Abstract
Objective
To examine the association between douching and four sexually transmitted infections (STIs).
Study Design
We followed 411 high-risk HIV-infected and uninfected female adolescents ages 12–19 over a median three-year period, both by time from study entry/first STI-free visit until an incident STI for participants who never, intermittently, and always douched, and also by reported douching at a given STI-free visit and incidence of STI at the next visit, using adjusted Cox proportional hazards models to calculate hazards ratios (HR).
Results
The time to STI was shorter for adolescents who always (HR=2.1; 95% CI, 1.2–3.4) and intermittently (HR=1.5; 95% CI:1.0–2.2) douched compared to never-douchers. An adjusted hazard for STI was 1.8 times larger for always-douchers (95%CI:1.1–3.1) and 1.4 times larger for intermittent-douchers (95%CI:0.9–2.0) compared to never-douchers. When classifying by follow-up post an STI-free visit, always-douchers had a shorter STI-free time than never-douchers (HRadj=2.1; 95%CI:1.5–3.1).
Conclusion
Counseling to discourage douching may reduce STI risk in adolescents.
INTRODUCTION
Vaginal douching is the practice of cleansing the vagina with a liquid solution for perceived hygienic reasons and/or therapeutic purposes.1,2 Many women douche in order to cleanse the vagina after menses or before or after sexual intercourse, prevent odor, alleviate vaginal symptoms, or to prevent pregnancy or sexually transmitted infections (STIs).3 While many women douche for perceived hygienic benefits, douching is potentially harmful.1,4,5 Douching has been linked to several adverse reproductive health consequences such as pelvic inflammatory disease,6,7,8 reduced fertility,9 ectopic pregnancy, 10 low birth weight,11 preterm delivery,12,13 cervical cancer,14 and other gynecologic health problems.15 Douching may also increase the risk for bacterial vaginosis 5,16,17 and STIs5,15,18–26 though very few prospective studies are published.27,28
Many factors and mechanisms are involved with STIs. Douching can disrupt the vaginal ecosystem. Oonderdonk et al report that douching caused partial elimination of the normal vaginal microflora with the transient reduction of bacterial counts. 29 Ness et al. report elimination of beneficial peroxidase-producing lactobacilli after douching.30 In a healthy vagina, hydrogen peroxide-producing lactobacilli protect against endogenous and exogenous pathogens by producing bacteriocins. Since douching causes the hydrogen peroxide-producing lactobacilli to decrease in concentration, an overgrowth of anaerobic and facultative aerobic bacteria may ensue.30 Bacterial vaginosis is associated with a higher prevalence of STIs26,31,32 and HIV-1.33
Prospective studies are better able to assess whether douching is a causal factor for STIs or, in contrast, if douching is initiated because of STI symptoms. Epidemiologic studies have suggested an association between douching and adverse outcomes, but a causal relationship is not yet established.34 Ness et al. conducted a prospective observational study of women at high risk for acquiring sexually transmitted infections, but did not find douching to be associated with acquiring gonococcal or chlamydial genital tract infections.27 Two prospective studies looked at douching and incidence of HIV-1 infection in African women, but had conflicting results.35,36 Myer et al. found no association between douching and incident HIV in an older population35 while McClelland et al. found a statistically significant association with various types of douching methods and incident HIV in younger women.36 Blythe et al. suggest that douching is more common among adolescents who are at an increased susceptibility for STIs.19 No prospective studies on douching and the incidence of STIs in adolescents have been published. We studied a cohort of HIV-infected and HIV-uninfected female adolescents with high-risk behavior to assess if douching was a risk factor for incident STIs. Our hypothesis was that douching increases the incidence of STIs in high-risk youth.
MATERIALS AND METHODS
Study Participants
This study included all adolescent females ages 12–19 years at baseline from an observational study called The Reaching for Excellence in Adolescent Care and Health (REACH) Project of the Adolescent Medicine HIV/AIDS Research Network. Investigators recruited both HIV-infected and HIV-uninfected adolescents at 16 locations in 13 U.S. cities from March 1996 through November 1999. The study cohort enlisted youth who were infected with HIV through sex or drug-taking behaviors, i.e., perinatally-acquired seropositive adolescents were excluded. The control group (HIV-uninfected) was frequency-matched on several sexual-risk and age characteristics with the HIV-infected adolescents. The HIV-infected adolescents were enrolled in an approximate 2:1 ratio compared to HIV-uninfected adolescents (the ratio for females was 1.8 at the end of study recruitment). Participants were eligible if they were enrolled in a comprehensive, adolescent-specific medical care center, enabling the management of any medical or social issues uncovered during the study.37 Detailed characteristics of participants, eligibility, methods, and study design have been reported elsewhere.37,38
Procedures
The study design was an observational, prospective cohort. REACH investigators obtained data for analysis from direct face-to-face interviews, Audio Computer-Assisted Self-Administered Interviews (ACASI) for sensitive questions such as sexual activity and drug use, and laboratory tests for STIs. The questions measured through face-to-face interviews and ACASI have been described in detail elsewhere.37,38,39 In the REACH study, participants came every three (HIV-infected) or six (HIV-uninfected) months for study visits and questionnaires, while laboratory assessments were completed approximately every six months.
