Abstract
Collagen type IV is the major structural component of the basement membrane and COL4A1 mutations cause adult small vessel disease, familial porencephaly and HANAC syndrome. Here we show that animals with a Col4a1 missense mutation (Col4a1+/Raw) display focal detachment of the endothelium from the media and age dependent defects in vascular function including a reduced response to nor-epinephrine. Age-dependent hypersensitivity to acetylcholine, is abolished by inhibition of nitric oxide synthase (NOS) activity, indicating that Col4a1 mutations affect vasorelaxation mediated by endothelium-derived nitric oxide (NO). These defects are associated with a reduction in basal NOS activity and the development of heightened NO sensitivity of the smooth muscle. The vascular function defects are physiologically relevant as they maintain in part the hypotension in mutant animals, which is primarily associated with a reduced red blood cell volume due to a reduction in red blood cell number, rather than defects in kidney function. To understand the molecular mechanism underlying these vascular defects we examined the deposition of collagen type IV in the basement membrane, and found it to be defective. Interestingly, this mutation also leads to activation of the unfolded protein response. In summary, our results indicate that mutations in Col4a1 result in a complex vascular phenotype encompassing defects in maintenance of vascular tone, endothelial cell function and blood pressure regulation.
Introduction
The basement membrane (BM) is a specialized extracellular matrix structure that compartmentalizes tissues, provides structural support and influences cell behaviour (1). Collagen type IV is the major structural component of the BM and in vertebrates, six different collagen type IV alpha chains (α1(IV) - α6(IV)) have been described which produce three different collagen type IV protomers (α1.α1.α2(IV), α3.α4.α5(IV) and α5.α5.α6(IV) (2).
The most abundant isoform, α1.α1.α2(IV), contributes to the formation of many basement membranes and its deficiency causes embryonic lethality in mouse (3). COL4A1 mutations have recently been identified as a cause of familial porencephaly, adult small vessel disease, hemorrhagic stroke, and a new syndrome called HANAC (hereditary angiopathy with nephropathy aneurysm and cramps) (for review see (4)). In mice deficiency in Col4a1 and Col4a2 and missense mutations in Col4a2 lead to haemorrhaging during development (3, 5) underscoring the important structural role of Col4a1 and Col4a2 in the vasculature.
In the vascular wall, the BM borders the vascular smooth muscle cells (VSMC) and separates them from the endothelium, which lines the vessel lumen (1). The vascular endothelium plays a crucial role in vasodilation through generating vasodilator agents including nitric oxide (NO), prostaglandins I2 and E2 and endothelium-derived hyperpolarizing factor (EDHF) (6). In large conduit vessels such as the descending aorta, the NO-cGMP pathway predominates whereby endothelium-derived NO activates soluble guanylate cyclase in the VSMC to generate cGMP, ultimately leading to smooth muscle relaxation (7, 8). The resulting vasodilation can be blocked by NOS inhibitors, such as L-Nω nitroarginine methyl ester (L-NAME). Clinically, endothelial cell dysfunction affects blood pressure control (9, 10) and promotes atherosclerosis (11).
Recently, we have characterised an allelic series of Col4a1 mouse mutants which display a spectrum of eye phenotypes combined with glomerulopathy (12, 13). In one of these mutants, the Col4a1+/Raw mutation (K950E) changes a lysine residue to glutamic acid at a Y residue of the Gly-X-Y collagen repeat in the collagen domain (12). Col4a1+/Raw animals are characterised by retinal arteriolar silvering due to focal BM defects (12, 13). However, the described function of Col4a1 in angiogenesis (for review see (1)), which can be mediated through integrin signaling (14), suggests additional functional roles in the vasculature.
To gain further insight into the role of Col4a1 in the vasculature, the vascular phenotypes of Col4a1+/Raw mice were explored including a structural and functional analysis of descending aortae. Mutant animals display haemorrhagic stroke and in descending aortae focal detachment of endothelial cells from the media occurs. Mutant animals have altered VSMC and EC function which affects the NO-cGMP pathway. Blood pressure analysis revealed hypotension in mutant animals that is associated with a reduced blood volume and is maintained in part by the described vasodilation defects. We also show that the Col4a1+/Raw mutation results in defective deposition of collagen type IV in the BM of descending aortae and leads to activation of the unfolded protein response. These results show for the first time that defects in the basement membrane in general, and collagen type IV in particular, affect the maintenance of vascular tone, endothelial cell function and blood pressure regulation.
Results
Col4a1+/Raw mutation results in perinatal cerebral haemorrhages and reduced viability
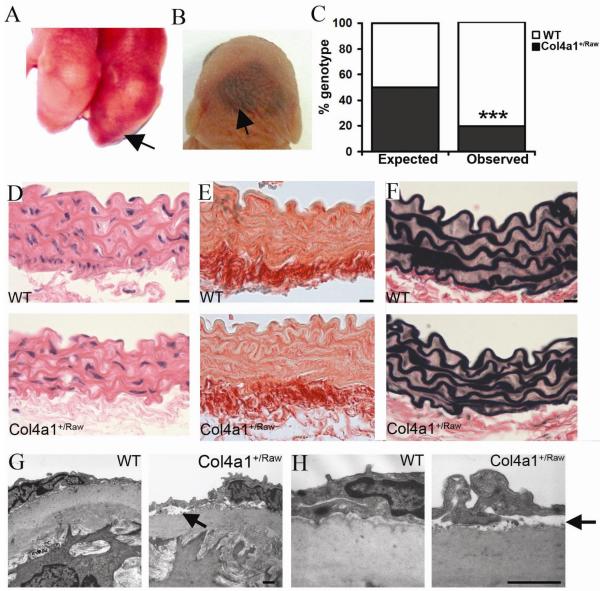
Analysis of newborn Col4a1+/Raw pups revealed mild cerebral haemorrhaging in mutant pups (Fig. 1A) with more severe haemorrhaging being observed in pups that died shortly after birth (Fig. 1B). The perinatal cerebral haemorrhaging is associated with reduced viability at weaning as we only detect 19.8% Col4a1+/Raw animals compared to the expected 50% (χ2 = 56.6, one degree of freedom, P<0.0001) (Fig. 1C).
