed to give a scale from 0 to 15 (Cronbach’s alpha = 0.83).
Statistical analysis
Univariate predictors of screening uptake were examined using t-tests and chi-square and a model of the potential mediators of SES and FOBt was tested using path modelling with the software EQS version 6.1. In line with recommendations three fit indices are reported: the Satorra-Bentler scaled chi-square (a correction to the chi-square statistic and the parameter estimates for the extent of non-normality) and robust statistics (also adjusted for non-normality) for one comparative fit index (the comparative fit index: CFI) and one absolute fit index (the root-mean-square error of approximation: RMSEA). The CFI and RMSEA were chosen because they are the most frequently reported fit indices (45) and hence provide a more useful comparison with other studies. A ratio of chi-square to degrees of freedom of less than two, and levels greater than 0.95 for CFI and less than 0.06 for RMSEA were adopted as indicating a good fitting model (45, 46). Two path equation models were tested: Model 1 where all proposed pathways were entered, and Model 2, where non-significant pathways were removed and only significant pathways included. Logistic regression was used (post-hoc) to examine which variables attenuated the relationship between self-efficacy and screening uptake.
Results
Participants
The sample for analysis (n=529) were compared with people who completed the survey but were excluded (n=489). The former were significantly younger (t = 1.98, df=1016; p=0.047, although the difference in the means was very small: 63.4 vs. 63.8) and had higher response efficacy beliefs (t=2.45 df=1006; p=0.014; means: 4.00 vs. 3.92), however the two groups did not differ on any of the other variables listed in Table 1 (SES, gender, ethnicity, self-rated health, depression, perceived cancer susceptibility, perceived cancer severity, cancer fear, self-efficacy, cancer fatalism).
Table 1. Univariate associations between demographic, psychological variables and uptake of FOBt screening (N=529).
| Predictor | Completed FOBt screening n=296 |
Did not complete FOBt screening n=233 |
|
|---|---|---|---|
| Demographics | |||
| SES [Mean (SD)] | 2.33 (0.96) | 2.05 (1.07) | t (527) = 3.21; p<0.001 |
| Age [Mean (SD)] | 64.7 (3.3) | 64.6 (3.2) | t (527) <1; ns |
| Sex [% (n)] Male Female |
57.6 (137) 54.6 (159) |
42.4 (101) 45.4 (132) |
Chi-square (1) = 0.50; ns |
| Ethnicity [% (n)] White Non-white Missing |
56.5 (271) 50.0 (17) 2.7 (8) |
43.5 (209) 50.0 (17) 3.0 (7) |
Chi-square (1) = 0.46; ns |
| Health and negative affect | |||
| Self-rated health1 [Mean (SD)] | 3.0 (0.7) | 2.7 (0.8) | t (521) = 4.3; p<0.001 |
| Depression1 [Mean (SD)] | 6.3 (5.6) | 7.2 (5.4) | t (525) = 1.79; p=0.07 |
| Threat and fear | |||
| Susceptibility1 [Mean (SD)] | 3.1 (0.7) | 3.0 (0.7) | t (499) = 1.0; ns |
| Severity1 [Mean (SD)] | 3.0 (0.6) | 3.0 (0.7) | t (509) <1; ns |
| Fear1 [Mean (SD)] | 2.7 (0.9) | 2.7 (0.9) | t (521) <1; ns |
| Efficacy and fatalism | |||
| Self-efficacy1 [Mean (SD)] | 3.8 (0.5) | 3.7 (0.6) | t (526) = 2.47; p=0.014 |
| Response efficacy1 [Mean (SD)] | 4.0 (0.5) | 3.9 (0.5) | t (526)=1.26; ns |
| Fatalism1 [Mean (SD)] | 2.6 (3.1) | 3.5 (3.4) | t (521) = 3.33; p<0.001 |
Out of the 529 cases there were missing data for self-rated health (6 cases missing), depression (2 cases missing), susceptibility (28 cases missing), severity (18 cases missing), fear (6 cases missing), self-efficacy (1 case missing), response-efficacy (1 case missing) and fatalism (6 cases missing).