Cervical, anal, and urine specimens were analyzed for the presence of the following STIs: Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and Herpes simplex virus type 2 (HSV-2).37,38,40 We tested first void urine, anal swab, and endocervical samples for Chlamydia trachomatis and Neisseria gonorrhoeae using the ligase chain reaction technique (LCX STD system™; Abbott Laboratories, Abbott Park, Illinois) at a central laboratory.41 Warm saline wet mount samples, culture (In Pouch TV™, Biomed Diagnostics, San Jose, California), and cytologic diagnosis were used to diagnosis Trichomonas vaginalis (a clear diagnosis on any of the three was considered an infection). Serologic testing specific for HSV-2 was done by the Centers for Disease Control and Prevention using a validated in-house enzyme linked immunoassay. REACH study investigators have published details of specimen processing, transport, and laboratory techniques previously.37,38, We excluded syphilis from the analysis as it was rare for participants to have active syphilis. Curable STIs were treated when diagnosed and follow-up exams were performed to confirm the clearing of infections. Although bacterial vaginosis was studied in these youth,40 we excluded this as an endpoint since it would have eliminated our ability to test our hypothesis that douching increases risk for STIs. While bacterial vaginosis is considered an STI by some, it is exceedingly common and therefore would have overwhelmed the aggregate STI endpoint (see below).
Study Variables
The principal outcome variable was any incident STI (T. vaginalis, C. trachomatis, N. gonorrhoeae, and/or HSV-2). The exposure variable was level of douching. Other douching-related variables examined in the study included age at baseline, race/ethnicity, HIV status at baseline, and self-reported sexual activity in the last 3 months at baseline.
Statistical Analysis
We used two types of analysis in this study. The first analysis type examined the association between douching behavior and time from study entry (if STI-free) or first STI-free visit (if STI noted at study entry) until advent of an incident STI. We also performed sensitivity analyses using only participants who were STI-free at baseline. We classified the female adolescents to the douching categories in two ways. The first method classified participants as never douching, intermittent douching, or always douching based on their ACASI responses at baseline and all subsequent visits. A participant was considered to never douche if she never reported “yes” to douching, to intermittently douche if she reported “yes” to douching at some of the visits, and to always douche if she reported “yes” to douching at every visit. We only included douching information from visits with laboratory assessments. The second method of classifying the adolescents allowed prospective analysis of the data: Because having a STI may alter douching behavior, we also classified adolescents as never, intermittent, or always douching based only on reported douching behavior during the interval of time during which the adolescent was STI-free. For example, if a participant had been STI-free from visits 1 to 4, but had a STI at visit 5, then in this second analysis, her douching status would only be based on reported douching behavior during visits 1–4. We computed Kaplan-Meier estimates of the probability of remaining STI-free as a function of time from baseline and compared douching categories using Cox proportional hazards.
The second type of analysis looked at the association between reported douching at the STI-free current visit and a positive or negative STI assessment at the next visit (up to 6 months after the current visit). Multiple outcomes were included for each subject. For example, if a subject had 3 visits, then the association between douching reported during visit 1 was assessed with STI at visit 2 and association between douching reported during visit 2 was assessed with STI at visit 3, and so on. Analyses were performed using generalized estimating equations with a logit link, adjusting for STI at current visit (yes/no), and assuming an exchangeable correlation structure.42 Such an approach permits the computation of odds ratios while accounting for correlation between multiple measurements taken on the same participant. Serial correlation was accounted for by including STI at current visit, representing a first-order Markov model as we are modeling STI in future (Yi) using STI at current visit (Yi-1). This model therefore accounts for serial correlation and then assumes that the residual correlation is constant within an individual over time by using an exchangeable correlation structure. Confidence intervals and p-values were computed using robust standard errors.
All statistical tests were performed using R (http://www.r-project.org) or SAS 9.1 (SAS, Inc, Cary, NC) software packages. Two-tailed tests were employed.
RESULTS
Demographic Characteristics
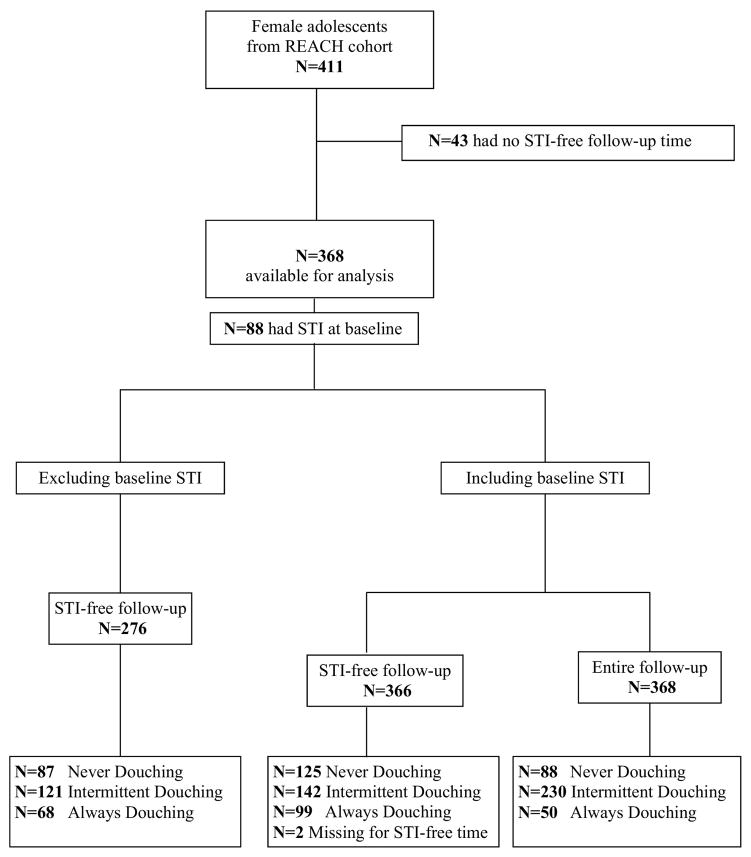
The original REACH cohort contained 411 non-virginal female adolescents, of whom 262 (63.7%) were HIV-positive and 147 were HIV-negative as per the selection criteria of the parent cohort study. The annual retention rate for HIV-infected youth in this cohort was 95%. The female annual retention rate for HIV-uninfected adolescents was 88%, with older HIV-uninfected female adolescents having the lowest retention rates.37 In our douching sub-study, 43 of the 411 participants were not eligible because they had no STI-free follow-up time (Figure 1).
