Abstract
Mounting evidence suggests that carbon monoxide (CO) exerts powerful cytoprotective actions. CO-releasing molecules (CORMs) offer an effective means of delivering CO to tissues in vivo. The goal of the present study was to determine whether a water-soluble CORM, tricarbonylchloro(glycinato)ruthenium(II) (CORM-3), induces delayed protection against myocardial infarction 24 h later and to explore the duration of this protection. Mice received a 60-min i.v. infusion of CORM-3 or inactive CORM-3 (which does not release CO) and then, 24, 72, or 120 h later, underwent a 30-min coronary occlusion followed by 24 h of reperfusion. Pretreatment with CORM-3 24 h prior to coronary occlusion markedly reduced infarct size (24.8% ± 2.9% of the risk region vs. 43.8% ± 4.4% with inactive CORM-3). The infarct-sparing effect of CORM-3 was still evident 72 h after administration of the CO donor (20.4% ± 3.7% of the risk region vs. 41.9% ± 2.5% with inactive CORM-3) but was no longer apparent at 120 h. Both at 24 and 72 h, the protective effects of CORM-3 were equivalent to those afforded by the late phase of ischemic preconditioning (PC; 27.0% ± 2.9% and 30.3% ± 3.9% of the risk region, respectively). We conclude that the novel CO-releasing compound, CORM-3, induces delayed protection against myocardial infarction which is similar to that afforded by the late phase of ischemic PC, and that this salubrious effect is sustained for 72 h. To our knowledge, this is the first report that exposure to CO causes the heart to shift to a preconditioned phenotype. In addition, this study provides the first evidence that the cardioprotective actions of ischemic PC persist for 72 h in the mouse.
Keywords: Carbon monoxide-releasing molecules, Preconditioning, Myocardial infarction
1. Introduction
Although CO has historically been viewed as toxic to biological systems [1], recent studies suggest that this byproduct of heme oxygenase-1 (HO-1) exerts an important regulatory role in many cellular and biological processes. Specifically, CO has been shown to promote vasorelaxation [2,3] and to inhibit proliferation of smooth muscle cells [4], transplant rejection [5], inflammation [6,7], platelet aggregation and microvascular thrombosis [8], cytokine production [9,10], and oxidative stress [11]. CO has also been shown to alleviate hypoxia/reoxygenation injury in isolated cells and ischemia/reperfusion injury in liver [12], in isolated hearts [5], and in an in vivo murine model of myocardial infarction [13]. In addition, numerous studies suggest that CO plays an important role in suppressing apoptosis [11,14–16].
Recent advances have enhanced the clinical feasibility of delivering CO in vivo. Thus far, most in vivo studies have utilized inhalation of CO to deliver this gas to tissues. However, administering CO gas is a very nonspecific approach, as most of the free CO reaching the bloodstream reacts rapidly with hemoglobin and other heme proteins prior to reaching the target tissue. This can potentially result in toxic effects. Carriers of CO that transport and deliver this gas to a target tissue would clearly increase both the clinical feasibility and the specificity of CO therapy. Motterlini et al. [5,17] have shown that transition metal carbonyls can deliver CO in biological systems and have recently developed a new molecule, namely, tricarbonyldichloro(glycinato)ruthenium(II), referred to as CO-releasing molecule-3 (CORM-3), which is water-soluble [5]. Clark et al. [5] has demonstrated that CORM-3 effectively delivers CO to tissues under physiological conditions and limits anoxia/reoxygenation or ischemia/reperfusion injury in isolated rat hearts and cardiomyocytes. We [13] have reported that administration of CORM-3 5 min before reperfusion in an in vivo murine model of myocardial ischemia/reperfusion results in a decrease in infarct size without increased CO levels in the blood. These results suggest that CORM-3 provides a clinically relevant tool for delivering therapeutic amounts of CO in an in vivo system; however, the long-term effects of CO donor administration on myocardial ischemia/reperfusion injury are unknown.
The powerful anti-apoptotic actions of CO suggest that, in addition to its immediate beneficial effects on myocardial ischemia/reperfusion injury [5,13], this gas may also produce sustained cytoprotection similar to that associated with the late phase of ischemic preconditioning (PC). Indeed, in non-cardiac cells CO has been reported to upregulate iNOS and HO-1 [18], two enzymes that are known to mediate the infarct-sparing actions of late PC [19,20]. In the heart, CO has been shown to participate in cardioprotective signaling during ischemia [21,22]. The potential clinical significance of late PC stems from the fact that it affords long-lasting (up to 72 h) protection against both myocardial stunning and myocardial infarction [23]. Previous studies have demonstrated that a delayed cardioprotective effect similar to the late phase of ischemic PC can be elicited by a variety of pharmacologic agents including nitric oxide donors [24–28], adenosine receptor agonists [29,30], opioid receptor agonists [31,32], reactive oxygen species [33], endotoxin derivatives [34,35], the KATP channel opener diazoxide [36,37], and nicorandil [38]. Unfortunately, most of these interventions are not clinically applicable or have significant side effects. Although CO donors are an effective means of delivering CO in vivo, the ability of these drugs to induce a delayed and sustained cardioprotective effect similar to that seen with the late phase of ischemic PC has not been tested.