Figure 1.
Vascular phenotypes of Col4a1+/Raw mice. (A,B) Mild and more severe cerebral haemorrhaging in Col4a1+/Raw pups (arrow). (C) Predicted (50%) and observed (19.8%) percentage of Col4a1+/Raw animals (31/156) (black) at weaning (χ2 = 56.6, one degree of freedom, p<0.0001). (D) Lack of gross histological phenotype in mutant aortae (E) Normal total collagen and elastin (F) deposition in mutant animals. (Data shown for 6 month old animals, see supplemental data for 3 and 9 month old animals). (G) Focal endothelial detachment in aortae from Col4a1+/Raw animals (arrow). (H) Higher magnification of EM analysis. Size 1μm (g,h), 10μm (d-f).
Mutations in Col4a1 results in ultrastructural changes in the vessel wall
Patients with COL4A1 mutations can suffer from aneurysm formation of the carotid artery (15) suggesting that COL4A1 mutations may affect the structure of large calibre vessels. We therefore performed histopathological and ultrastructural analysis on descending aortae of 6 month old Col4a1+/Raw animals which revealed no gross morphological defects and normal total collagen and elastin deposition was observed (Fig. 1D-F, Supplemental Fig. 1). However ultrastructurally Col4a1+/Raw mice displayed areas of focal detachment of the intact endothelium from the underlying media (Fig. 1G and H).
Col4a1+/Raw animals have defects in vascular smooth muscle cell function
To analyse if the structural BM defects affect vascular function, myography was performed on rings from descending aortae of 3, 6 and 11-15 month old animals. VSMC function, assessed by contraction in response to KCl containing-physiological salt solution (K-PSS (125mM KCl), was impaired in Col4a1+/Raw aortae (Fig. 2A; n=6, p = 0.001). This indicates reduced contractile strength independent of endothelial function (16) and suggests that BM defects may weaken the vessel thereby increasing the susceptibility to cerebral haemorrhaging; observed in patients and mice with COL4A1 mutations.
Figure 2.
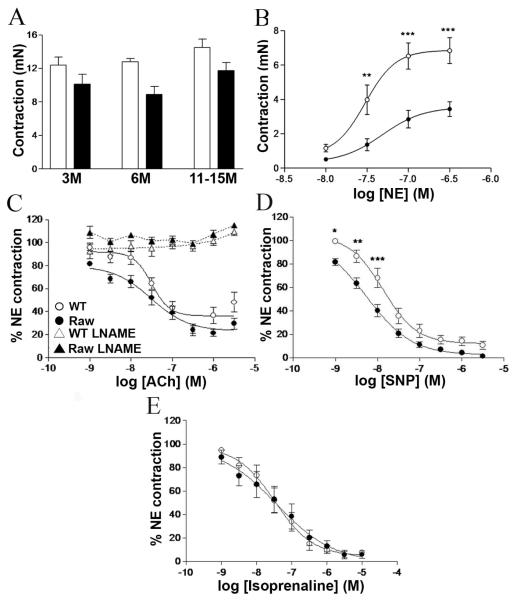
Assessment of vascular function. (A) Mutant animals (black bars) have reduced amounts of contraction (mN) in K-PSS (p=0.0012 two way Anova, n=6 for 3 and 6 month old animals, n=10 for 11-15 month old). (B) Reduced contraction to Nor-Epinephrine in 6 month old mutant animals (n=6) (two way Anova p<0.001, 6 months). (C) 11-15 Month old mutant animals display increased vasodilation to low concentrations of ACh (two way Anova, n=10, p<0.0001). Incubation of vessels in LNAME (labelled +L-NAME) before administration of ACh abolishes vasodilation. (D) Vasodilation to SNP in 11-15 month old mutant animals shows increased relaxation (two way Anova, n=10, p<0.0001). (E) Vasodilation to Iso-Prenaline in 11-15 month old animals (n=6). WT (open circles), Col4a1+/Raw (filled circles). Error bars indicate standard error of measurement. * p< 0.05, ** p< 0.01, *** p<0.001 post hoc test
Reduced contractile responses to the adrenoceptor agonist, nor-epinephrine (NE), were observed in 6 month (Fig. 2B) and 11-15 month (Supplemental Fig. 2B) old mutant animals (two way ANOVA p < 0.0001) but were normal in 3 month old animals (Supplemental Fig. 2A). These data would suggest that an age-dependent defect in VSMC function develops between 3 and 6 months of age after the onset of reduced vessel wall strength.