Figure 1.
Recruitment of participants into the REACH study and features of the prospective douching sub-study cohort.
Of the 368 participants with STI-free follow-up time, the average age was 16.9 years, 73.2% were black, 64.8% were HIV-infected, and 74.9% reported being sexually active within the three months prior to baseline. The median follow-up time was 3 years (interquartile range: 1.7 to 3.8 years). Over the entire follow-up period, 88 (23.9%) never reported douching and 50 (13.6%) reported douching at every visit. Only considering STI-free follow-up time, 125 (34.2%) never reported douching whereas 99 (27.0%) reported douching at each visit. Douching information was missing for 2 individuals (0.5%) over their STI-free follow-up time. Demographic characteristics according to douching classifications are shown in Table 1. Black/non-Hispanic race and infection with HIV tended to be associated with higher levels of douching (Chi-square P = 0.004 and P = 0.005, respectively, when defining douching categories based on all follow-up time; P = 0.02 and P = 0.06, respectively, when defining douching categories based on STI-free follow-up time).
Table 1.
Demographic characteristics of adolescent girls and young women in the REACH douching sub-study
| How the douching category was defined | ||||||
|---|---|---|---|---|---|---|
| Over all follow-up (n=368) | Over STI-free follow-up (n=366) | |||||
| Never Douche | Intermittingly Douche | Always Douche | Never Douche | Intermittingly Douche | Always Douche | |
| (n = 88) | (n = 230) | (n = 50) | (n = 125) | (n = 142) | (n = 99) | |
| Mean Age in years ± standard deviation | 17.1 ± 1.3 | 16.9 ± 1.3 | 16.9 ± 1.1 | 17.0 ± 1.3 | 16.9 ± 1.1 | 16.8 ± 1.3 |
| Race/Ethnicity | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| White/Non-Hispanic | 4 (4.5) | 12 (5.2) | 3 (6) | 4 (3.2) | 9 (6.3) | 6 (6.1) |
| White/Hispanic | 6 (6.8) | 4 (1.7) | 0 (0) | 7 (5.6) | 3 (2.1) | 0 (0) |
| Black/Non-Hispanic | 51 (58.0) | 172 (74.8) | 40 (80) | 80 (64.0) | 100 (70.4) | 81 (81.8) |
| Black/Hispanic | 3 (3.4) | 3 (1.3) | 1 (2) | 3 (2.4) | 1 (0.7) | 3 (3.0) |
| Other/Non-Hispanic | 10 (11.4) | 15 (6.5) | 2 (4) | 13 (10.4) | 10 (7.0) | 4 (4.0) |
| Other/Hispanic | 14 (15.9) | 24 (10.4) | 4 (8) | 18 (14.4) | 19 (13.4) | 5 (5.1) |
| HIV Status | ||||||
| Infected | 45 (51.1) | 162 (70.4) | 31 (62) | 71 (56.8) | 100 (70.4) | 66 (66.7) |
| Uninfected | 43 (48.9) | 68 (29.6) | 19 (38) | 54 (43.2) | 42 (29.6) | 33 (33.3) |
| Sex in Last 3 Months | ||||||
| Yes | 61 (69.3) | 178 (77.4) | 36 (72) | 88 (70.4) | 108 (76.1) | 78 (78.8) |
| No | 26 (29.5) | 48 (20.9) | 13 (26) | 36 (28.8) | 32 (22.5) | 19 (19.2) |
| Unknown/Refused | 1 (1.1) | 4 (1.7) | 1 (2) | 1 (0.8) | 2 (1.4) | 2 (2.0) |
NOTE: Percentages may not sum to 100% due to rounding; STI denotes sexually transmitted infection
STI Prevalence
We have reported on baseline STI prevalence in the parent REACH cohort previously.40 Briefly, N. gonorrhoeae in any specimen (first void urine, anal swab, endocervical swab) was detected in 8.8% of 317 females screened at baseline. C. trachomatis in any specimen (first void urine, anal swab, endocervical swab) was noted in 22.4% of 321 screened females. (Both gonorrhea and chlamydia were noted in 5.4% of 317 females who had both assessed at baseline.) We diagnosed T. vaginalis by wet mount, culture, and/or cytology in 9.4% of 406 females evaluated at baseline. The baseline seroprevalence of HSV-2 was 39.4% of 416 screened females.40
STI Incidence
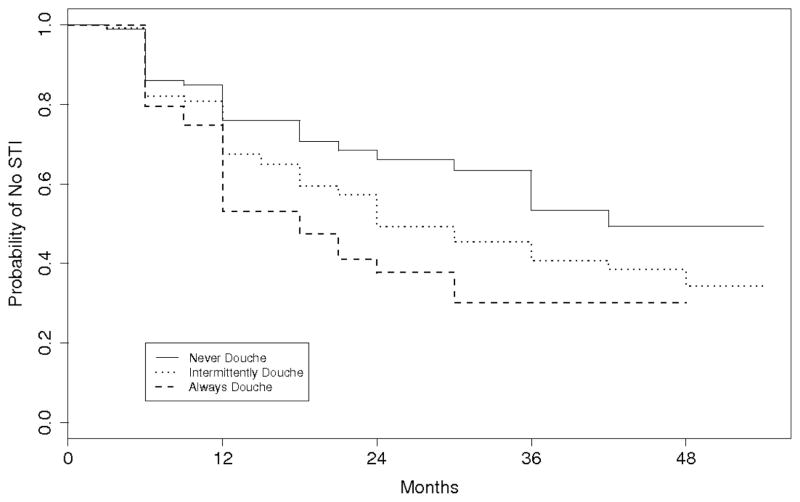
The probability of remaining STI-free as a function of time is shown in Figure 2 for participants in the never douching, intermittent douching, and always douching categories. Compared to females who never douched, the time to STI was shorter for those who always douched (hazard ratio (HR), 2.1; 95% confidence interval (CI), 1.2–3.4; P = 0.007) and for those who intermittently douched (HR, 1.5; 95% CI, 1.0–2.2; P = 0.05). After adjusting for HIV status, race, baseline sexual history, and age, the hazard of STI was 1.8 times larger for participants who always douched than participants who never douched (95% CI, 1.1–3.1; P = 0.02; Table 2), while the hazard of STI was 1.4 times larger for participants who intermittently douched than participants who never douched (95% CI, 0.9–2.0; P = 0.13). Race and age were also good predictors of STI: the hazard of STI was 1.6 times greater for blacks than non-blacks (95% CI, 1.1–2.2; P = 0.011), and for every year of age, the hazard of STI decreased 15% (HR, 0.85; 95% CI, 0.75–0.96; P = 0.01). Results were similar when the analysis was repeated using only the subgroup of participants who initiated the study STI free (N=276); for always versus never douching, unadjusted HR was 1.9 (95% CI, 1.03–3.3; P = 0.04) and adjusted HR was 1.7 (95% CI, 0.93–3.0; P = 0.09).