Accordingly, the goal of the present study was to determine whether pretreatment with CORM-3, in the absence of ischemia, can reproduce the protective effect of the late phase of ischemic PC against myocardial infarction. All experiments were performed in a well-established murine model of ischemia/reperfusion injury [39].
2. Materials and methods
This study was performed in accordance with the guidelines of the Animal Care and Use Committee of the University of Louisville School of Medicine (Louisville, KY) and with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, National Institutes of Health, Publication No. 86-23).
2.1. Animals
Male ICR mice (body wt. 35.5 ± 1.3 g, age 10.2 ± 0.3 weeks) were used for this study. All mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in microisolator cages under specific pathogen-free conditions in a room with a temperature of 24 °C, 55–65% relative humidity, and a 12-h light–dark cycle.
2.2. Experimental protocol
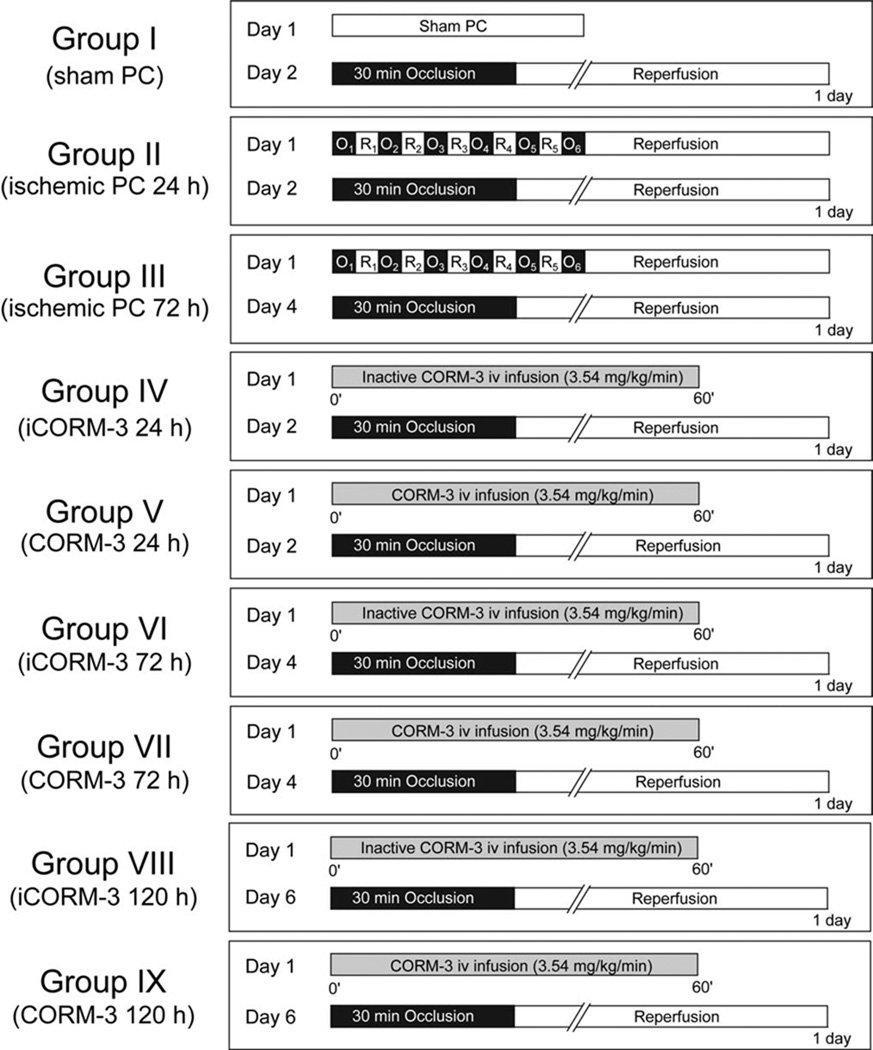
The overall experimental design is summarized in Fig. 1. Mice were assigned to nine groups. On day 1, mice in group I received sham PC (1 h of open-chest state with no coronary occlusion), whereas mice in groups II and III underwent six cycles of 4-min coronary occlusion/4-min reperfusion according to the methods described below. On day 1, mice in groups V, VII, and IX received a 60-min i.v. infusion of CORM-3 (total dose, 3.54 mg/kg); to preserve the CO-releasing activity, CORM-3 was dissolved in distilled water (pH 7.0). This dose of CORM-3 was selected because it affords robust cardioprotection in this murine model [13]. Mice in groups IV, VI, and VIII received inactive CORM-3 at the same dose used in groups V, VII, and IX. CORM-3 was inactivated by dissolving it in PBS (0.35 mg/ml) and leaving it at room temperature for 24 h; under these conditions, 1 mol of CO per mole of compound is released in the solution and, as a result, no additional CO is liberated upon administration of the drug [5]. On day 2 (24 h after the six occlusion/reperfusion cycles, after sham PC, or after CORM-3 or inactive CORM-3 infusion), mice in groups I, II, IV, and V underwent a 30-min coronary occlusion followed by 24 h of reperfusion [39]. Mice in groups III, VI and VII underwent the 30-min occlusion/24-h reperfusion sequence 72 h after the six occlusion/reperfusion cycles, after infusion of inactive CORM-3, or after infusion of CORM-3. Mice in groups VIII and IX underwent the 30-min occlusion/24-h reperfusion sequence 120 h after infusion of inactive CORM-3 or CORM-3.