Predictors of uptake
Screening uptake was 56% and, as predicted, was higher among people with higher SES. Furthermore there was a gradient in uptake by SES with 43% uptake among people scoring 0 on the SES scale, 48% uptake among those scoring 1, 51% uptake among people scoring 2 and 62% uptake among people scoring 3. In line with predictions, screening uptake was also higher among people with better self-rated health, higher self-efficacy beliefs and lower cancer fatalism, and the association between depression and uptake approached significance (p<0.10) (see Table 1). There were no associations between uptake and any of the threat constructs (fear, perceived susceptibility, perceived severity) or between uptake and response-efficacy, age, gender or ethnicity.
Mediators of the SES-uptake relationship
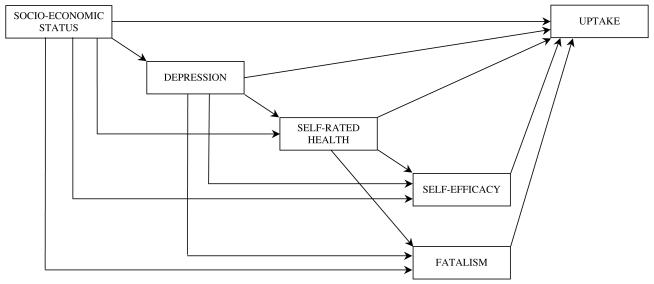
Variables that correlated significantly with both SES and uptake, or approached significance, were entered into the initial model shown in Figure 1 (Model 1). Correlations between the variables are shown in Table 2. The estimation technique used was maximum likelihood robust method as the data were not normally distributed (45). Data were missing for 14 participants on one or more questionnaire items included in the path model, leaving 515 as the sample for analysis.
Figure 1.
Proposed model (Model 1)
Table 2.
Correlations between variables entered into the path model
| Socio- economic status (high values indicate higher SES) |
Depression | Self-rated health |
Self-efficacy | Fatalism | |
|---|---|---|---|---|---|
| Depression | −.247d (n=527) |
- | |||
| Self-rated health |
.337d (n=523) |
−.456d (n=521) |
- | ||
| Self-efficacy | 0.135c (n=528) |
−0.179d (n=526) |
0.249d (n=522) |
- | |
| Fatalism | −.338d (n=523) |
.185d (n=521) |
−.194d (n=517) |
−0.144d (n=523) |
- |
| Uptake | .140d (n=529) |
−.078a (n=527) |
.184d (n=523) |
0.107b (n=528) |
−.140d (n=523) |
p<0.10
p<0.05
p<0.01
p<0.001
The model fit for Model 1 was very good with a CFI = 0.993, RMSEA = 0.061; and Satorra-Bentler chi-square = 2.93, df= 1; p=0.087. Residuals were small, with the largest between self-efficacy and fatalism (−0.073). Participants with complete data had higher SES than participants with incomplete data (t = 2.11; df= 527; p=0.04). However the two groups did not differ on any of the other variables entered in the model and re-running the analysis imputing missing data using maximum likelihood estimation did not alter the results. Similarly, omitting one participant who made a very large contribution to normalized multivariate kurtosis did not alter the results.
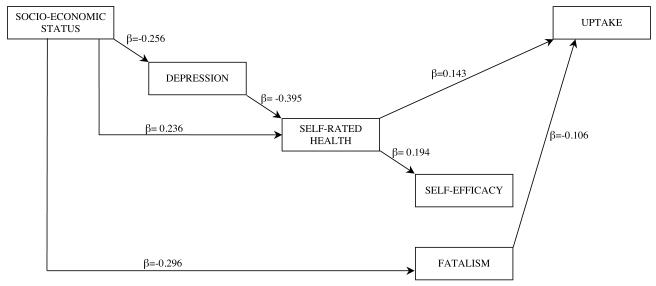
The analysis was re-run including only the significant pathways (Model 2: see Figure 2) and the total and indirect effects of these pathways on screening uptake are shown in Table 3. Both poorer self-rated health and higher cancer fatalism were associated with lower uptake of FOBt screening. While there was no direct pathway from SES to screening uptake, there were indirect pathways from SES to uptake via depression, self-rated health and fatalism. Depression did not have a direct effect on uptake but had an indirect effect via poorer self-rated health. Contrary to predictions, efficacy beliefs did not predict uptake in multivariate analyses and hence did not mediate associations between SES and FOBt uptake (see Figure 2). A series of models were tested using logistic regression to see which variables attenuated the relationship between self-efficacy and screening uptake (see Table 4). The association between self-efficacy and uptake remained significant when SES was entered into the model, approached significance when depression was also added in, but became non-significant with the further addition of self-rated health. Examination of the effect of each individual predictor on the association between self-efficacy and uptake showed that while both SES and depression reduced the odds ratio between self-efficacy and screening uptake, self-efficacy remained an independent predictor. However when either fatalism or self-rated health were included, the association between self-efficacy and screening uptake became non-significant (data not shown).