Figure 2.
Kaplan-Meier estimates of not having an incident sexually transmitted infection (STI) over the entire follow-up time.
Table 2.
Multivariable Cox proportional hazards analysis of sexually transmitted infection (STI) incidence.
| Variable | Defining douching category follow-up time over all
|
Defining douching category only over STI-free follow-up
|
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI1 | p-value | Hazard Ratio | 95% CI1 | p-value | |
| Age | 0.85 | 0.75–0.96 | 0.010 | 0.86 | 0.76–0.97 | 0.016 |
| Race | ||||||
| Black | 1.57 | 1.11–2.22 | 0.011 | 1.46 | 1.03–2.07 | 0.035 |
| Non-black | 1.00 | 1.00 | ||||
| HIV Status | ||||||
| Infected | 1.09 | 0.79–1.51 | 0.60 | 1.20 | 0.87–1.67 | 0.28 |
| Uninfected | 1.00 | 1.00 | ||||
| Sex in Last 3 Months | ||||||
| Yes | 1.06 | 0.75–1.50 | 0.76 | 1.13 | 0.80–1.60 | 0.49 |
| No | 1.00 | 1.00 | ||||
| Douching Status | ||||||
| Always | 1.84 | 1.09–3.10 | 0.022 | 2.12 | 1.47–3.06 | <0.0001 |
| Intermittent | 1.36 | 0.91–2.03 | 0.13 | 0.51 | 0.35–0.74 | 0.0005 |
| Never | 1.00 | 1.00 | ||||
CI denotes Confidence Interval
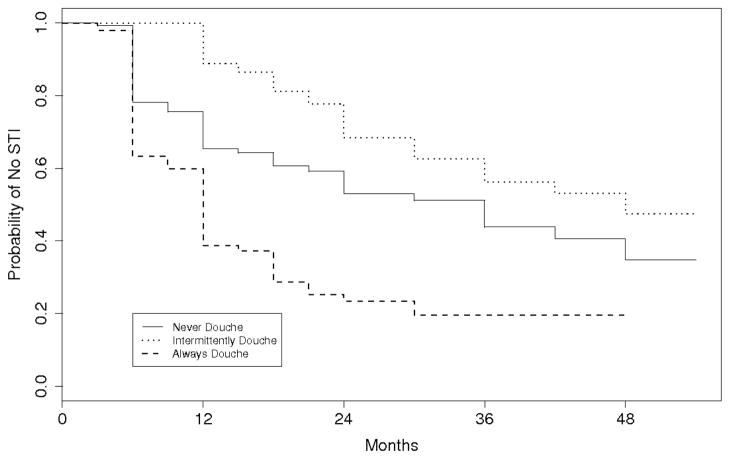
We also performed analyses that classified adolescents as never, intermittent, or always douching based only on their reported douching behavior during the interval of time when they were STI-free. The probability of remaining STI-free as a function of time for the re-defined douching categories is shown in Figure 3. Participants who always douched had a shorter STI-free time than participants who never douched over the STI-free follow-up period (HR, 2.3; 95% CI, 1.6–3.4; P<0.0001). After adjusting for HIV status, race, baseline sexual history, and age douching-related variables, the HR was 2.1 (95% CI, 1.5–3.1; Table 2). We found similar results when performing the analysis excluding adolescents with a baseline STI; unadjusted HR was 2.7 (95% CI, 1.7–4.2; P<0.0001) and adjusted HR was 2.5 (95% CI, 1.6–3.9; P<0.0001).
Figure 3.
Kaplan-Meier estimates of not having an incident sexually transmitted infection (STI) over the STI-free follow-up time
In contrast to Figure 2, Figure 3 shows that females in the intermittent douching category had the longest STI-free time (compared to never douching, adjusted HR, 0.51; 95% CI, 0.35–0.74; P<0.0001). This is likely to be an artifact of how the douching categories were defined. The longer a participant was followed, the more likely the participant was to be classified as intermittently douching, a point that is discussed later.