Fig. 1.
Experimental protocol. Nine groups of mice were used. On day 1, mice in group I (n = 6, sham PC) received sham PC, while mice in group II (n = 10, late PC 24 h) and group III (n = 10, late PC 72 h) underwent six cycles of 4-min coronary occlusion (O)/4-min reperfusion (R). Mice in groups IV (n = 9),VI (n = 9), and VIII (n = 7) received 3.54 mg/kg of inactive CORM-3 (iCORM-3) as an i.v. infusion for 60 min. Mice in groups V (n = 10), VII (n = 10), and IX (n = 11) received 3.54 mg/kg of CORM-3 as an i.v. infusion for 60 min. In groups I, II, IV, and V, mice underwent a 30-min coronary occlusion followed by 24 h of reperfusion on day 2 (24 h after sham PC, ischemic PC, inactive CORM-3, or CORM-3). In groups III, VI, and VII, mice underwent a 30-min coronary occlusion followed by 24 h of reperfusion on day 4 (72 h after ischemic PC, inactive CORM-3 or CORM-3). In groups VIII and IX, mice underwent a 30-min coronary occlusion followed by 24 h of reperfusion on day 6 (120 h after inactive CORM-3 or CORM-3).
2.3. Studies of myocardial infarction
The experimental preparation has been described in detail previously [20,39]. Briefly, mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.), intubated, and ventilated with room air supplemented with oxygen at a rate of 105 strokes per min and with a tidal volume of 0.6 ml using a small rodent ventilator. These respiratory settings result in physiological values of arterial pH (7.39 ± 0.01) and adequate oxygenation [20,39]. After administration of antibiotics, the chest was opened through a midline sternotomy, and a non-traumatic balloon occluder was implanted around the mid-left anterior descending coronary artery by using an 8-0 nylon suture [39]. To prevent hypotension, blood from donor mice was given during surgery. Physiologic variables, including heart rate, core body temperature, and arterial pH were monitored and carefully maintained within the normal range throughout the experiments [20,39]. In all groups, myocardial infarction was produced by a 30-min coronary occlusion followed by 24 h of reperfusion [20,39]. After the coronary occlusion/reperfusion protocol, the chest was closed in layers and the mice were allowed to recover.
2.4. Postmortem tissue analysis
At the conclusion of the study, the heart was excised and perfused with Krebs–Henseleit solution through an aortic cannula. To delineate infarcted from viable myocardium, the heart was then perfused with 1% 2,3,5-triphenyltetrazolium chloride in phosphate buffer. To delineate the occluded/reperfused bed, the coronary artery was tied at the site of the previous occlusion and the aortic root was perfused with 10% Phthalo blue dye [20,39]. As a result of this procedure, the region at risk was identified by the absence of blue dye, whereas the rest of the LV was stained dark blue. The left ventricle was cut into five to seven transverse slices, which were fixed in 10% neutral buffered formaldehyde, weighed, and photographed under a microscope. Infarct size was calculated by computerized planimetry of the infarcted, ischemic/reperfused and nonischemic regions in serial heart slices [20,39]. From these measurements, infarct size was calculated as a percentage of the region at risk [20,39].
2.5. Statistical analysis
Data are reported as mean ± S.E.M. Heart rate, risk region, and infarct size were analyzed with a one-way or a two-way repeated-measures (time and group) ANOVA, as appropriate, followed by Student’s t-tests for unpaired data with the Bonferroni correction [40]. The relationship between infarct size and risk region size was compared between groups with ANCOVA, with the size of the risk region as the covariate. The correlation between infarct size and risk region size was assessed by linear regression with the least-squares method. P < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS (version 8.0) statistical software (SPSS Inc., Chicago, IL).
3. Results
A total of 94 mice were used. Twelve mice were excluded for the reasons specified in Table 1.
Table 1.
