Abstract
Introduction
Pneumatosis intestinalis is a rare condition affecting 0.03% of the population. It has a myriad of aetiological causes and hence presentation can vary immensely. The management of symptomatic pneumatosis intestinalis in an acute and outpatient setting remains a challenge to both physicians and surgeons.
Case presentation
We present a case of a 79 year old who presented in a gastroenterology outpatients department with a history suggestive of intermittent small bowel obstruction associated with abdominal pain aggravated by eating and posture. He was found to have signs suggestive of Marfan's syndrome. Computed tomography demonstrated extensive pneumatosis intestinalis of the small bowel. Due to deterioration in symptoms, an exploratory laparotomy was performed demonstrating segmental small bowel pneumatosis intestinalis secondary to a hypermobile mesentery.
Conclusion
This case highlights the importance of both surgical and gastroenterology expertise in successfully managing symptomatic pneumatosis intestinalis.
Keywords: Pneumatosis intestinalis, Small bowel, Marfan's
1. Introduction
Pneumatosis intestinalis (PI) is a rare condition that was first described by Du Vernoy in 1730 from cadaveric dissections.1 It remains a difficult condition to successfully manage and presents a challenge to surgeons in both an acute and outpatient setting.1 The overall incidence of PI in the general population has been reported to be 0.03%.2 It is thought that the increased availability and use of computed tomography (CT) has resulted in greater detection rates with an incidence of 0.37% being reported CT studies.3,4 PI has numerous aetiological causes resulting in a spectrum of clinical presentations ranging from subacute bowel obstruction to no overt symptoms. In early case reports, PI was considered as either primary or secondary in origin, with a quoted 85% of cases secondary to necrotic, non-necrotic gastrointestinal or pulmonary pathology.5 We describe a rare case of segmental PI of the small bowel presenting with acute abdominal pain.
2. Case report
A 74 year old Caucasian male was referred to the gastroenterology outpatient with a very gradual onset of abdominal distension and pain over a period of 1 year associated with intermittent absolute constipation and occasional vomiting. His symptoms were aggravated by meals and by posture particularly when driving for long periods of time. He described a significant loss of weight thought to be approximately 13 kg over a year. His medical background included atrial fibrillation, polymyalgia rheumatica, anaemia and hypertension. Surgical history included an open bilateral inguinal hernia repair as well as recurrent umbilical hernia repairs. Upon examination, he was found to be 6 ft 4 in. tall with a weight of 92 kg (BMI 25). He was also noted to have signs suggestive of Marfan's syndrome including pectus carinatum, ectopia lentis and a high arched palate. The abdomen was distended but soft and non-tender with normal bowel sounds. Routine blood tests revealed a normocytic anaemia with a haemoglobin of 11.3 g/dl and a mean corpuscular volume of 82. Liver, kidney and bone profiles were unremarkable. Endoscopic investigations were undertaken, an OGD demonstrated Barrett's and a subsequent colonoscopy was found to be normal. An initial plain chest radiograph demonstrated bilateral free sub diaphragmatic air (Fig. 1). A computed tomography (CT) abdomen/pelvis demonstrated extensive pneumatosis intestinalis involving his small bowel with free intraperitoneal air reported as possibly secondary to a malrotation of his proximal small bowel (Fig. 2).
Fig. 1.
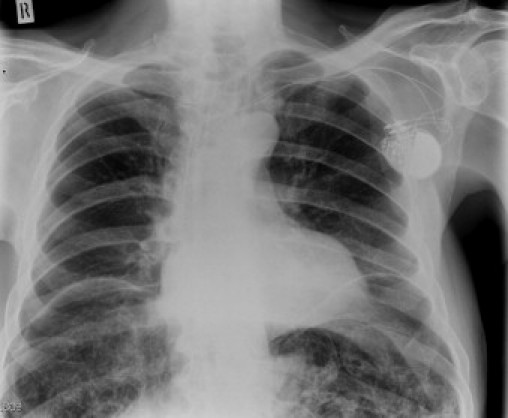
Erect chest radiograph demonstrating free air under both hemidiaphragms.
Fig. 2.
