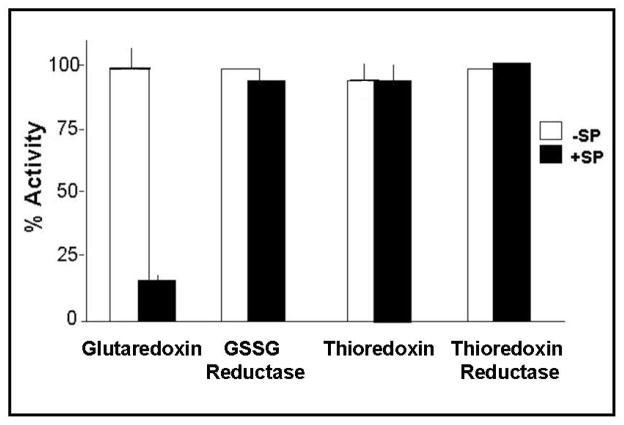
Figure 4. Selective inactivation of GRx1 by sporidesmin.
Thiol-disulfide oxidoreductase enzymes were incubated with 1 mM sporidesmin at 30 °C, 0.1 M K phosphate (5% ethanol), pH 7.5 for 20 min. The remaining enzyme activity was expressed as percent of the control activity. Human GRx1 (1.8 μM) activity was determined by GSSG-reductase mediated NADPH oxidation coupled to GSSG formation. Yeast GSSG reductase (0.125 μM) activity was measured by the loss of NADPH absorbance at 340 nm. E.coli thioredoxin (0.2 mg.ml−1) and thioredoxin reductase (0.1 mg.ml−1) were assayed by NADPH oxidation coupled to thioredoxin oxidation using GSSG as the substrate. Mammalian thioredoxin was assayed by a turbidometric assay using insulin as the substrate. Each bar represents the mean value, plus and minus the standard error, for at least three determinations. Where errors are not shown the value represents a single experiment carried out after optimization of conditions.