Mutations in Col4a1 affect endothelial cell function and NO/cGMP mediated vasodilatation
Endothelial cell-mediated vasodilatation was assessed in 3, 6 and 11-15 month old animals through the vasodilation responses to acetylcholine (ACh) on NE (EC80)-preconstricted aortic rings. Endothelium-dependent vasorelaxation was not different between 3 month and 6 month old WT and mutant animals (Supplemental Fig. 2C, 2D). However, in contrast aortae of 11-15 month old mutant animals displayed significantly increased vasodilatation to ACh (two way ANOVA; p <0.0001; Fig. 2C), indicating that this age-dependent phenotype becomes significant after 6 months. The response to ACh was mediated through the NO-cGMP pathway as blockade of NOS activity by L-NAME abolished Ach-induced vasodilation both in Col4a1+/Raw and WT aortic rings (Fig. 2C). Because changes in NO generation can be accompanied by alterations in the response of the smooth muscle cells to NO (17), aortic rings were exposed to the NO donor, sodium nitroprusside (SNP). No differences were observed in 3 and 6 month old mutant and WT animals (Supplemental Fig. 2E, 2F). In contrast, a significant increase in SNP-induced vasodilation was observed in the 11-15 month old animals (Fig. 2D), confirming the development of increased NO sensitivity in smooth muscle. These results show that Col4a1 mutations can affect NO-mediated vasodilation and NO-sensitivity, suggesting an upregulation of the NO/cGMP pathway.
The observed vasodilation defects are specific to the NO-cGMP pathway as the response to the β2 adrenoceptor agonist, isoprenaline, which induces cAMP-mediated vasodilatation (18), was similar between WT and mutant animals (Fig. 2E). These results also indicate that the smooth muscle of mutant vessels is able to maintain tension.
Col4a1 mutant animals have reduced basal NOS function
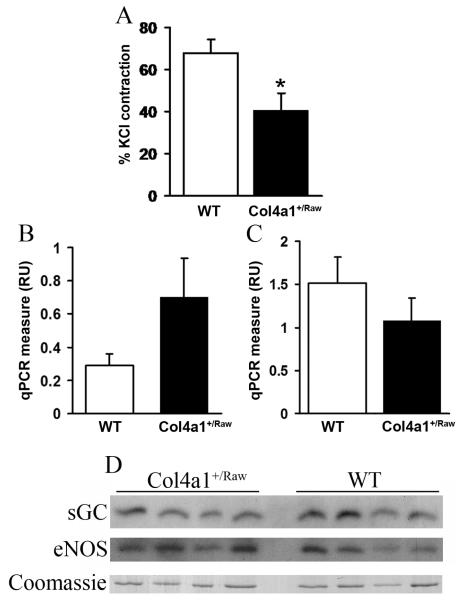
To further investigate the NO-cGMP pathway we assessed basal NOS function in 12 month old aortae by measuring the constriction induced by L-NAME (19). Mutant aortae showed a 40% reduction in LNAME-induced contraction (p < 0.05) (Fig. 3A) indicating that mutant animals have reduced basal NOS function. This reduction in activity is independent of changes in eNOS mRNA and protein expression as shown by quantitative RT-PCR and western blot analysis (Fig. 3C, 3D). Similarly the increased NO-sensitivity of the smooth muscle is independent of changes in expression of cGMP-dependent protein kinase 1 (Fig. 3B) and soluble guanylate cyclase respectively (Fig. 3D).
Figure 3.
Reduced basal NOS function in Col4a1+/Raw. (A) Contraction obtained by administration of L-NAME as measure of amount of NO in 11-15 month animals expressed as percentage of contraction obtained with KCl. (n= 14 WT, n=11 Col4a1+/Raw, t-test p <0.05). (B) qRT-PCR for PRKG1 (cGMP dependant protein kinase I). Expressed as relative units to 18S RNA levels. (n=6) (C). eNOS mRNA levels in 12 month old WT (white) and Col4a1+/Raw (black) (n=6) 3 animals as determined by qRT-PCR relative to 18S RNA levels (p = 0.17 t-test). (D) eNOS, and sGC protein expression levels in aortae of 11-15 month old animals. Predominant band of coomassie gel is given as loading control (entire gel is provided in Supplemental Fig. 3). Error bars indicate standard error of measurement. * p< 0.05 t-test.
Collagen type IV influences vascular homeostasis
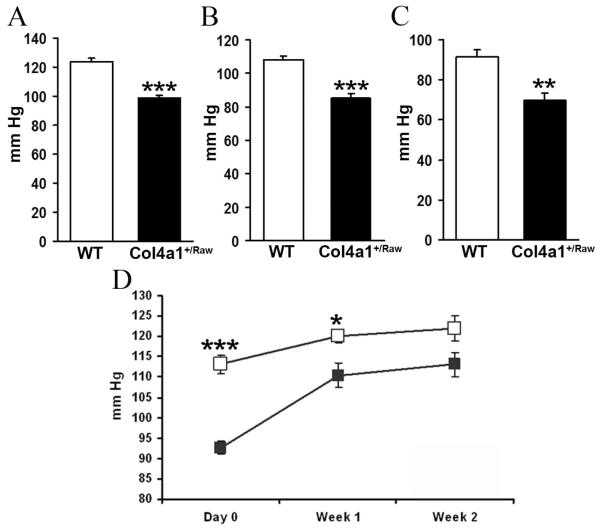
Vascular resistance is one of the key mechanisms involved in blood pressure regulation, which can be altered through alterations in vasodilation (9) and/or vasoconstriction (20). To investigate if the structural defects of Col4a1+/Raw animals affect vascular homeostasis, the systolic blood pressure of 4-6 and 14 month old mice was measured by tail plethysmography. This revealed that in both cohorts Col4a1+/Raw animals have a reduction of 20-25 mm Hg in their systolic blood pressure (P<0.001 for both cohorts) (Fig. 4A, 4B) indicating that mutations in collagen type IV can result in hypotension. Our results differ from previous reports in which animals with a deletion of exon 40 in Col4a1 were normotensive using tail plethysmography (21). It is known that different mutations in the same collagen alpha chain can affect the displayed phenotypes and phenotype severity (12, 22). Moreover tail cuff recording of blood pressure in mice can be imprecise, particularly in hypotensive states, and is best confirmed using direct measurement (23). We therefore measured the blood pressure of Col4a1+/Raw animals using direct arterial cannulation. This confirmed that the blood pressure was 20-25 mm Hg lower in mutant animals and extended the observation to animals at 40 days of age (Fig. 4C).