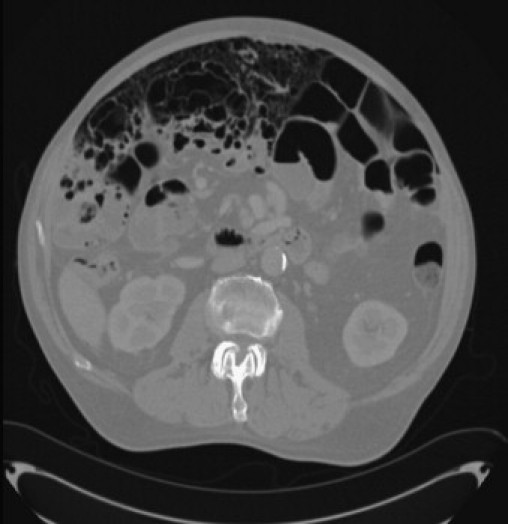
Axial computed tomography demonstrating extensive pneumatosis intestinalis of small bowel and free intra-peritoneal air.
Due to a lack of improvement in his symptoms he was referred for a surgical opinion. Following a surgical review it was elected to perform an elective exploratory laparotomy. Intra-operatively, he was found to have extensive and bulky pneumatosis intestinalis extending from the duodenal–jejenal flexure to the terminal ileum with only few very short segments of normal bowel found (Fig. 3). There was no evidence of a malrotation, bands or strictures. The small bowel mesentery was noted to be especially long along its entire length. The colon was entirely normal as well as the rest of the intra-peritoneal organs. Small bowel resection was not undertaken in view of the absence of any significant length of normal bowel. The post-operative recovery was uneventful.
Fig. 3.
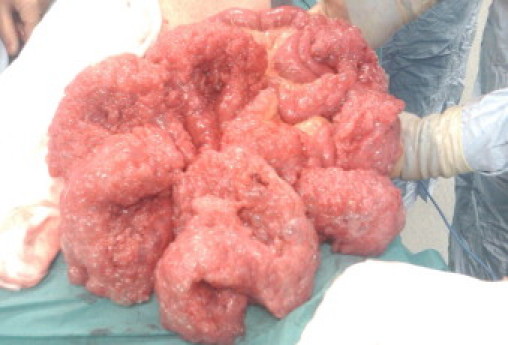
Segmental pneumatosis intestinalis of the small bowel.
3. Discussion
As our case demonstrates, in the majority of cases the provisional diagnosis of PI is made based on the classic radiological findings on CT demonstrating extra-luminal gas in the bowel wall. The pathogenesis remains uncertain, but it is believed to be as a result of multiple contributing factors.6 It is thought that it can occur as a result of two different processes. It can either occur due to intra-luminal gas traversing microbreaks in the bowel mucosa secondary to inflammation or ischaemia. Alternatively, it may be as a result of direct gas diffusion across an intact mucosal membrane, such as in instances of increased abdominal pressure.7 As previously mentioned secondary PI is associated with many different aetiological factors although not an exhaustive list, known associations include mechanical causes such as volvulus, autoimmune disease such as Crohn's disease, infective agents such as HIV, connective tissue disorders such as scleroderma, respiratory aetiologies for example COPD, drug toxicities, graft versus host disease, transplantation and gut ischaemia.1 Our case is of interest as the cause of his PI was secondary to small bowel volvulus secondary to a long and hypermobile small bowel mesentery. We believe that the patient's small bowel mesenteric length could be related to the patient's suspected Marfan's syndrome. It is well recognised that PI can be secondary to connective tissue disorders, however, there are no reports of an association with Marfan's syndrome.1 Interestingly, our hypothesis is supported by the fact that bowel obstruction in Marfan's syndrome has been reported secondary to long caecal and sigmoid mesenteries.8 The location of PI varies and may be anywhere from the stomach to the rectum. The location of the disease can act as a good guide to the aetiology with proximal disease usually being secondary to pyloric stenosis, gastric malignancy or ulcers whereas distal disease may be as a result of diverticulitis or mesenteric ischaemia.9 Most reports of PI describe continuous portions of diseased bowel secondary to an intramural disease process or a mechanical obstruction. Interestingly, segmental disease as was seen in our case is rarely found. The initial priority in the management of PI is to clarify whether the underlying pathology is life threatening or a benign cause. As was seen in our case, it is common for imaging such as plain radiographs or CTs to demonstrate features suggestive of free intraperitoneal gas (Fig. 1). This is thought to be as a result of the perforation of individual gas filled cysts.10 However, it is important to consider the possibility of a perforated viscus and it is thus crucial to correlate radiographic signs with clinical findings. Involvement of a radiologist at an early stage is advisable as certain radiographic features such as crescentic or linear gas collections can be indicative of a bowel infarction.11 In the case of a suspected life threatening condition such as bowel ischaemia, surgical intervention in the form of an emergency exploratory laparotomy is mandatory. In view of this, features of low-flow vascular states such as sepsis, congestive heart failure, use of ionotropic agents, and other causes of hypotension in the acute setting should lead to a high index of suspicion for ischaemic bowel and a low threshold for surgery.9 Conservative management involving nasogastric bowel decompression maybe appropriate if a serious underlying cause of the PI has been excluded.12 However, with high associated mortality rates of between 22 and 50% non-surgical management should be used with caution.13,14
4. Conclusion
This case demonstrates that both surgical and gastroenterology expertise are required to successfully manage this challenging condition. In addition, our case highlights that although pre-operative imaging has an important role in PI, clinical judgement is paramount in tackling this rare condition.