Figure 2.
Final model of direct and indirect pathways from socio-economic status to FOBt uptake (N=515) (Model 2)
Table 3. Significant total and indirect effects on FOBt uptake (standardized coefficients) (n=515).
| Variable | Indirect | Total |
|---|---|---|
| Socio-economic status | 0.093 | 0.093 |
| Depression | −0.060 | −0.060 |
| Self-rated health | - | 0.153 |
| Fatalism | - | −0.123 |
| Self-efficacy | - | - |
Table 4.
Variables affecting the association between self-efficacy and FOBt uptake (Odds ratios and confidence intervals in brackets)
| Univariate associations with uptake |
Model 1: Self- efficacy and SES |
Model 2: Self- efficacy, SES and depression |
Model 3: Self- efficacy, SES, depression and self-rated health |
Model 4: (full model) Self- efficacy, SES, depression, self- rated health and fatalism |
|
|---|---|---|---|---|---|
| Self-efficacy | 1.49 (1.08-2.05) p=0.015 |
1.41 (1.01-1.95) p=0.041 |
1.37 (0.98-1.90) p=0.064 |
1.26 (0.90-1.77) ns |
1.18 (0.83-1.67) ns |
| SES | - | 1.28 (1.09-1.53) p=0.004 |
1.28 (1.07-1.52) p=0.007 |
1.21 (1.00-1.46) p=0.044 |
1.14 (0.94-1.38) Ns |
| Depression | - | - | 0.99 (0.96-1.02) ns |
1.01 (0.98-1.0) ns |
1.02 (0.98-1.05) ns |
| Self-rated health | - | - | - | 1.49 (1.14-1.95) p=0.003 |
1.47 (1.12-1.93) p=0.005 |
| Fatalism | - | - | - | - | 0.94 (0.89-0.99) p=0.022 |
Discussion
This prospective study of the demographic and psychological predictors of uptake of FOBt-based CRC screening in England showed that better self-rated health and lower cancer fatalism were associated with greater participation in screening and mediated the effect of individual-level SES on uptake of FOBt. Importantly, these associations remained significant after controlling for depression, suggesting they do not merely reflect a greater tendency to endorse negative statements about cancer or personal health due to negative mood, important given lower SES is associated with greater pessimism in general (47).
The association between better self-rated health and higher uptake of FOBt is consistent with research on predictors of attendance at both FS and FOBt screening (7, 26), but in addition we found that poor self-rated health significantly mediated the relationship between SES and uptake. Self-rated health is associated with numerous physical and psycho-social factors, including physical health, psychological health (e.g. depression), social support, health expectations and behaviors (37). Depression was a significant predictor of self-rated health and mediated part of the association between SES, self-rated health and uptake. However self-rated health remained a significant independent predictor of screening uptake with depression in the model which suggests that other correlates of self-rated health also play a role in FOBt uptake such as the presence of other health problems, health expectations (e.g. not expecting to live for long enough to benefit from early detection) or psycho-social factors such as social support. Future research could examine the contribution of these factors to FOBt uptake in more detail. This would be particularly important for the development of future interventions to address deterrents that relate to this variable.