Figure 4.
Collagen type IV and blood pressure regulation. (A) Systolic blood pressure of 4-6 months old animals. (Col4a1+/Raw 99 mm Hg (n=6), WT 124 mm Hg (n=5), p<0.001 t-test) (B) Systolic blood pressure of 14 month old animals. (Col4a1+/Raw 85.1mm Hg, WT 108.1mm Hg, n=6, p< 0.001, t-test). (C) Mean arterial blood pressure of 40 day old animals (Col4a1+/Raw 70 mm Hg (n=5), WT 91 mm Hg (n=7), p<0.01, t-test). (D) Systolic blood pressure of 14 month old animals before and during treatment with L-NAME. Measurements were collected at day 0, day 7 and day 14 of treatment. (WT, n=9, Col4a1+/Raw n=7) * p< 0.05, ** p< 0.01, *** p<0.001 t- test
The different ages of onset of the hypotension and vasodilation defects data indicate that the vasodilation defects do not cause the hypotension. However once established the vasodilation defects may be important for maintaining the hypotension by reducing vascular resistance. Consequently L-NAME was administered chronically to 14 month old animals and systolic blood pressure measurements were collected before and during treatment. Two weeks of L-NAME treatment resulted in an increase in blood pressure in WT animals of 8.8mm Hg compared to 20.5mm Hg in mutant animals (p< 0.01) (Fig. 4D). Consequently the treatment resulted in a partial rescue of the hypotension with systolic blood pressure of 121.9 mmHg and 113.1 mm HG for WT and mutant animals respectively. These data confirm the hypothesis that the vasodilation defect is important for the maintenance of the hypotension.
Hypotension in Col4a1 mutant animals is associated with a reduced blood volume
Since the hypotension is evident before the onset of the vascular function defects, we investigated alternative causes of low blood pressure. Given the pivotal role of the kidney in blood pressure homeostasis (24), sodium excretion was analysed in 40 day old animals (Fig. 5A) which showed a marked reduction in sodium clearance in mutant animals compared to WT (P< 0.01), indicating that renal sodium wasting does not maintain the hypotension. There was a significant increase in plasma aldosterone in mutant animals (Fig. 5B), suggesting activation of the renin-angiotensin-aldosterone system (RAAS) which would promote sodium retention in the distal nephron.
Figure 5.
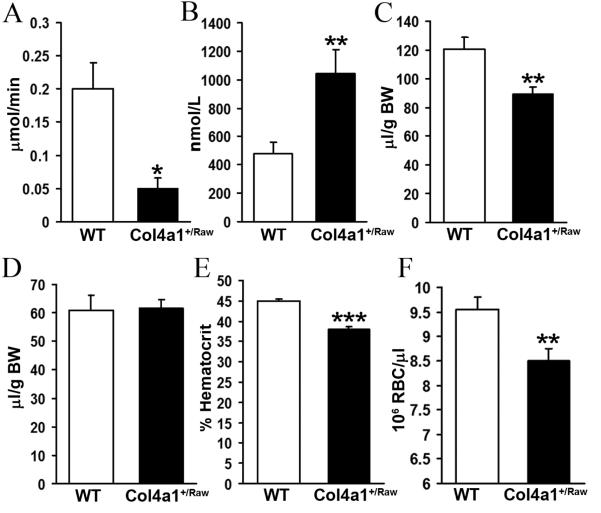
Low blood pressure is associated with reduced blood volume. (A) Sodium excretion in 40 day old animals (Col4a1+/Raw 0.05 μmol/min (n=5), WT 0.2 μmol/min (n=7), p<0.05 t-test) (B) Plasma aldosterone levels in 40 days old animals. (Col4a1+/Raw 1044 nmol/L (n=5), WT 476 nmol/L (n=7), p<0.01 t-test) (C) Blood volume in animals (Col4a1+/Raw 88.9 μl/g body weight (n=10), WT 120 μl/g body weight (n=13), p<0.01 t-test). (D) Plasma volume of animals. (E) Reduced hematocrit in mutant animals (Col4a1+/Raw 38% (n=15), WT 45% (n=19), p<0.001 t-test). (F) Reduced red blood cell number in Col4a1+/Raw animals. (Col4a1+/Raw 8.55 × 106 RBC/μl (n=10), WT 9.50 × 106 RBC/μl (n=12), p<0.01 t-test). Error bars indicate standard error of measurement. * p< 0.05, ** p< 0.01, *** p<0.001 t- test
Given the close relation between blood volume and blood pressure, we calculated the blood volume in 40 day to 8 month old animals following measurement of plasma volume and hematocrit. These analyses revealed a ~26% reduction in blood volume in mutant animals (Fig. 5C, P< 0.01) which is consistent with activation of the RAAS and increased circulating aldosterone levels. However, Col4a1+/Raw animals have normal plasma volume (Fig. 5D) and the reduction in blood volume reflects a lower average hematocrit of 38% compared to 45% for WT animals (Fig. 5E, P < 0.001). These data indicate that the lower blood volume is caused by a decrease in packed cell volume. The decreased packed cell volume is due to a reduction in red blood cell number in mutant animals. Measurement of red blood cell number (RBC) showed an 11% reduction in red blood cell number in mutant animals (Fig. 5F, P< 0.01) as Col4a1+/Raw animals have 8.55 × 106 RBC/μl compared to 9.50 × 106 RBC/μl for WT animals. Thus the red blood cell volume reflects a reduction in red blood cell number.