Reasons for excluding mice
| Group I | Group II | Group III | Group IV | GroupV | Group VI | Group VII | Group VIII | Group IX | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Death | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 6 |
| Technical problems | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 4 |
| Poor postmortem staining | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Mice excluded | 0 | 4 | 1 | 3 | 3 | 1 | 0 | 0 | 0 | 12 |
| Mice operated upon | 6 | 14 | 11 | 12 | 13 | 10 | 10 | 7 | 11 | 94 |
| Mice included in study | 6 | 10 | 10 | 9 | 10 | 9 | 10 | 7 | 11 | 82 |
| Mice included in study (%) | 100 | 71 | 91 | 75 | 77 | 90 | 100 | 100 | 100 | 87 |
3.1. Body temperature and heart rate
By experimental design [20,39], rectal temperature remained within a narrow, physiologic range (36.7–37.3 °C) in all groups. Five minutes before the 30-min coronary occlusion, the average heart rate in groups I–IX ranged from 490 to 636 beats/min (Table 2). In group II, the average heart rate 5 min before occlusion was higher (636 ± 33 beats/min, P < 0.05 vs. other groups), likely due to the trauma from the surgery that had been performed 24 h previously [39]. During the 30-min occlusion and ensuing reperfusion, heart rate did not differ significantly among groups I and III–IX but remained elevated in group II compared with the other groups (Table 2).
Table 2.
Rectal temperature and heart rate on the day of the 30-min coronary occlusion
| Group | Preocclusion | Occlusion | Reperfusion | ||
|---|---|---|---|---|---|
| 5 min | 30 min | 5 min | 15 min | ||
| Temperature (°C) | |||||
| I | 37.2 ± 0.1 | 37.2 ± 0.2 | 37.0 ± 0.2 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| II | 36.8 ± 0.0 | 37.0 ± 0.0 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 |
| III | 37.0 ± 0.1 | 37.2 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 |
| IV | 37.0 ± 0.1 | 37.2 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 |
| V | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 |
| VI | 36.8 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 |
| VII | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.2 ± 0.1 | 36.9 ± 0.0 |
| VIII | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 |
| IX | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.0 |
| Heart rate (bpm) | |||||
| I | 584 ± 16 | 635 ± 17 | 639 ± 23 | 641 ± 30 | 626 ± 32 |
| II | 636 ± 33* | 675 ± 25* | 689 ± 17* | 679 ± 11* | 662 ± 13* |
| III | 550 ± 25 | 620 ± 20 | 635 ± 25 | 634 ± 20 | 603 ± 19 |
| IV | 580 ± 20 | 600 ± 16 | 579 ± 20 | 560 ± 16 | 566 ± 23 |
| V | 600 ± 22 | 628 ± 22 | 608 ± 28 | 600 ± 31 | 612 ± 31 |
| VI | 503 ± 14 | 583 ± 22 | 552 ± 25 | 557 ± 21 | 534 ± 17 |
| VII | 526 ± 27 | 561 ± 28 | 544 ± 23 | 579 ± 24 | 558 ± 26 |
| VIII | 490 ± 20 | 569 ± 15 | 541 ± 23 | 552 ± 25 | 537 ± 34 |
| IX | 538 ± 18 | 549 ± 22 | 531 ± 22 | 542 ± 22 | 534 ± 25 |
Mice underwent a 30-min coronary occlusion followed by 24 h of reperfusion. Heart rate was measured 5 min before coronary occlusion (preocclusion), at 5 and 30 min into the 30-min coronary occlusion, and at 5 and 15 min after reperfusion. The experimental protocols are specified in the legend to Fig. 1. Data are mean ± S.E.M. Note that the heart rate in group II was higher than that in groups I and III–IX, possibly reflecting the effect of surgical trauma 24 h earlier.
P < 0.05 vs. groups I, III–IX).
3.2. Infarct size
There were no significant differences among the nine groups with respect to heart weight or weight of the region at risk (Table 3). In the sham PC mice (group I), infarct size averaged 49.9% ± 4.0% of the region at risk (Fig. 2). As expected [39], at 24 h after ischemic PC (group II) infarct size was smaller than in the sham PC group (27.0% ± 2.9%, P < 0.05). A similar reduction in infarct size was noted 72 h after ischemic PC (group III) (30.3% ± 3.9%, P < 0.05).
Table 3.