The results are also consistent with previous research showing that higher cancer fatalism is associated with lower uptake of colorectal screening (25, 26) and that changing cancer fatalism can increase uptake of colorectal cancer screening (48). Cancer fatalism has also been linked with lower levels of other cancer prevention behaviors (exercise and fruit and vegetable intake) (27) and is therefore emerging as a key target for intervention in cancer control. However the construct is poorly understood at present and has been measured differently across different studies. In addition, while fatalism is often used as an adjunct to other theories, little consensus has emerged about how it should be integrated. Some research has proposed that fatalism influences screening through effects on cancer fear (44), whereas others have shown that fatalism affects uptake via perceived barriers to screening (e.g. embarrassment, concerns about getting a cancer diagnosis) although pathways have also been shown to differ across samples (49, 50). The Powe Fatalism Index used in the current study was developed to examine the construct in African Americans although has been used by Powe on white samples as well (25). Future research could explore the relationship between cancer fatalism and other related constructs such as pessimism and locus of control (e.g. fate/ chance), and examine the stability of its factor structure across different populations with a view to further understanding the construct and determining how educational efforts addressed towards reducing fatalism might best be developed.
Unlike previous findings (7, 51) we did not observe gender, age or ethnicity effects on uptake. For age and ethnicity the proportions were in the same direction as previous research (i.e. higher uptake among older and white participants) and non-significant findings may reflect a lack of power to detect small effects, however for gender uptake levels were in the opposite direction (i.e. higher in men rather than women) (5). As women were much more likely to complete the questionnaire than men (38), the male participants were almost certainly less representative of men in general than the women, and probably had higher levels of health interest and motivation relative to men in the general population.
None of the variables that play a central role in theories of health motivation (risk, severity, response- and self-efficacy beliefs) predicted screening uptake in multivariate analyses. While there was a univariate association between self-efficacy beliefs and screening uptake, this became non-significant once either self-rated health or cancer fatalism were added into the model. This suggests that, for at least some of the respondents, physical limitations associated with poor self-rated health may have reduced their confidence to complete the test. Similarly the mediation by cancer fatalism suggests that poor self-efficacy relates to a more general sense of being unable to affect cancer-related outcomes. However in both cases the lack of specificity of the self-efficacy measure may have resulted in greater conceptual overlap with other measures. Future studies could address this by using measures of self-efficacy which assess a person’s confidence to adhere to behavioural requirements of CRC screening more directly (e.g. (52)) and see whether similar relationships with poor self-rated health and cancer fatalism remain when such measures are used.
While the lack of association between threat and response efficacy beliefs and screening uptake are consistent with some other research (23), methodological limitations may have played a role. Predictors were measured two to three years prior to receiving an invitation, which may have attenuated relationships, particularly for self-efficacy beliefs, which may be more state-like in comparison with variables such as depression or fatalism which may be more stable over time.
Lack of specificity in the measures may also have contributed because the questions addressed cancer in general and not bowel cancer per se. However both limitations also apply to cancer fatalism yet associations between the latter and screening uptake were still observed. However it has been argued that associations between perceived risk and disease severity need to take into account behavioral intentions, for example people may rate their risk as low because they intend to go for screening, thereby weakening any link between perceived cancer risk and screening uptake (53). In the present study, though, there was no immediate prospect of CRC screening, and no prospect of screening of any kind for men (women would be eligible for mammography) at the time of the survey, so this methodological limitation is unlikely to account for the absence of associations between risk and behavior in this particular study.
A key limitation of the study is that the postal survey was designed to examine attitudes to health and cancer worry rather than predictors of screening uptake. This means that factors identified as potential barriers to FOBt uptake were not assessed. Previous research, albeit cross-sectional, has shown that the association between fatalistic beliefs and mammography uptake are either partially or fully mediated by perceived barriers (50) and such relationships could not be explored here. A further limitation of this study was the attrition rate, however there were few differences between people who completed the survey and formed the sample for analysis and those who completed the survey but were excluded. The main limitation in terms of the sample therefore lies with people not having completed the original postal survey, and hence this limitation is shared with other questionnaire studies. Previous research has shown that people who complete questionnaires have higher SES than the average population (54) and so the results cannot be generalised to the whole population.