Col4a1 mutation result in activation of the unfolded protein response
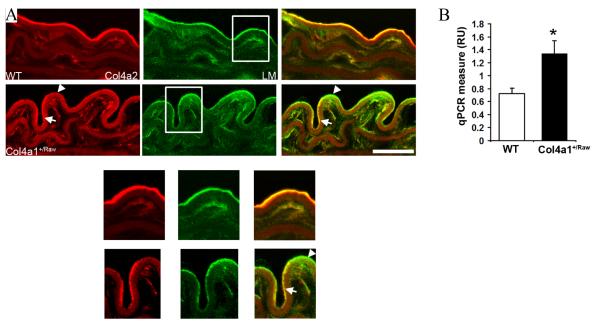
To further characterize the molecular consequences of the Col4a1+/Raw mutation and how it may lead to the vascular phenotypes, immunostaining was performed on descending aortae from 12 month old animals. This revealed interrupted staining for the α1.α1.α2(IV) network compared to continuous laminin staining, suggesting a defective deposition of collagen type IV and altered BM composition (Fig.6A).
Figure 6.
Col4a1+/Raw mutation results in UPR activation. (A) Interrupted staining for Col4a2 in vascular BM of descending aortae from mutant animals in contrast to continuous laminin (LM) staining. (arrow indicate presence of Col4a2 in BM. Arrowhead indicate absence of Col4a2 as indicated by green staining in merged image) Size 20 μm. Detail (white square) is provided below panel A. (B) Increased Bip mRNA expression levels in mutant animals. Expressed as relative units to 18S RNA levels (p< 0.05 t-test, n=9).
In the ER three collagen type IV alpha chains interact and form a collagen type IV protomer. Mutations in proteins that affect protein folding can lead to ER stress which may lead to activation of the unfolded protein response (UPR) (25). The presence of swollen ER vesicles and increased expression of ER chaperones in Col4a1 mutant animals (12, 26) suggest the occurrence of ER stress. To investigate if this occurs within the vasculature and leads to UPR activation, quantitative RT-PCR analysis was performed to assess Bip (also known as GRP78) mRNA expression levels, a marker for UPR activation. This revealed a 1.7 fold increased Bip mRNA expression level in mutant animals (Fig. 6B, p < 0.05 t-test) indicative of ER stress and UPR activation.
Discussion
The identification of COL4A1 mutations in patients with familial porencephaly (27-29) and HANAC syndrome (15) combined with vascular phenotypes in mice (for review see (4)) underline the important structural role of type IV collagen in the vasculature. However, the role of collagen type IV in angiogenesis implies that collagen type IV and the basement membrane possess additional non-structural roles (1). Here we describe that a Col4a1 mutation affects vascular function and homeostasis thereby highlighting a potential novel functional role for type IV collagen.
The Col4a1+/Raw mutation affects both the capillaries and large calibre vessels, which in neonates results in the occurrence of cerebral haemorrhaging. Although in another Col4a1 animal model these haemorrhages can lead to the occurrence of porencephaly (28), no evidence of porencephaly was observed in the Col4a1+/Raw animals (12). This milder phenotype has also been observed in the eye (12) and is probably due to the less severe effects of a mutation affecting a lysine residue at the Y position of the Gly-X-Y repeat compared to the effects of a Gly mutation at the Gly position.
In descending aortae the mutation results in the focal separation of the endothelium from the media. These structural defects are associated with a complex functional phenotype affecting both VSMC and EC function. The reduced contraction in K-PSS in Col4a1+/Raw animals indicates a reduction of contractile strength independent of endothelial function (16). These data confirm that the structural BM defects weaken the vessel which would increase the susceptibility to cerebral haemorrhaging observed in patients and mice with COL4A1 mutations (15, 21, 27-31).
In addition to a reduction in contractile strength, Col4a1+/Raw animals develop a late onset VSMC phenotype and complex EC phenotype. Basal endothelial activity is reduced as shown by a blunted contraction in response to L-NAME (19). However stimulated NO generation is increased as illustrated by the increased sensitivity to ACh which is abolished by L-NAME-mediated inhibition of NOS, indicating that the mutation affects NO-mediated vasodilation. Moreover the vessels develop an increased response to the NO donor, SNP, suggestive of heightened NO sensitivity of the smooth muscle. Taken together these results suggest an up regulation of the downstream relaxative mechanism, possibly in response to reduced basal NO production as observed in eNOS deficient animals (32, 33). Our data suggests that the sensitisation of this pathway does not compensate the endothelial dysfunction under basal conditions but becomes apparent upon endothelium stimulation. In addition these results support the hypothesis that vascular smooth muscle cells have the capacity to increase their NO sensitivity as a protective measure to counter the influence of partially reduced endothelial function. This hypothesis is also supported by the heightened NO sensitivity of heterozygous eNOS deficient animals (17). In eNOS deficient animals, the increased NO sensitivity is mediated through elevated activity of soluble guanylate cyclase (32) and cGMP-dependent protein kinase (PKG1) (33). It is likely that a similar mechanism occurs in Col4a1+/Raw animals as no changes in expression levels were observed for sGC, eNOS and PKG1.
