Abstract
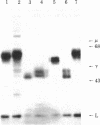
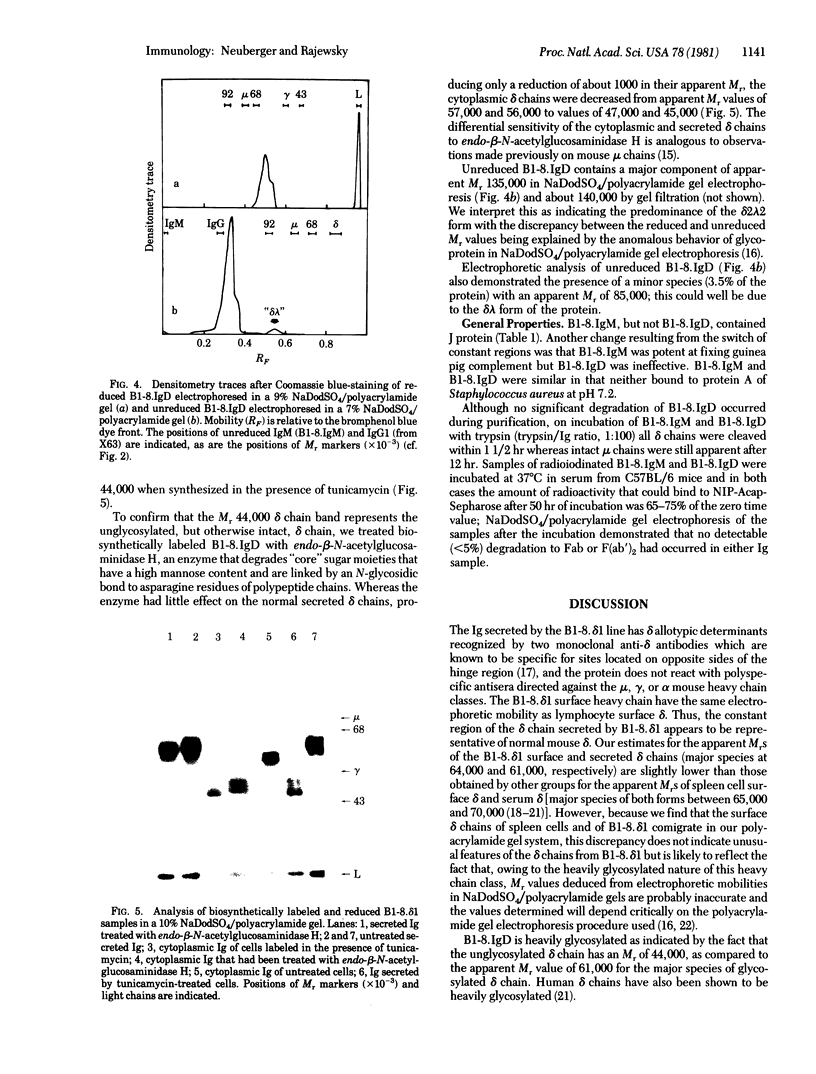
From a hybrid mouse cell line (B1-8) that secreted an IgM, lambda 1 anti-(4-hydroxy-3-nitrophenyl)acetyl antibody but that had no detectable surface IgM, selection for a variant with lambda 1 chains on the surface resulted in the isolation of a line that had switched from mu to delta expression. The surface and secreted Igs of this line were typed as IgD with two monoclonal antibodies, and the parental IgM and variant IgD molecules carried the same variable regions as judged by hapten-binding and idiotypic analysis. The surface and secreted delta chains of the IgD variant have apparent molecular weights of 64,000 and 61,000, respectively. However, the unglycosylated secreted delta polypeptide chain has a molecular weight of only 44,000. The secreted IgD exists predominantly in the delta 2 lambda A2 form, does not contain J protein, is relatively stable in serum, and does not fix complement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Hunter I. R., Parkhouse R. M. Preparation and characterisation of an antiserum to the mouse candidate for immunoglobulin D. Nature. 1976 Feb 5;259(5542):404–406. doi: 10.1038/259404a0. [DOI] [PubMed] [Google Scholar]
- Bargellesi A., Corte G., Cosulich E., Ferrarini M. Presence of serum IgD and IgD-containing plasma cells in the mouse. Eur J Immunol. 1979 Jun;9(6):490–492. doi: 10.1002/eji.1830090614. [DOI] [PubMed] [Google Scholar]
- Bazin H., Beckers A., Urbain-Vansanten G., Pauwels R., Bruyns C., Tilkin A. F., Platteau B., Urbain J. Transplantable IgD immunoglobulin-secreting tumors in rat. J Immunol. 1978 Nov;121(5):2077–2082. [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Eidels L. IgD is present on the cell surface of murine lymphocytes in two forms: delta 2L2 and delta L. J Immunol. 1979 Aug;123(2):896–902. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Complement-fixing IgG1 constitutes a new subclass of mouse IgG. Nature. 1979 Oct 11;281(5731):492–493. doi: 10.1038/281492a0. [DOI] [PubMed] [Google Scholar]
- Haustein D. Effective radioiodination by lactoperoxidase and solubilisation of cell-surface proteins of cultured murine T lymphoma cells. J Immunol Methods. 1975 Apr;7(1):25–38. doi: 10.1016/0022-1759(75)90127-1. [DOI] [PubMed] [Google Scholar]
- Honjo T., Kataoka T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W., Woods V. L., Finkelman F. D., Scher I. Membrane orientation and location of multiple and distinct allotypic determinants of mouse lymphocyte IgD. J Immunol. 1979 Dec;123(6):2772–2778. [PubMed] [Google Scholar]
- Liesegang B., Radbruch A., Rajewsky K. Isolation of myeloma variants with predefined variant surface immunoglobulin by cell sorting. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3901–3905. doi: 10.1073/pnas.75.8.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Melcher U., Uhr J. W. Cell surface immunoglobulin. XVI. Polypeptide chain structure of mouse IgM and IgD-like molecule. J Immunol. 1976 Feb;116(2):409–415. [PubMed] [Google Scholar]
- Mescher M. F., Pollock R. R. Murine cell surface immunoglobulin: two forms of delta-heavy chain. J Immunol. 1979 Sep;123(3):1155–1161. [PubMed] [Google Scholar]
- Mosmann T. R., Gravel Y., Williamson A. R., Baumal R. Modification and fate of J chain in myeloma cells in the presence and absence of polymeric immunoglobulin secretion. Eur J Immunol. 1978 Feb;8(2):94–101. doi: 10.1002/eji.1830080205. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Patterson R. A., Hartley B. S. Purification and properties of Klebsiella aerogenes D-arabitol dehydrogenase. Biochem J. 1979 Oct 1;183(1):31–42. doi: 10.1042/bj1830031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Pearson T., Galfrè G., Ziegler A., Milstein C. A myeloma hybrid producing antibody specific for an allotypic determinant on "IgD-like" molecules of the mouse. Eur J Immunol. 1977 Oct;7(10):684–690. doi: 10.1002/eji.1830071006. [DOI] [PubMed] [Google Scholar]
- ROWE D. S., FAHEY J. L. A NEW CLASS OF HUMAN IMMUNOGLOBULINS. I. A UNIQUE MYELOMA PROTEIN. J Exp Med. 1965 Jan 1;121:171–184. doi: 10.1084/jem.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A., Liesegang B., Rajewsky K. Isolation of variants of mouse myeloma X63 that express changed immunoglobulin class. Proc Natl Acad Sci U S A. 1980 May;77(5):2909–2913. doi: 10.1073/pnas.77.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979 Dec;9(12):1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]