Size of left ventricle, risk region, and infarct
| Group | Body Wt (g) |
Heart Wt (mg) |
LVWt (mg) |
Heart Wt/body Wt |
Risk region Wt (mg) |
Infarct Wt (mg) |
Risk region (% of LV) |
Infarct (% of risk region) |
Infarct (% of LV) |
|---|---|---|---|---|---|---|---|---|---|
| I | 30.7 ± 1.3 | 141.5 ± 7.2 | 101.4 ± 5.9 | 0.47 ± 0.03 | 40.0 ± 5.7 | 20.2 ± 3.3 | 39.3 ± 4.7 | 49.9 ± 4.0 | 19.9 ± 3.1 |
| II | 34.3 ± 0.8 | 164.1 ± 5.8 | 122.3 ± 4.1 | 0.48 ± 0.01 | 53.8 ± 4.9 | 14.1 ± 1.7* | 44.0 ± 3.9 | 27.0 ± 2.9* | 11.4 ± 1.1* |
| III | 35.1 ± 1.9 | 172.8 ± 6.4 | 122.0 ± 4.2 | 0.50 ± 0.02 | 46.0 ± 2.6 | 14.0 ± 1.9* | 37.5 ± 1.3 | 30.3 ± 3.9* | 11.5 ± 1.6* |
| IV | 40.0 ± 0.7 | 171.9 ± 6.1 | 125.7 ± 3.5 | 0.43 ± 0.02 | 53.2 ± 2.4 | 23.9 ± 3.5 | 42.3 ± 1.5 | 43.8 ± 4.4 | 18.9 ± 2.4 |
| V | 37.8 ± 1.3 | 162.2 ± 4.3 | 114.2 ± 4.5 | 0.43 ± 0.01 | 45.2 ± 2.9 | 11.4 ± 1.6# | 39.5 ± 1.7 | 24.8 ± 2.9# | 9.8 ± 1.2# |
| VI | 37.2 ± 0.9 | 161.9 ± 5.8 | 113.8 ± 4.1 | 0.44 ± 0.01 | 48.8 ± 3.3 | 20.6 ± 2.1 | 42.6 ± 2.0 | 41.9 ± 2.5 | 18.0 ± 1.7 |
| VII | 35.1 ± 0.5 | 148.4 ± 6.3 | 105.8 ± 3.9 | 0.42 ± 0.02 | 43.5 ± 3.0 | 9.11 ± 1.8^ | 41.0 ± 2.2 | 20.4 ± 3.7^ | 8.4 ± 1.5^ |
| VIII | 35.4 ± 1.0 | 169.4 ± 7.1 | 118.7 ± 6.2 | 0.48 ± 0.02 | 41.2 ± 1.9 | 18.4 ± 1.6 | 34.9 ± 1.4 | 44.6 ± 3.0 | 15.6 ± 1.1 |
| IX | 36.6 ± 0.9 | 165.1 ± 6.4 | 118.0 ± 5.4 | 0.45 ± 0.02 | 49.2 ± 1.7 | 22.3 ± 1.7 | 42.3 ± 1.8 | 45.5 ± 3.4 | 19.2 ± 1.7 |
Data are mean ± S.E. Heart Wt, total heart weight (ventricles and atria); LV, left ventricle.
P < 0.05 vs. group I.
P < 0.05 vs. group IV.
P < 0.05 vs. group VI.
Fig. 2.
Myocardial infarct size in groups I–IX. Infarct is expressed as a percentage of the region at risk of infarction, ○, Individual mice; ●, mean ± S.E.M. for respective groups; CORM, CORM-3; iCORM, inactive CORM-3.
In mice pretreated with inactive CORM-3 (groups IV and VI), infarct size was similar to the sham PC group (group I) (43.8% ± 4.4%, 41.9% ± 2.5%, and 49.9% ± 4.0% of the region at risk, respectively), indicating that administration of CORM-3 alone does not affect the extent of cell death. However, in mice pretreated with CORM-3 and subjected to coronary occlusion 24 h later (group V), infarct size (24.8% ± 2.9% of the region at risk) was significantly (P < 0.05) smaller than that observed in group IV (Fig. 2). This infarct-sparing effect persisted at 72 h after CORM-3 (20.4% ± 3.7% of the region at risk vs. 41.9% ± 2.5% in mice pretreated with inactive CORM-3), but was no longer observed at 120 h (45.5% ± 3.4% of the region at risk vs. 44.6% ± 3.0% in mice pretreated with inactive CORM-3). The infarct size measured in groups V and VII was similar to that measured in groups II and III, indicating that administration of CORM-3 24 or 72 h before ischemia/reperfusion resulted in a protective effect that was comparable to that induced by the late phase of ischemic PC at corresponding time-points (24 and 72 h).
In groups IV–IX, the size of the infarction was positively and linearly related to the size of the region at risk (r = 0.75, 0.41, 0.83, 0.26, 0.49, and 0.77, respectively). The regression line, however, was shifted down and to the right in group V compared with group IV (P < 0.05) and in group VII compared with group VI (P < 0.05; Fig. 3), indicating that for any given size of the region at risk, the resulting infarction was smaller in CORM-3-treated mice than in inactive CORM-3-treated mice at both 24 and 72 h. The relationship between infarct size and region of risk did not differ between groups VIII and IX (data not shown).
Fig. 3.
Relationship between size of the region at risk and size of the infarction. Panel A. Individual values and regression lines obtained by linear regression analysis in groups IV and V. In both groups, infarct size was positively and linearly related to risk region size. The linear regression equations were as follows: group IV, y = −31 + 1.2x, r = 0.75; group V, y = −1.4 + 0.28x, r = 0.41. Analysis of covariance demonstrated that the regression line for group IV was significantly different from group V (P < 0.05), indicating that for any given risk region size, infarct was smaller in CORM-3-treated mice than in inactive CORM-3-treated mice at 24 h after treatment. Panel B. Individual values and regression lines obtained by linear regression analysis in groups VI and VII. In both groups, infarct size was positively and linearly related to risk region size. The linear regression equations were as follows: group VI, y = −11.5 + 0.69x, r = 0.83; group VII, y = 0.72 + 0.72x, r = 0.26. Analysis of covariance demonstrated that the regression line for group VI was significantly different from group VII (P < 0.05), indicating that for any given risk region size, infarct was smaller in CORM-3-treated mice than in inactive CORM-3-treated mice at 72 h after treatment; CORM, CORM-3; iCORM, inactive CORM-3.