The chronic administration of L-NAME and partial rescue of the hypotension indicates that the vasodilation defects contribute to the maintenance of the low blood pressure. However, they are unlikely to be causal as the hypotension precedes the onset of the vasodilation defect. Renal salt wasting causes low blood pressure in several genetic disorders (for review see (24)) but in this analysis sodium excretion was reduced in Col4a1+/Raw animals. Thus the kidney is adapting appropriately to low blood pressure by activation the RAAS (24).
Interestingly we showed that the hypotension is associated with a reduction in blood volume due to a decreased packed cell volume caused by a reduction in red blood cell number. The reduced hematocrit and red blood cell number are supported by other mouse models with Col4a1 mutations (5) although no vascular function and blood pressure regulation was measured in these mice. These results suggest that the Col4a1 mutation is associated with hypotension at least in part due to a reduction in blood volume and that the hypotension is maintained at least in part by the vasodilation defect.
The mechanism underlying the reduced red blood cell number remains unknown. Because Col4a1 mutations lead to haemorrhaging (our data and (21, 28)), chronic haemorrhaging may underlie this phenotype. Given that no severe haemorrhaging has been observed in mutant animals (unpublished data), this would be caused by multiple microbleeds. Alternatively, the Col4a1 mutation may affect erythropoiesis as blood progenitor cells need to transmigrate a basement membrane before being released into the peripheral blood and these cells express collagen type IV degrading gelatinases (34). Thus this migration may be affected due to mutations in type IV collagen. In addition erythroid differentiation requires interaction with extracellular matrix proteins such as fibronectin, mediated through integrin alpha4beta1 (35), but erythroid progenitor cells also express integrin receptors which can bind the BM protein laminin (36). Further research is required to determine the mechanism by which the Col4a1 mutations lead to a reduction in red blood cell number and blood volume.
One important question is how a mutation affecting a basement membrane protein can affect endothelial cell function and vasodilation. ER stress and UPR activation can be triggered by mutations that affect protein folding. The triple helical domain of collagen type IV contains numerous (> 20) interruptions which are thought to be important for its flexibility and function given that their location within the alpha chain is highly conserved (37). The sequences flanking the interruptions are atypical suggesting that they may play an important role at the molecular level related to triple helix stability (38). The Col4a1+/Raw mutation changes a lysine residue to glutamic acid at the Y position of a Gly-X-Y triplet that flanks a major interruption (12). Although most mutation in collagen proteins affect Glycine residues, the effects of the Col4a1+/Raw mutation combined with the inherent instability of the interruption may severely affect protein folding. Our data show that the Col4a1+/Raw mutation leads to UPR activation, a coordinated response activated by and aimed at alleviating ER stress (25). It is thus tempting to suggest that the defective deposition of collagen type IV in the BM is an indication of reduced secretion with intracellular retention of collagen type IV causing ER stress. This is supported by analysis of other Col4a1 mutant mouse models that revealed intracellular retention of Col4a1, increased chaperone expression and presence of swollen ER vesicles (12, 26, 28). UPR activation has also been observed in response to mutations in collagen type X (39) where it results in cellular reprogramming. Chronic ER stress is a pathologic mechanism that may lead to diseases such as diabetes (40). Thus our data raise the interesting hypothesis that the vascular defects may be associated with and possibly due to UPR activation. In this context it is interesting to note that ER stress and UPR activation has been observed in endothelium in areas prone to atherosclerosis (41), in which endothelial cell dysfunction plays an important role and is one of the earliest markers. The identification of UPR activation due to Col4a1 mutations is also of clinical relevance as modulation of UPR activation provides an attractive target for intervention. Indeed, UPR activation has recently been modified successfully in a mouse model of diabetes (40).
In conclusion, we have identified that mutations in collagen type IV are associated with UPR activation and defects in vascular function and blood pressure regulation. As collagen type IV is part of the basement membrane, it is tempting to speculate that other basement membrane proteins and their receptors may have similar roles in vascular biology. In this regard it is interesting to note that endostatin, a proteolytically cleaved fragment of collagen XVIII (42), induces endothelium-dependent vasorelaxation (43), although the role of collagen XVIII itself has not been investigated. Finally, it is now important to assess these phenotypes in patients with COL4A1 mutations in order to gain a complete understanding of the spectrum of disease phenotypes and processes affected by COL4A1 mutations.
Materials and Methods
Animals
Studies were performed under guidance from the UK Home Office. Animals were backcrossed for 5 generations on a Bl/6 background and control animals were WT littermates.
Ultrastructural analysis
Tissue were collected from 3 adult animals (3 and 6 months old) and processed as previously described (12).
Histopathology
Aortae were fixed overnight in 10% neutral buffered formalin or 4% paraformaldehyde and embedded in paraffin wax. Sections were stained with Hematoxylin-Eosin, Sirius Red or Verhoeff stain using standard protocols (n=3).
Systolic Blood Pressure Analysis
Systolic blood pressure was measured using tail cuff plethysmography on restrained conscious animals using an IITC model 129 analyser (Woodland Hills, CA, USA). All animals underwent three periods of training prior to recording measurements. Per animal, the data were generated from the average of 4 readings taken on three independent occasions.
In vivo studies
Mice were anesthetized with Inactin (50mg/kg IP) and prepared surgically for renal function experiments as described (44). Mice were infused IV with isotonic saline at a rate of 200ul/h/10g body weight. After a 40 minute equilibration period, MABP was recorded for 30-40 minutes on a Powerlab, sampling at a rate of 2Hz and urine was collected for 1 hour. Following this, Evans Blue (1μl/g of a 0.5% solution w:v) was injected for measurement of plasma volume: blood volume was calculated from this and hematocrit (44). At the end of the experiment, a 500-μl blood sample was taken for measurements of aldosterone. Sodium concentration in urine and plasma was measured by ISE (Roche). Red blood cell number was determined using blood samples collected from the tail or cheek vein and blood counts were performed using a coulter counter (Beckman). Statistical analysis was done by Student’s t-test.