Conflict of interest statement
None.
Funding
None.
Ethical approval
The patient has provided both verbal and written consent for the publication of this article.
Author contributions
All authors were involved in the researching, writing and editing of the manuscript. PHP and SCD contributed equally to the writing of this manuscript.
References
- 1.Braumann C., Menenakos C., Jacobi C.A. Pneumatosis intestinalis—a pitfall for surgeons? Scand J Surg. 2005;94(1):47–50. doi: 10.1177/145749690509400112. [DOI] [PubMed] [Google Scholar]
- 2.Heng Y., Schuffler M.D., Haggitt R.C. Pneumatosis intestinalis: a review. Am J Gastroenterol. 1995;90:1747–1758. [PubMed] [Google Scholar]
- 3.Neumayer L., Wako E., Fergestaad J. Impact of journal articles and grand rounds on practice: CT scanning in appendicitis. J Gastrointest Surg. 2002;6:338–341. doi: 10.1016/s1091-255x(01)00088-9. [DOI] [PubMed] [Google Scholar]
- 4.Morris M.S., Gee A.C., Cho S.D. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679–683. doi: 10.1016/j.amjsurg.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Stovall J.M. Pneumatosis coli: a case presentation and review of literature. J Natl Med Assoc. 1983;75(6) [PMC free article] [PubMed] [Google Scholar]
- 6.Peter S.D.S., Abbas M.A., Kelly K.A. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68–75. doi: 10.1001/archsurg.138.1.68. [DOI] [PubMed] [Google Scholar]
- 7.Pieterse A.S., Leong A.S.Y., Rowland R. The mucosal changes and pathogenesis of pneumatosis cystoides intestinalis. Hum Pathol. 1985;16(7):683–688. doi: 10.1016/s0046-8177(85)80152-0. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G.P., Purkayastha S., Athanasiou T., Darzi A. General surgical manifestations of Marfan's syndrome. Br J Hosp Med (Lond) 2008;69(May (5)):270–274. doi: 10.12968/hmed.2008.69.5.29359. [DOI] [PubMed] [Google Scholar]
- 9.Donovan S., Cernigliaro J., Nancy Dawson N. Pneumatosis intestinalis: a case report and approach to management. Case Rep Med. 2011;5 doi: 10.1155/2011/571387. [Article ID 571387] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams N.M.A., Watkin D.F.L. Spontaneous pneumoperitoneum and other nonsurgical causes of intraperitoneal free gas. Postgrad Med J. 1997;73:531–537. doi: 10.1136/pgmj.73.863.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soyer P., Martin-Grivaud S., Boudiaf M. Linear or bubbly: a pictorial review of CT features of intestinal pneumatosis in adults. J Radiol. 2008;89(12):1907–1920. doi: 10.1016/s0221-0363(08)74786-3. [DOI] [PubMed] [Google Scholar]
- 12.Morris M.S., Gee A.C., Cho S.D. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195:679–683. doi: 10.1016/j.amjsurg.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz N.S., Cohn D.E., Herzog T.J. The significance of pneumatosis intestinalis or bowel perforation in patients with gynecologic malignancies. Gynecol Oncol. 2002;86:79–84. doi: 10.1006/gyno.2002.6728. [DOI] [PubMed] [Google Scholar]
- 14.Knechtle S.J., Davidoff A.M., Rice R.P. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212:160–165. doi: 10.1097/00000658-199008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]