STI at the current visit was associated with reported douching at the current visit, which is a measure of douching during the months prior to the visit (odds ratio (OR), 1.3; 95% CI, 1.0–1.6; P = 0.05). Reported douching at the current visit was also associated with a STI at the next 6-month visit (OR, 1.3; 95% CI, 0.98–1.7; P = 0.07). After adjusting for whether or not an adolescent had a current STI, douching at the current visit was still associated with incidence of STI at the next visit (OR, 1.3; 95% CI, 1.0–1.7; P = 0.07). Adjusting for baseline HIV status, age, and race weakened the association between douching and a future STI (OR, 1.2; 95% CI, 0.9–1.6; P = 0.2, Table 3).
Table 3.
Factors associated with having a sexually transmitted infection (STI) at the next visit by douching status.
| Odds Ratio (95% CI1) | p-value | |
|---|---|---|
| Douching since last visit | 1.20 (0.89, 1.61) | 0.23 |
| STI at current visit | 1.64 (1.21, 2.24) | 0.0016 |
| HIV at baseline | 0.90 (0.68, 1.20) | 0.47 |
| Age | 0.90 (0.83, 0.98) | 0.016 |
| Black | 1.91 (1.39, 2.63) | <0.0001 |
CI denotes Confidence Interval
COMMENT
In this prospective cohort of HIV-infected and high-risk uninfected adolescent girls and young women followed for a median of three years, douching was an independent risk factor for STI acquisition. This finding supports the hypothesis that regular douching contributes to the risk of STI acquisition among high-risk female adolescents since the douching behaviors measured in this prospective study antedated the incident STI. The study addresses directly one of the principal limitations in much of the douching and health research literature, namely that cross-sectional studies may be measuring increases in women’s douching activities due to their douching in response to STI symptoms, rather than douching having increased the women’s probability of actually acquiring an STI.3,34 We found that the adjusted hazard of STI for female adolescents who always douched was about two times the hazard for those who never douched. The positive findings of this study support the hypothesis that douching and STI acquisition are causally related, namely that prior douching in risk factor for acquiring a subsequent STI.
The advantage and strength of the design of our study is that it allowed prospective examination of the influence of douching behavior on incidence of STIs. Only a few prospective studies have examined douching and STI27,28 Ness et al. did not find an association between douching and development of a STI among their cohort. 27 Hawes et al. found evidence of association between douching and bacterial vaginosis, but did not find an association between douching and acquiring a STI.28
Many cross-sectional studies support the relationship between douching and STI5,15,18–20,22–26 while other studies report conflicting results.15,19,30 Some cross-sectional studies suggest the incidence of STIs are highest among women who douche more frequently,3,20,21,26 but other studies show that women who have a history of STIs are less likely to douche.3,7 Annang et al. found that women who douched during menses and those who douched to alleviate itching were more likely to be infected with C. trachomatis, while those who douched after sex were less likely to be infected with N. gonorrhoeae.15 Ness et al. reported in a cross-sectional study that while women with intermediate disturbed vaginal flora (a Nugent score43 of 4 to 6) had a higher prevalence of STI, they did not find that douching was associated with a STI.30 However, Chacko et al. showed that a history of douching was associated with cervical gonorrheal infection22 and Beck-Sague et al. showed that douching during the prior month had a significant association with the prevalence of chlamydial infection.23 Our prospective study was underpowered to look at each STI outcome separately which is why we used an aggregate “any of the four STIs” outcome.
A unique aspect to this study was that it followed a cohort of female adolescents. No prospective studies looking at the effects of douching on incidence of STI in female adolescents have been published. Another strength of the study is that results were consistent whether or not those with a STI at baseline in the analysis were included. While the disadvantage of including those with baseline STI is that there could be some intervention altering behavior in the cohort, both analyses provided similar results. We used baseline sexual behavior in our models because sexual behaviors did not change substantially in our cohort of HIV-infected and high risk HIV-uninfected youth, despite intensive educational efforts to reduce high risk behaviors.44
Using the intermittent douching category as a comparison group for the Cox proportional hazards regression analysis was a methodologic concern. Female adolescents in the intermittent douching group were extremely diverse in their douching patterns and not enough detail was obtained in douching behavior in the REACH study to tease out these differences. The advantage of limiting comparisons to female adolescents who always or never douche is that it gives a better idea of consistent douching practices.
In addition to categorizing female adolescents to douching categories using self-reported douching behavior at all follow-up visits, adolescents were categorized using self-reported douching behavior over STI-free follow-up times only. The advantage of the second categorization is that it accounts for the prospective nature of the data by not including post-STI douching behavior. A limitation of this second approach is that female adolescents classified as always douching or never douching have shorter follow-up times. A female who regularly douches is more likely to be classified as always douching if she is only STI-free for a few months of follow-up. The same holds true for a female who rarely douches in being classified as never douching. Using this second method of categorization, many of those classified as never or always douching may actually be females who intermittently douche. Hence, the longer time to STI among intermittent douchers (Figure 3) is likely an artifact of how we defined the intermittent douching category, and implies that the hazard ratio comparing those who always (or never) douche to those who intermittently douche is likely biased. For this reason, we focus on the comparison between those who never douche and those who always douche, where this potential bias is limited. Our study results comparing participants who always versus never douche were consistent whether categorizing douching behavior over all follow-up time or over all STI-free follow-up time.
We found an association between STI at current visit and self-reported douching. However, similar to cross-sectional studies, a limitation of this analysis is that causality is unknown as douching could have been precipitated by the STI itself as a response to discomfort or vaginal discharge. Therefore, we examined the association between douching reported at the current visit and STI at the next visit. We found evidence of an association even after adjusting for STI at the current visit, though this association was weakened when we further adjusted for HIV-status, race, and age. This type of analysis may underestimate the effect of douching on STI as STI at current visit may be on the causal pathway between reported douching at the current visit and STI at the next visit. In other words, douching may lead to STI at current visit which may in turn lead to STI at next visit. By adjusting for STI at current visit, we are being conservative and in essence assuming that STI at current visit were not caused by douching. Therefore, although the adjusted association in this conservative analysis was not strongly statistically significant, the estimated odds ratios are consistent with douching being a risk factor for future STI acquisition.