4. Discussion
In recent years there has been a remarkable paradigm shift with respect to our understanding of the role of CO in biological systems. Mounting evidence indicates that this gas, which has been traditionally regarded as a toxic byproduct of HO-1 activity, exerts an important homeostatic function and plays a cytoprotective role in many pathophysiological conditions [16]. In the heart, the effects of CO have been examined only in the context of acute CO treatment [5,13]. The present study is the first to explore the long-term effects of CO exposure on myocardial ischemia/reperfusion injury.
Our results demonstrate that administration of the water-soluble CO donor CORM-3 24 or 72 h prior to ischemia/reperfusion reduces infarct size in vivo. The magnitude of the delayed cardioprotective effects of CORM-3 was similar to that observed during the late phase of ischemic PC, both at 24 and 72 h. We have previously shown that this dose of CORM-3 has no effect on arterial pressure [13], and in the present study heart rate during coronary occlusion was similar in CORM-3-treated and inactive CORM-3-treated mice (Table 2). Consequently, the late PC-mimetic actions of CORM-3 cannot be attributed to alterations in arterial pressure or heart rate, nor can they be ascribed to nonspecific actions of the drug, since administration of the same moiety lacking the ability to release CO (inactive CORM-3) had no effect on infarct size. Previous investigations have documented the ability of CO to elicit immediate cardioprotective effects [5,13]. To our knowledge, this is the first study to demonstrate that a relatively brief exposure to CO can produce a sustained, long-term cardioprotective effect that mimics the late phase of ischemic PC. These findings advance our understanding of the role of CO in cardiovascular homeostasis by shifting the focus from its immediate, short-lived actions to its long-term effects.
To better assess the cardioprotective actions of CORM-3, we compared them with those of ischemic PC, both at 24 and 72 h. Since all previous studies of ischemic PC in murine models have been performed at 24 h after the stimulus [20,39,41–49], the duration of ischemia-induced late PC in this species is unknown. To our knowledge, the data obtained in group III provide the first evidence that the cardioprotective actions of ischemic PC persist for 72 h in the mouse. Fig. 2 clearly demonstrates that the infarct-sparing actions of CORM-3 are at least as robust as those of ischemic PC at 24 and 72 h, further supporting the potential utility of CO donors for cardioprotection.
The implicit goal of studying PC is to exploit this phenomenon for the protection of ischemic myocardium in patients with coronary artery disease. Although various pharmacological agents have been shown to induce a late PC phenotype in a variety of animal models, many of these agents are not clinically applicable or have significant side effects [24–38]. The recent development of CORMs has enabled administration of CO in biological systems in a predictable, effective, and safe manner. We have previously shown that CORM-3, administered in vivo in this same murine model, releases CO at concentrations sufficient to elicit protection without increasing blood carboxyhemoglobin levels [13]. The present investigation suggests that, in addition to their immediate infarct-sparing actions [13], CO donors may also be useful as pharmacologic tools to promote the shift of the heart from a naïve to a defensive (preconditioned) phenotype. Unlike other late PC-mimetic agents, CORM-3 reduces infarct size even when given at the time of reperfusion [13]. These facts provide a rationale for testing the potential utility of CO donors as late PC mimetics in patients at risk for myocardial infarction.
The mechanism whereby brief exposure to CO induces sustained cardioprotection remains to be elucidated. CO modulates many potentially protective signaling pathways. For example, CO may alleviate ischemia/reperfusion injury by activating mitochondrial ATP-sensitive (mitoKATP) potassium channels. This mechanism has been suggested by Clark et al. [5], who demonstrated that the cardioprotection induced by CORM-3 in cardiac cells and isolated hearts was abrogated by 5-hydroxydecanoic acid. Whether this immediate effect of CO can account for its delayed cytoprotective actions, however, is unknown. Because CO exerts powerful anti-apoptotic effects in many cell types [11,14–16], it is also plausible that CO-induced cardioprotection may be associated with upregulation of anti-apoptotic proteins and/or downregulation of pro-apoptotic proteins. Another pathway that may potentially mediate CO donor-induced cardioprotection is the p38 MAPK signaling pathway, which has been previously implicated in the protective effects of ischemic PC [50]. Activation of p38 MAPK has been shown to underlie CO-dependent alleviation of hepatic ischemia/reperfusion injury [12] and CO-dependent inhibition of apoptosis during lung ischemia/reperfusion injury [51]. Considerable work will be necessary to unravel the molecular basis of CO-induced delayed cardioprotection.