Myography
Aortic rings (2 mm) were prepared for myography on a wire myograph (DMT myographs, Denmark) in PSS (NaCl, KCl, MgSO4, NaHCO3, KH2PO4, EDTA, Glucose, CaCl2), contracted 3 times in KPSS (PSS plus 125mM KCl) and contracted in 30μM Nor-epinephrine(NE) followed by a dilation in 1μM Acetylcholine (ACh). The following concentration-response curves were performed: NE (10−8M – 3×10−6 M), ACh (10−9M – 3×10−6 M), SNP (10−9M – 3×10−6 M) and Isoprenaline (10−9M – 3×10−5 M). Dilation response curves were performed following contraction (80% of maximum determined by NE concentration-response curve) with NE. ACh concentration-response curves were performed following incubation (15min) with 200 μM L-NAME. Basal NO levels were determined by measuring contraction following incubation (12min) with 200μM L-NAME after contraction with NE (30% of maximum. The amount of contraction was expressed as a percentage of the KCl contraction. All drugs were from Sigma (Dorset, UK). Statistical analysis was performed using Two-Way ANOVA followed by post hoc test.
RT-PCR
RNA was isolated using Tri-Reagent (Sigma, UK). First strand synthesis was performed using AMV-RT (Roche Applied Science) and quantitative real time PCR analysis was performed in triplicate using TaqMan Gene Expression (Applied Biosystems) assays on ABI System 7700 (eNOS) or using Stratagene Brilliant Sybrgreen QPCR (Prkg1, Bip) on MJ Research Chromo4 Thermal Cycler. mRNA levels were corrected using 18sRNA mRNA levels and statistical analysis was performed using Student t-test.
Immunoblotting
Protein samples were extracted from descending aorta in the presence of RIPA buffer containing EDTA protease (Roche Applied Science) and phosphatase inhibitors (Phostop Roche) using standard protocols. Primary antibodies eNOS( I/1000 BD Transduction Laboratories) and, sGC (1/2000 Sigma). Protein levels were corrected for coomassie staining of a predominant protein band and measured using Biorad GS700 Image Analyser software analysis. Statistical analysis was performed using unpaired t-test.
Immunohistochemistry
Cryosections were fixed for 10 minutes in acetone followed by antigen retrieval using 0.1M HCl/KCl for 10 minutes and standard protocols. The Laminin (1/1000) antibody was a kind gift from Dr. Ulrike Mayer and the H22 antibody against Col4a2 (1/100) from Dr. Yoshikazu Sadu.
Supplementary Material
Acknowledgements
We would like to acknowledge A. Taggart for technical assistance, C Kenyon for advice on tail cuff measurement; P Hadoke, N Gray, E. Plaisier and P. Ronco for critical reading of the manuscript; and Glaxo Smith Kline for generation of the Col4a1+/Raw animal model. This work was supported by a CVRI Wellcome Trust Intermediate Fellowhip and a MRC New Investigator Research Grant to TVA.
Abbreviations
- NO
nitric oxide
- BM
basement membrane
- eNOS
endothelial nitric oxide synthase
- ACh
Acetylcholine
- VSMC
vascular smooth muscle cell
- HANAC
hereditary angiopathy with nephropathy aneurysm and cramps
- EDHF
endothelial cell hyperpolarizing factor
- L-NAME
L-Nω nitroarginine methyl ester
- NE
nor-epinephrine
References
- 1.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 2.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–56. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 3.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 4.Van Agtmael T, Bruckner-Tuderman L. Basement membranes and human disease. Cell Tissue Res. 2009 doi: 10.1007/s00441-009-0866-y. [DOI] [PubMed] [Google Scholar]
- 5.Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhauser-Klaus A, Pretsch W, Schmahl W, Quintanilla-Fend L. Type IV Procollagen Missense Mutations Associated With Defects of the Eye, Vascular Stability, the Brain, Kidney Function and Embryonic or Postnatal Viability in the Mouse, Mus musculus: An Extension of the Col4a1 Allelic Series and the Identification of the First Two Col4a2 Mutant Alleles. Genetics. 2007;175:725–36. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int. 2006;70:840–53. doi: 10.1038/sj.ki.5001680. [DOI] [PubMed] [Google Scholar]
- 7.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 8.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 10.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–81. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 12.Van Agtmael T, Schlotzer-Schrehardt U, McKie L, Brownstein DG, Lee AW, Cross SH, Sado Y, Mullins JJ, Poschl E, Jackson IJ. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum Mol Genet. 2005;14:3161–8. doi: 10.1093/hmg/ddi348. [DOI] [PubMed] [Google Scholar]
- 13.Thaung C, West K, Clark BJ, McKie L, Morgan JE, Arnold K, Nolan PM, Peters J, Hunter AJ, Brown SD, et al. Novel ENU-induced eye mutations in the mouse: models for human eye disease. Hum Mol Genet. 2002;11:755–67. doi: 10.1093/hmg/11.7.755. [DOI] [PubMed] [Google Scholar]
- 14.Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, Cosgrove D, Kalluri R. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J Clin Invest. 2005;115:2801–10. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, Marro B, Desmettre T, Cohen SY, Roullet E, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–95. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 16.Budzyn K, Marley PD, Sobey CG. Chronic mevastatin modulates receptor-dependent vascular contraction in eNOS-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R342–8. doi: 10.1152/ajpregu.00156.2004. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol. 1998;274:H564–70. doi: 10.1152/ajpheart.1998.274.2.H564. [DOI] [PubMed] [Google Scholar]