As this point will not be an obvious one to many readers, we reiterate that by focusing on the association between douching and STI at a future visit after adjusting for STI at current visit, we are in essence assuming that none of the STIs at current visit are caused by douching. That we still see an association, despite this extremely conservative assumption, is evidence consistent with douching being a risk factor for STI. A familiar example can illustrate our point further. Oncogenic types of human papillomavirus (HPV) can lead to cervical cancer that can lead to death. If one adjusts for cervical cancer and estimates the effect of HPV on death, one is adjusting away some or much of its true effect. If HPV is still associated with death, even after adjusting for cervical cancer, then that can be construed as even stronger evidence for an association between HPV and death.
The study had other limitations. The study was conducted in a defined population of female adolescents with high-risk behaviors, two-thirds of whom had HIV infection. The primary finding may not be generalizable to lower-risk adolescent populations with different sociodemographic, immunologic, and/or behavioral characteristics. Also, the study utilized self-report for the main exposure variable to classify the level of douching for each female. The use of ACASI may help decrease social desirability bias, but there may be potential concerns about reliability and accuracy. However, the directness and simplicity of the douching questions may have mitigated any potential misunderstanding of what was meant.39 As with all observational studies, the results could have been influenced by some unmeasured confounding variables.
Because two-thirds of the adolescents in the cohort were HIV-infected, HIV status may have influenced incidence of STI. McClelland et al. reported that HIV-1 infection was associated with a significantly higher incidence of genital tract infections in commercial sex workers in Kenya.45 In our study, HIV infection status was not a risk factor for STI acquisition, and HIV status did not modify our douching and STI associations.
Given the weight of the evidence in this study, important public health and clinical action is suggested. Public health initiatives are needed to educate adolescent females to not douche and to urge women who douche to modify the behavior. Adolescent girls who douche often start douching for non-medical reasons and that douching behavior is currently being initiated at earlier ages than ever before.46 The most common pro-douching influences are mothers, friends, and relatives.1,2 Many women see douching as a preventative measure. A study of 1500 low-income women in Missouri showed that nearly 10% douched at certain times to try to prevent HIV infection.47 Women who douched were more likely to practice other preventive hygienic activities, such as brushing their teeth or obtaining a Pap smear in one survey in the southeastern U.S.1 Persistence of douching may also be due to aggressive advertising by manufacturers of douching products and the absence of cautionary statements by authoritative medical and public health organizations.2
In a randomized clinical trial in urban Alabama, Grimley et al. found that douching risk reduction counseling succeeded in reducing douching among 275 mostly black, high risk adolescent girls/young women ages 14–23.45 Given these results, clinical counseling to avoid douching should be considered an appropriate standard of care.45 The weight of the evidence suggests that anti-douching counseling should be provided in multiple venues to reach youth in order to discourage douching. These venues include public health campaigns, school health courses, popular media publications and media programs targeting girls and women (e.g., “Oprah” and “The View”, programs popular at present in 2008), and, importantly, anticipatory counseling by all health professionals who care for pubescent girls, adolescents, and young adults. Given their experience and credibility, gynecologists can provide special leadership in this effort. Future research should assess whether douching also increases risk of other reproductive tract infections and should study how to implement effective douching reduction education in clinical, school, and home venues.
Acknowledgments
Financial Support: The REACH study was funded by grant U01-HD32842 from the National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, National Institute of Allergy and Infectious Diseases, and National Institute of Mental Health. Support for the analytic work was received from the Vanderbilt-Meharry Center for AIDS Research, grant P30AI054999 from the National Institute of Allergy and Infectious Diseases.
The authors acknowledge the contributions of the investigators and staff of the Adolescent Medicine HIV/AIDS Research Network (1994–2001) and the youth who participated in the research. Participating investigators and staff are listed in J Adolesc Health 2001; 29 (suppl): 5–6.
Footnotes
Cities in which the study was conducted: 16 locations in 13 U.S. cities as follows: University of Miami (two locations), Miami, FL; Children’s Hospital of Philadelphia, Philadelphia, PA; Tulane Medical Center, New Orleans, LA; Children’s National Medical Center, Washington, DC; Montefiore Medical Center, Bronx, NY; University of Maryland, Baltimore, MD; Children’s Hospital of Los Angeles, Los Angeles, CA; Cook County Hospital/University of Chicago, Chicago, IL; Mt. Sinai Medical Center, New York, NY; Alabama Children’s Hospital, Birmingham, AL; Emory University, Atlanta, GA; St. Jude Children’s Research Hospital, Memphis, TN; SUNY Health Science Center at Brooklyn, Brooklyn, NY; Children’s Diagnostic and Treatment Center, University of Puerto Rico, San Juan, PR; University of Medicine and Dentistry of New Jersey, Newark, NJ.