In conclusion, our understanding of the cardiovascular functions of CO continues to evolve rapidly. The results of this study indicate, for the first time, that CO induces a sustained cardioprotective phenotype that confers resistance to myocardial ischemia/reperfusion injury. We propose the novel idea that CO plays a critical role in the adaptation of the heart to stress by virtue of its ability to reprogram the heart in a manner that promotes cell survival. This concept implies that, far from being a toxic waste product, CO exerts a fundamental (and heretofore unrecognized) function in protecting the heart against ischemia. Besides these conceptual implications, the present findings have potential practical reverberations, since CO donors may become a new class of antiischemic drugs [5]. The fact that CORM-3 has now been shown to induce cardioprotection not only when administered at the time of reperfusion [13] but also when given 24 or 72 h prior to coronary occlusion suggests that CO confers a broad range of salubrious actions in myocardial ischemia/reperfusion. The observations reported herein, coupled with prior reports [13], suggest that CO-releasing agents may be potentially useful for inducing a sustained cardioprotective phenotype in patients at risk for myocardial infarction.
Acknowledgments
The authors would like to thank Professor Brian Mann for the synthesis of CORM-3. This study was supported in part by AHA Postdoctoral Fellowship Award 0325372B, AHA Ohio Valley Affiliate Grant 0265087B, AHA Scientist Development Grant 0130146N, NIH grants R01 HL-55757, HL-68088, HL-70897, HL-76794, HL-65660, and HL-72410, the Medical Research Grant Program of the Jewish Hospital Foundation, Louisville, KY, and the Commonwealth of Kentucky Research Challenge Trust Fund.
References
- 1.Chance B, Erecinska M, Wagner M. Mitochondrial responses to carbon monoxide toxicity. Ann NY Acad Sci. 1970;174:193–204. doi: 10.1111/j.1749-6632.1970.tb49786.x. [DOI] [PubMed] [Google Scholar]
- 2.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 3.Sammut IA, Foresti R, Clark JE, Exon DJ, Vesely MJ, Sarathchandra P, et al. Carbon monoxide is a major contributor to the regulation of vascular tone in aortas expressing high levels of haeme oxygenase-1. Br J Pharmacol. 1998;125:1437–1444. doi: 10.1038/sj.bjp.0702212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song R, Mahidhara RS, Liu F, Ning W, Otterbein LE, Choi AM. Carbon monoxide inhibits human airway smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Am J Respir Cell Mol Biol. 2002;27:603–610. doi: 10.1165/rcmb.4851. [DOI] [PubMed] [Google Scholar]
- 5.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 6.Otterbein LE. Carbon monoxide: innovative anti-inflammatory properties of an age-old gasmolecule. Antioxid Redox Signal. 2002;4:309–319. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- 7.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 8.Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 9.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, et al. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AM. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol. 2002;27:739–745. doi: 10.1165/rcmb.4816. [DOI] [PubMed] [Google Scholar]
- 11.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 12.Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, et al. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunther L, Berberat PO, Haga M, Brouard S, Smith RN, Soares MP, et al. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002;51:994–999. doi: 10.2337/diabetes.51.4.994. [DOI] [PubMed] [Google Scholar]
- 15.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otterbein L, Soares M, Yamashita K, Bach F. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 17.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerbraun BS, Billiar TR, Otterbein SL, Kim PK, Liu F, Choi AM, et al. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med. 2003;198:1707–1716. doi: 10.1084/jem.20031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, Xuan YT, Wu WJ, Zhu X, Tan W, Zhu Y, et al. Hemeoxygenase-1 mediates three different types of late preconditioning. Circulation. 2003;108(IV):93. [Abstract]. [Google Scholar]
- 20.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maulik N, Engelman DT, Watanabe M, Engelman RM, Das DK. Nitric oxide—a retrograde messenger for carbon monoxide signaling in ischemic heart. Mol Cell Biochem. 1996;157:75–86. doi: 10.1007/BF00227883. [DOI] [PubMed] [Google Scholar]
- 22.Maulik N, Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE, 3rd, et al. Nitric oxide/carbon monoxide. A molecular switch for myocardial preservation during ischemia. Circulation. 1996;94:II398–II406. [PubMed] [Google Scholar]
- 23.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee S, Tang XL, Qiu Y, Takano H, Manchikalapudi S, Dawn B, et al. Nitroglycerin induces late preconditioning against myocardial stunning via a PKC-dependent pathway. Am J Physiol. 1999;277:H2488–H2494. doi: 10.1152/ajpheart.1999.277.6.H2488. [DOI] [PubMed] [Google Scholar]