- 18.Maurice DH, Crankshaw D, Haslam RJ. Synergistic actions of nitrovasodilators and isoprenaline on rat aortic smooth muscle. Eur J Pharmacol. 1991;192:235–42. doi: 10.1016/0014-2999(91)90048-u. [DOI] [PubMed] [Google Scholar]
- 19.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y, Yokoyama M. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest. 1998;102:2061–71. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–8. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 21.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–96. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 22.Prockop DJ, Constantinou CD, Dombrowski KE, Hojima Y, Kadler KE, Kuivaniemi H, Tromp G, Vogel BE. Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable disorders of connective tissue. Am J Med Genet. 1989;34:60–7. doi: 10.1002/ajmg.1320340112. [DOI] [PubMed] [Google Scholar]
- 23.Van Vliet BN, Chafe LL, Antic V, Schnyder-Candrian S, Montani JP. Direct and indirect methods used to study arterial blood pressure. J Pharmacol Toxicol Methods. 2000;44:361–73. doi: 10.1016/s1056-8719(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 24.Mullins LJ, Bailey MA, Mullins JJ. Hypertension, kidney, and transgenics: a fresh perspective. Physiol Rev. 2006;86:709–46. doi: 10.1152/physrev.00016.2005. [DOI] [PubMed] [Google Scholar]
- 25.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 26.Gould DB, Marchant JK, Savinova OV, Smith RS, John SW. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum Mol Genet. 2007;16:798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- 27.Breedveld G, de Coo IF, Lequin MH, Arts WF, Heutink P, Gould DB, John SW, Oostra B, Mancini GM. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet. 2006;43:490–5. doi: 10.1136/jmg.2005.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–71. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 29.van der Knaap MS, Smit LM, Barkhof F, Pijnenburg YA, Zweegman S, Niessen HW, Imhof S, Heutink P. Neonatal porencephaly and adult stroke related to mutations in collagen IV A1. Ann Neurol. 2006;59:504–11. doi: 10.1002/ana.20715. [DOI] [PubMed] [Google Scholar]
- 30.Sibon I, Coupry I, Menegon P, Bouchet JP, Gorry P, Burgelin I, Calvas P, Orignac I, Dousset V, Lacombe D, et al. COL4A1 mutation in Axenfeld-Rieger anomaly with leukoencephalopathy and stroke. Ann Neurol. 2007;62:177–84. doi: 10.1002/ana.21191. [DOI] [PubMed] [Google Scholar]
- 31.Vahedi K, Kubis N, Boukobza M, Arnoult M, Massin P, Tournier-Lasserve E, Bousser MG. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–4. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- 32.Brandes RP, Kim D, Schmitz-Winnenthal FH, Amidi M, Godecke A, Mulsch A, Busse R. Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase knockout mice: role of soluble guanylyl cyclase. Hypertension. 2000;35:231–6. doi: 10.1161/01.hyp.35.1.231. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Rikitake Y, Inoue N, Hirata K, Akita H, Yokoyama M. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension. 2000;36:97–102. doi: 10.1161/01.hyp.36.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Janowska-Wieczorek A, Marquez LA, Nabholtz JM, Cabuhat ML, Montaño J, Chang H, Rozmus J, Russell JA, Edwards DR, Turner AR. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34(+) cells and their transmigration through reconstituted basement membrane. Blood. 1999;15:3379–3390. [PubMed] [Google Scholar]
- 35.Eshghi S, Vogelezang MG, Hynes RO, Griffith LG, Lodish HF. Alpha4beta1 integrin and erythropoietin mediate temporally distinct steps in erythropoiesis: integrins in red cell development. J Cell Biol. 2007;177:871–880. doi: 10.1083/jcb.200702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;20:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 37.Muthukumaran G, Blumberg B, Kurkinen M. The complete primary structure for the alpha 1-chain of mouse collagen IV. Differential evolution of collagen IV domains. J Biol Chem. 1989;264:6310–7. [PubMed] [Google Scholar]
- 38.Long CG, Thomas M, Brodsky B. Atypical Gly-X-Y sequences surround interruptions in the repeating tripeptide pattern of basement membrane collagen. Biopolymers. 1995;35:621–628. doi: 10.1002/bip.360350608. [DOI] [PubMed] [Google Scholar]
- 39.Tsang KY, Chan D, Cheslett D, Chan WC, So CL, Melhado IG, Chan TW, Kwan KM, Hunziker EB, Yamada Y, et al. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol. 2007;5:e44. doi: 10.1371/journal.pbio.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr., Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–61. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 43.Wenzel D, Schmidt A, Reimann K, Hescheler J, Pfitzer G, Bloch W, Fleischmann BK. Endostatin, the proteolytic fragment of collagen XVIII, induces vasorelaxation. Circ Res. 2006;98:1203–11. doi: 10.1161/01.RES.0000219899.93384.ed. [DOI] [PubMed] [Google Scholar]
- 44.Bailey MA, Paterson JM, Hadoke PW, Wrobel N, Bellamy CO, Brownstein DG, Seckl JR, Mullins JJ. A switch in the mechanism of hypertension in the syndrome of apparent mineralocorticoid excess. J Am Soc Nephrol. 2008;19:47–58. doi: 10.1681/ASN.2007040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.