Reprints are not available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Funkhouser E, Pulley L, Lueschen G, et al. Douching Beliefs and Practices among Black and White Women. J Womens Health Gend Based Med. 2002;11:29–37. doi: 10.1089/152460902753473435. [DOI] [PubMed] [Google Scholar]
- 2.Martino J, Youngpairoj S, Vermund SH. Vaginal Douching: Personal Practices and Public Policies. J Womens Health. 2004;13:1048–65. doi: 10.1089/jwh.2004.13.1048. [DOI] [PubMed] [Google Scholar]
- 3.Martino J, Vermund SH. Vaginal douching: Evidence for risks or benefits to women’s health. Epidemiol Rev. 2002;24:109–24. doi: 10.1093/epirev/mxf004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ness R, Hillier S, Richter H, et al. Why women douche and why they may or may not stop. Sex Transm Dis. 2003;30:71–4. doi: 10.1097/00007435-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Cottrell B. Vaginal Douching Practices of Women in Eight Florida Panhandle Counties. J Obstet Gynecol Neonatal Nurs. 2006;35:24–33. doi: 10.1111/j.1552-6909.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- 6.Ness R, Soper D, Holley R, et al. Douching and endometritis: results from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2001;28:240–5. doi: 10.1097/00007435-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Wolner-Hanssen P, Eschenbach D, Paavonen J, et al. Association between vaginal douching and acute pelvic inflammatory disease. JAMA. 1990;263:1936–41. doi: 10.1001/jama.1990.03440140062032. [DOI] [PubMed] [Google Scholar]
- 8.Aral S, Wasserheit J. Social and behavioral correlates of pelvic inflammatory disease. Sex Transm Dis. 1998;25:378–85. doi: 10.1097/00007435-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Baird D, Weinberg C, Voigt L, et al. Vaginal douching and reduced fertility. Am J Public Health. 1996;86:844–50. doi: 10.2105/ajph.86.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendrick J, Atrash H, Strauss L, et al. Vaginal douching as a risk factor of ectopic pregnancy among black women. Am J Obstet Gynecol. 1997;176:991–7. doi: 10.1016/s0002-9378(97)70391-0. [DOI] [PubMed] [Google Scholar]
- 11.Fiscella K, Franks P, Kendrick J, et al. The risk of low birth weight associated with vaginal douching. Obstet Gynecol. 1998;92:913–7. doi: 10.1016/s0029-7844(98)00325-1. [DOI] [PubMed] [Google Scholar]
- 12.Bruce F, Fiscella K, Kendrick J. Vaginal douching and preterm birth: An intriguing hypothesis. Med Hypotheses. 2000;54:448–52. doi: 10.1054/mehy.1999.0875. [DOI] [PubMed] [Google Scholar]
- 13.Fiscella K, Franks P, Kendrick J, et al. Risk of preterm birth that is associated with vaginal douching. Am J Obstet Gynecol. 2002;186:1345–50. doi: 10.1067/mob.2002.122406. [DOI] [PubMed] [Google Scholar]
- 14.Gardner J, Schuman K, Slattery M, et al. Is vaginal douching related to cervical carcinoma? Am J Epidemiol. 1991;133:368–75. doi: 10.1093/oxfordjournals.aje.a115890. [DOI] [PubMed] [Google Scholar]
- 15.Annang L, Grimley D, Hook E. Vaginal Douche Practices Among Black Women at Risk: Exploring Douching Prevalence, Reasons for Douching, and Sexually Transmitted Disease Infection. Sex Transm Dis. 2006;33:215–9. doi: 10.1097/01.olq.0000205046.11916.c5. [DOI] [PubMed] [Google Scholar]
- 16.Fonck K, Kaul R, Keli F, Bwayo JJ, Ngugi EN, Moses S, et al. Sexually transmitted infections and vaginal douching in a population of female sex workers in Nairobi, Kenya. Sex Transm Infect. 2001;77:271–5. doi: 10.1136/sti.77.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzman C, Leventhal JM, Jones NM, Wang J BV Study Group. Factors linked to bacterial vaginosis in nonpregnant women. Am J Public Health. 2001;91:1664–70. doi: 10.2105/ajph.91.10.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholes D, Stergachis A, Ichikawa L, et al. Vaginal Douching as a risk factor for cervical Chlamydia trachomatis infection. Obstet Gynecol. 1998;91:993–7. doi: 10.1016/s0029-7844(98)00095-7. [DOI] [PubMed] [Google Scholar]
- 19.Blythe M, Fortenberry J, Orr D. Douching behaviors reported by adolescent and young adult women at high risk for sexually transmitted infections. J Pediatr Adolesc Gynecol. 2003;16:95–100. doi: 10.1016/s1083-3188(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 20.Joesoef M, Sumampouw H, Linnan M, Schmid S, Idajadi A, St Louis M. Douching and sexually transmitted diseases in pregnant women in Surabaya, Indonesia. Am J Obstet Gynecol. 1996;174:115–9. doi: 10.1016/s0002-9378(96)70382-4. [DOI] [PubMed] [Google Scholar]
- 21.Myer L, Denny L, De Souza M, Barone M, Wright T, Kuhn L. Intravaginal practices, HIV and other sexually transmitted diseases among South African women. Sex Transm Dis. 2004;31:174–9. doi: 10.1097/01.olq.0000114942.41998.58. [DOI] [PubMed] [Google Scholar]
- 22.Chacko M, Kozinetz C, Regard M, Smith P. The relationship between vaginal douching and lower genital tract infection in young women. Adolesc Pediatr Gynecol. 1992;5:171–6. [Google Scholar]
- 23.Beck-Sague C, Farshy C, Jackson T, et al. Detection of Chlamydia trachomatis cervical infection by urine tests among adolescents. J Adolesc Health. 1998;22:197–204. doi: 10.1016/S1054-139X(97)00209-7. [DOI] [PubMed] [Google Scholar]
- 24.Stergachis A, Scholes D, Heidrich FE, Sherer DM, Holmes KK, Stamm WE. Selective screening for Chlamydia trachomatis infection in a primary care population of women. Am J Epidemiol. 1993;138:143–53. doi: 10.1093/oxfordjournals.aje.a116840. [DOI] [PubMed] [Google Scholar]