The present study found that cancer fatalism and poor self-rated health predict screening uptake and that there are beliefs about cancer that could be addressed in the years prior to a screening invitation that may enhance uptake among people with lower SES. Previous research has shown that while fatalism played a role in motivation (or intention) to attend for colorectal screening, poorer self-rated health played a role in intention-translation (rather than motivation) (26). The separate pathways observed between SES and FOBt uptake via self-rated health and fatalism in the present study could therefore reflect ways in which both intention and intention-translation can be affected by SES. Future research could examine the reasons why poorer self-rated health is associated with lower uptake of screening and develop interventions to reduce cancer fatalism in order to reduce socioeconomic disparities in screening uptake.
Acknowledgements
We would like to thank the James Wigg Practice, the Elizabeth Avenue Group Practice, and the Keats Group Practice who allowed us to contact their patients and invite them to participate in this research. We would also like to thank Mark Stewart for his work linking the survey data with data on screening uptake and Dennis Wright for supporting the project in its early stages.
This work was undertaken with the support of Camden and Islington Primary Care Trusts who received a proportion of funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive.
Support: This work was funded by Cancer Research UK and the NHS Bowel Cancer Screening Programme. The sponsors were not involved in the conduct of the research or the preparation of the article.
Footnotes
Conflict of interest: None.
Reference List
- (1).WHO. World Health Organization . Cancer Fact Sheet no 297. http://www.whoint/mediacentre/factsheets/fs297/en/index.html [date accessed: 2010 Jan. 22] [Google Scholar]
- (2).Aarts MJ, Lemmens VE, Louwman MW, Kunst AE, Coebergh JW. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer. 2010;46:2681–95. doi: 10.1016/j.ejca.2010.04.026. [DOI] [PubMed] [Google Scholar]
- (3).Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–49. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- (4).Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122:1357–67. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- (5).von Euler-Chelpin M, Brasso K, Lynge E. Determinants of participation in colorectal cancer screening with faecal occult blood testing. J Public Health. 2010;32:395–405. doi: 10.1093/pubmed/fdp115. [DOI] [PubMed] [Google Scholar]
- (6).Stock C, Brenner H. Utilization of lower gastrointestinal endoscopy and fecal occult blood test in 11 European countries: evidence from the Survey of Health, Aging and Retirement in Europe (SHARE) Endoscopy. 2010;42:546–56. doi: 10.1055/s-0029-1244127. [DOI] [PubMed] [Google Scholar]
- (7).von Wagner C, Good A, Wright D, Rachet B, Obichere A, Bloom S, et al. Inequalities in colorectal cancer screening participation in the first round of the national screening programme in England. Br J Cancer. 2009;101(Suppl 2):S60–3. doi: 10.1038/sj.bjc.6605392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lian M, Schootman M, Yun S. Geographic variation and effect of area-level poverty rate on colorectal cancer screening. BMC Public Health. 2008;8:358. doi: 10.1186/1471-2458-8-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Schootman M, Jeffe DB, Baker EA, Walker MS. Effect of area poverty rate on cancer screening across US communities. J Epidemiol Community Health. 2006;60:202–7. doi: 10.1136/jech.2005.041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).von Wagner C, Baio G, Raine R, Snowball J, Morris S, Atkin W, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol. 2011 doi: 10.1093/ije/dyr008. EPub ahead of print. [DOI] [PubMed] [Google Scholar]
- (11).Chapple A, Ziebland S, Hewitson P, McPherson A. What affects the uptake of screening for bowel cancer using a faecal occult blood test (FOBt): a qualitative study. Soc Sci Med. 2008;66:2425–35. doi: 10.1016/j.socscimed.2008.02.009. [DOI] [PubMed] [Google Scholar]