- 25.Hill M, Takano H, Tang XL, Kodani E, Shirk G, Bolli R. Nitroglycerin induces late preconditioning against myocardial infarction in conscious rabbits despite development of nitrate tolerance. Circulation. 2001;104:694–699. doi: 10.1161/hc3201.092218. [DOI] [PubMed] [Google Scholar]
- 26.Ping P, Takano H, Zhang J, Tang XL, Qiu Y, Li RC, et al. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res. 1999;84:587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- 27.Takano H, Tang XL, Qiu Y, Guo Y, French BA, Bolli R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang XL, Kodani E, Takano H, Hill M, Shinmura K, Vondriska TM, et al. Protein tyrosine kinase signaling is necessary for NO donor-induced late preconditioning against myocardial stunning. Am J Physiol Heart Circ Physiol. 2003;284:H1441–H1448. doi: 10.1152/ajpheart.00789.2002. [DOI] [PubMed] [Google Scholar]
- 29.Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 h after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- 30.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, et al. A1 or A3 adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 31.Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res. 1999;84:846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- 32.Fryer RM, Hsu AK, Nagase H, Gross GJ. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J Pharmacol Exp Ther. 2000;294:451–457. [PubMed] [Google Scholar]
- 33.Tsuchida A, Miura T, Tanno M, Sakamoto J, Miki T, Kuno A, et al. Infarct size limitation by nicorandil: roles of mitochondrial K(ATP) channels, sarcolemmal K(ATP) channels, and protein kinase. C JAm Coll Cardiol. 2002;40:1523–1530. doi: 10.1016/s0735-1097(02)02268-4. [DOI] [PubMed] [Google Scholar]
- 34.Xi L, Jarrett NC, Hess ML, Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 1999;99:2157–2163. doi: 10.1161/01.cir.99.16.2157. [DOI] [PubMed] [Google Scholar]
- 35.Yao Z, Auchampach JA, Pieper GM, Gross GJ. Cardioprotective effects of monophosphoryl lipid A, a novel endotoxin analogue, in the dog. Cardiovasc Res. 1993;27:832–838. doi: 10.1093/cvr/27.5.832. [DOI] [PubMed] [Google Scholar]
- 36.Ockaili R, Emani VR, Okubo S, Brown M, Krottapalli K, Kukreja RC. Opening of mitochondrial KATP channel induces early and delayed cardioprotective effect: role of nitric oxide. Am J Physiol Heart Circ Physiol. 1999;277:H2425–H2434. doi: 10.1152/ajpheart.1999.277.6.H2425. [DOI] [PubMed] [Google Scholar]
- 37.Takashi E, Wang Y, Ashraf M. Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res. 1999;85:1146–1153. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- 38.Tang XL, Xuan YT, Zhu Y, Shirk G, Bolli R. Nicorandil induces late preconditioning against myocardial infarction in conscious rabbits. Am J Physiol Heart Circ Physiol. 2004;286(4):1273–1280. doi: 10.1152/ajpheart.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Fisher SG, Marber MS. An in vivo model of ischaemia–reperfusion injury and ischaemic preconditioning in the mouse heart. J Pharmacol Toxicol Methods. 2002;48:161–169. doi: 10.1016/S1056-8719(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 42.Guo Y, Bao W, Wu WJ, Shinmura K, Tang XL, Bolli R. Evidence for an essential role of cyclooxygenase-2 as amediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–484. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maulik N, Yoshida T, Das DK. Regulation of cardiomyocyte apoptosis in ischemic reperfused mouse heart by glutathione peroxidase. Mol Cell Biochem. 1999;196:13–21. doi: 10.1007/978-1-4615-5097-6_2. [DOI] [PubMed] [Google Scholar]
- 44.Miller DL, Van Winkle DM. Ischemic preconditioning limits infarct size following regional ischemia–reperfusion in in situ mouse hearts. Cardiovasc Res. 1999;42:680–684. doi: 10.1016/s0008-6363(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 45.Sumeray MS, Yellon DM. Ischaemic preconditioning reduces infarct size following global ischaemia in the murine myocardium. Basic Res Cardiol. 1998;93:384–390. doi: 10.1007/s003950050106. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Kudo M, Xu M, Ayub A, Ashraf M. Mitochondrial K(ATP) channel as an end effector of cardioprotection during late preconditioning: triggering role of nitric oxide. J Mol Cell Cardiol. 2001;33:2037–2046. doi: 10.1006/jmcc.2001.1468. [DOI] [PubMed] [Google Scholar]
- 47.Xi L, Hess ML, Kukreja RC. Ischemic preconditioning in isolated perfused mouse heart: reduction in infarct size without improvement of post-ischemic ventricular function. Mol Cell Biochem. 1998;186:69–77. [PubMed] [Google Scholar]
- 48.Yamaura G, Turoczi T, Yamamoto F, Siddqui MA, Maulik N, Das DK. STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H476–H482. doi: 10.1152/ajpheart.00079.2003. [DOI] [PubMed] [Google Scholar]
- 49.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA. 2001;98:9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulz R, Belosjorow S, Gres P, Jansen J, Michel MC, Heusch G. p38 MAP kinase is a mediator of ischemic preconditioning in pigs. Cardiovasc Res. 2002;55:690–700. doi: 10.1016/s0008-6363(02)00319-x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, et al. Carbon monoxide inhibition of apoptosis during ischemia–reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]