- 25.La Ruche G, Messou N, Ali-Napo L. Vaginal douching: association with lower genital tract infections in African pregnant women. Sex Transm Dis. 1999;26(4):191–6. doi: 10.1097/00007435-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Peters SE, Beck-Sague CM, Farshy CE, et al. Behaviors associated with Neisseria gonorrhoeae and Chlamydia trachomatis: Cervical infection among young women attending adolescent clinics. Clin Pediatr(Phila) 2000;39:173–7. doi: 10.1177/000992280003900307. [DOI] [PubMed] [Google Scholar]
- 27.Ness B, Hillier S, Kip K, et al. Douching, pelvic inflammatory disease, and incident gonococcal and chlamydial genital infection in a cohort of high-risk women. Am J Epidemiol. 2005;161:186–195. doi: 10.1093/aje/kwi025. [DOI] [PubMed] [Google Scholar]
- 28.Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–63. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 29.Onderdonk A, Delaney M, Hinkson P, DuBois A. Quantitative and qualitative effects of douche preparations on vaginal microflora. Obstet Gynecol. 1992;80:333–8. [PubMed] [Google Scholar]
- 30.Ness R, Hillier S, Richter H, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol. 2002;100:765–72. doi: 10.1016/s0029-7844(02)02184-1. [DOI] [PubMed] [Google Scholar]
- 31.Hillier SL, Krohn MA, Nugent RP, Gibbs RS. Characteristics of three vaginal flora patterns assessed by gram stain among pregnant women. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1992;166:938–944. doi: 10.1016/0002-9378(92)91368-k. [DOI] [PubMed] [Google Scholar]
- 32.Hillier SL, Krohn MA, Klebanoff SJ, Eschenbach DA. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet Gynecol. 1992;79:369–373. doi: 10.1097/00006250-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Sewankambo N, Gray R, Wawer M, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–50. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 34.Monif G. The great douching debate: To douche, or not to douche. Obstet Gynecol. 1999;94:630–1. [PubMed] [Google Scholar]
- 35.Myer L, Denny L, de Souza M, et al. Distinguishing the temporal association between women’s intravaginal practices and risk of human immunodeficiency virus infection: a prospective study of South African women. Am J Epidemiol. 2006;163(6):552–60. doi: 10.1093/aje/kwj071. [DOI] [PubMed] [Google Scholar]
- 36.McClelland R, Lavreys L, Hassan W, et al. Vaginal washing and increased risk of HIV-1 acquisition among African women: a 10-year prospective study. AIDS. 2006;20(2):269–73. doi: 10.1097/01.aids.0000196165.48518.7b. [DOI] [PubMed] [Google Scholar]
- 37.Wilson C, Houser J, Partlow C, et al. The REACH (Reaching for Excellence in Adolescent Care and Health) Project: Study design, methods, and population profile. J Adolesc Health. 2001;29:8–18. doi: 10.1016/s1054-139x(01)00291-9. [DOI] [PubMed] [Google Scholar]
- 38.Rogers A, Futterman D, Moscicki A, Wilson C, Ellenberg J, Vermund S. The REACH Project of the Adolescent Medicine HIV/AIDS Research Network: design, methods and selected characteristics of participants. J Adolesc Health. 1998;22:300–11. doi: 10.1016/s1054-139x(97)00279-6. [DOI] [PubMed] [Google Scholar]
- 39.Vermund S, Sarr M, Murphy DA, et al. Douching practices among HIV-infected and uninfected adolescents in the United States. J Adolesc Health. 2001;295:80–6. doi: 10.1016/s1054-139x(01)00284-1. [DOI] [PubMed] [Google Scholar]
- 40.Vermund SH, Wilson CM, Rogers AS, Partlow C, Moscicki AB. Sexually transmitted infections among HIV infected and HIV uninfected high-risk youth in the REACH study: Reaching for Excellence in Adolescent Care and Health. J Adolesc Health. 2001;29(3 Suppl):49–56. doi: 10.1016/s1054-139x(01)00296-8. [DOI] [PubMed] [Google Scholar]
- 41.Peralta L, Durako SJ, Yong M Adolescent Medicine HIV/AIDS Research Network. Correlation between urine and cervical specimens for the detection of cervical Chlamydia trachomatis and Neisseria gonorrhoeae using ligase chain reaction in a cohort of HIV infected and uninfected adolescents. J Adolesc Health. 2001;29(Suppl):87–92. doi: 10.1016/s1054-139x(01)00283-x. [DOI] [PubMed] [Google Scholar]
- 42.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 43.Nugent B, Krohn M, Hillier S. Reliability of diagnosing bacterial vaginosis by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy DA, Durako SJ, Moscicki AB, Vermund SH, Ma Y, Schwarz DF, Muenz LR. No change in health risk behaviors over time among HIV-infected adolescents in care: role of psychological distress. J Adolesc Health. 2001;29(3 Suppl):57–63. doi: 10.1016/s1054-139x(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 45.McClelland R, Lavreys L, Katingima C, et al. Contribution of HIV-1 infection to acquisition of sexually transmitted disease: a 10-year prospective study. J Infect Dis. 2005;191:333–338. doi: 10.1086/427262. [DOI] [PubMed] [Google Scholar]
- 46.Grimley D, Oh K, Desmond R, Hook E, Vermund S. An intervention to reduce vaginal douching among adolescent and young adult women: a randomized, controlled trial. Sex Transm Dis. 2005;32:752–8. doi: 10.1097/01.olq.0000190018.58079.05. [DOI] [PubMed] [Google Scholar]
- 47.Crosby R, Yarber W, Meyerson B. Prevention strategies other than male condoms employed by low-income women to prevent HIV infection. Public Health Nurs. 2000;17:53–60. doi: 10.1046/j.1525-1446.2000.00053.x. [DOI] [PubMed] [Google Scholar]