- (12).O’Sullivan I, Orbell S. Self-sampling in screening to reduce mortality from colorectal cancer: a qualitative exploration of the decision to complete a faecal occult blood test (FOBT) J Med Screen. 2004;11:16–22. doi: 10.1177/096914130301100105. [DOI] [PubMed] [Google Scholar]
- (13).Maiman LA, Becker MH. The Health Belief Model: Origins and correlates in psychological theory. Health Educ Monogr. 1974;2:336–53. [Google Scholar]
- (14).Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974;2:1–8. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- (15).Rogers RW. A protection motivation theory of fear appeals and attitude change. J Psychol. 1975;91:93–114. doi: 10.1080/00223980.1975.9915803. [DOI] [PubMed] [Google Scholar]
- (16).Rogers RW. Cognitive and physiological processes in fear appeals and attitude change: a revised theory of protection motivation. In: Cacioppo JT, Petty RE, editors. Social Psychophysiology: A sourcebook. The Guildford Press; New York: 1983. pp. 153–76. [Google Scholar]
- (17).Witte K. Putting the fear back into fear appeals: the Extended Parallel Process Model. Comm Monogr. 1992;59:329–49. [Google Scholar]
- (18).Hay JL, McCaul KD, Magnan RE. Does worry about breast cancer predict screening behaviors? A meta-analysis of the prospective evidence. Prev Med. 2006;42:401–8. doi: 10.1016/j.ypmed.2006.03.002. [DOI] [PubMed] [Google Scholar]
- (19).Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- (20).Wardle J, McCaffery K, Nadel M, Atkin W. Socioeconomic differences in cancer screening participation: comparing cognitive and psychosocial explanations. Soc Sci Med. 2004;59:249–61. doi: 10.1016/j.socscimed.2003.10.030. [DOI] [PubMed] [Google Scholar]
- (21).Robb KA, Miles A, Wardle J. Demographic and psychosocial factors associated with perceived risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:366–72. [PubMed] [Google Scholar]
- (22).McQueen A, Vernon SW, Myers RE, Watts BG, Lee ES, Tilley BC. Correlates and predictors of colorectal cancer screening among male automotive workers. Cancer Epidemiol Biomarkers Prev. 2007;16:500–9. doi: 10.1158/1055-9965.EPI-06-0757. [DOI] [PubMed] [Google Scholar]
- (23).Sutton S, Wardle J, Taylor T, McCaffery K, Williamson S, Edwards R, et al. Predictors of attendance in the United Kingdom flexible sigmoidoscopy screening trial. J Med Screen. 2000;7:99–104. doi: 10.1136/jms.7.2.99. [DOI] [PubMed] [Google Scholar]
- (24).Leganger A, Kraft P. Control constructs: Do they mediate the relation between educational attainment and health behaviour? J Health Psychol. 2003;8:361–72. doi: 10.1177/13591053030083006. [DOI] [PubMed] [Google Scholar]
- (25).Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs. 1995;18:385–92. [PubMed] [Google Scholar]
- (26).Power E, van Jaarsveld CH, McCaffery K, Miles A, Atkin W, Wardle J. Understanding intentions and action in colorectal cancer screening. Ann Behav Med. 2008;35:285–94. doi: 10.1007/s12160-008-9034-y. [DOI] [PubMed] [Google Scholar]
- (27).Niederdeppe J, Levy AG. Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer Epidemiol Biomarkers Prev. 2007;16:998–1003. doi: 10.1158/1055-9965.EPI-06-0608. [DOI] [PubMed] [Google Scholar]
- (28).Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psych Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- (29).Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- (30).Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–39. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- (31).Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA. 1990;264:1541–5. [PubMed] [Google Scholar]
- (32).Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol. 1995;14:88–90. doi: 10.1037//0278-6133.14.1.88. [DOI] [PubMed] [Google Scholar]
- (33).Aggarwal A, Freund K, Sato A, Adams-Campbell LL, Lopez AM, Lessin LS, et al. Are depressive symptoms associated with cancer screening and cancer stage at diagnosis among postmenopausal women? The Women’s Health Initiative observational cohort. J Womens Health. 2008;17:1353–61. doi: 10.1089/jwh.2007.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ludman EJ, Ichikawa LE, Simon GE, Rohde P, Arterburn D, Operskalski BH, et al. Breast and cervical cancer screening specific effects of depression and obesity. Am J Prev Med. 2010;38:303–10. doi: 10.1016/j.amepre.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Price MA, Butow PN, Charles M, Bullen T, Meiser B, McKinley JM, et al. Predictors of breast cancer screening behavior in women with a strong family history of the disease. Breast Cancer Res Treat. 2010;124:509–19. doi: 10.1007/s10549-010-0868-1. [DOI] [PubMed] [Google Scholar]
- (36).Cohen S, Kaplan GA, Salonen JT. The role of psychological characteristics in the relation between socioeconomic status and perceived health. J Appl Soc Psychol. 1999;29:445–68. [Google Scholar]
- (37).Sargent-Cox KA, Anstey KJ, Luszcz MA. Determinants of self-rated health items with different points of reference: implications for health measurement of older adults. J Aging Health. 2008;20:739–61. doi: 10.1177/0898264308321035. [DOI] [PubMed] [Google Scholar]
- (38).Miles A, Voorwinden S, Chapman S, Wardle J. Psychologic predictors of cancer information avoidance among older adults: the role of cancer fear and fatalism. Cancer Epidemiol Biomarkers Prev. 2008;17:1872–9. doi: 10.1158/1055-9965.EPI-08-0074. [DOI] [PubMed] [Google Scholar]
- (39).Grundy E, Holt G. The socioeconomic status of older adults: how should we measure it in studies of health inequalities? J Epidemiol Community Health. 2001;55:895–904. doi: 10.1136/jech.55.12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Prescott-Clarke P, Primatesta P. Health survey for england. London, HMSO: 1994. [Google Scholar]
- (41).Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult. Arch Intern Med. 1999;159:1701–4. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- (42).Witte K, Cameron KA, McKeon JK, Berkowitz JM. Predicting risk behaviors: development and validation of a diagnostic scale. J Health Commun. 1996;1:317–41. doi: 10.1080/108107396127988. [DOI] [PubMed] [Google Scholar]
- (43).UK CRC Screening Pilot Evaluation Team . Evaluation of UK Colorectal Cancer Screening Pilot: Report Supplement 2003. [2007 Dec. 11]. http://www.cancerscreening.nhsuk/bowel/reportsupplement.pdf [date accessed: 2007 Dec. 11] [Google Scholar]
- (44).Champion VL, Skinner CS, Menon U, Rawl S, Giesler RB, Monahan P, et al. A breast cancer fear scale: psychometric development. J Health Psychol. 2004;9:753–62. doi: 10.1177/1359105304045383. [DOI] [PubMed] [Google Scholar]
- (45).Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Allyn and Bacon; Boston: 2007. [Google Scholar]
- (46).Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- (47).Robb KA, Simon AE, Wardle J. Socioeconomic disparities in optimism and pessimism. Int J Behav Med. 2009;16:331–8. doi: 10.1007/s12529-008-9018-0. [DOI] [PubMed] [Google Scholar]
- (48).Philip EJ, DuHamel K, Jandorf L. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: a preliminary study. Cancer Causes Control. 2010;21:1685–91. doi: 10.1007/s10552-010-9597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Baron-Epel O, Friedman N, Lernau O. Fatalism and Mammography in a Multicultural Population. Oncol Nurs Forum. 2009;36:353–61. doi: 10.1188/09.ONF.353-361. [DOI] [PubMed] [Google Scholar]
- (50).Baron-Epel O. Attitudes and beliefs associated with mammography in a multiethnic population in Israel. Health Educ Behav. 2010;37:227–42. doi: 10.1177/1090198109339460. [DOI] [PubMed] [Google Scholar]
- (51).Szczepura A, Price C, Gumber A. Breast and bowel cancer screening uptake patterns over 15 years for UK south Asian ethnic minority populations, corrected for differences in socio-demographic characteristics. BMC Public Health. 2008;8:346. doi: 10.1186/1471-2458-8-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).von Wagner C, Semmler C, Good A, Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: The role of information processing. Patient Educ Couns. 2009;75:352–7. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- (53).Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26:136–45. doi: 10.1037/0278-6133.26.2.136. [DOI] [PubMed] [Google Scholar]
- (54).Wardle J, Williamson S, McCaffery K, Sutton S, Taylor T, Edwards R, et al. Increasing attendance at colorectal cancer screening: testing the efficacy of a mailed, psychoeducational intervention in a community sample of older adults. Health Psychol. 2003;22:99–105. doi: 10.1037//0278-6133.22.1.99. [DOI] [PubMed] [Google Scholar]