Abstract
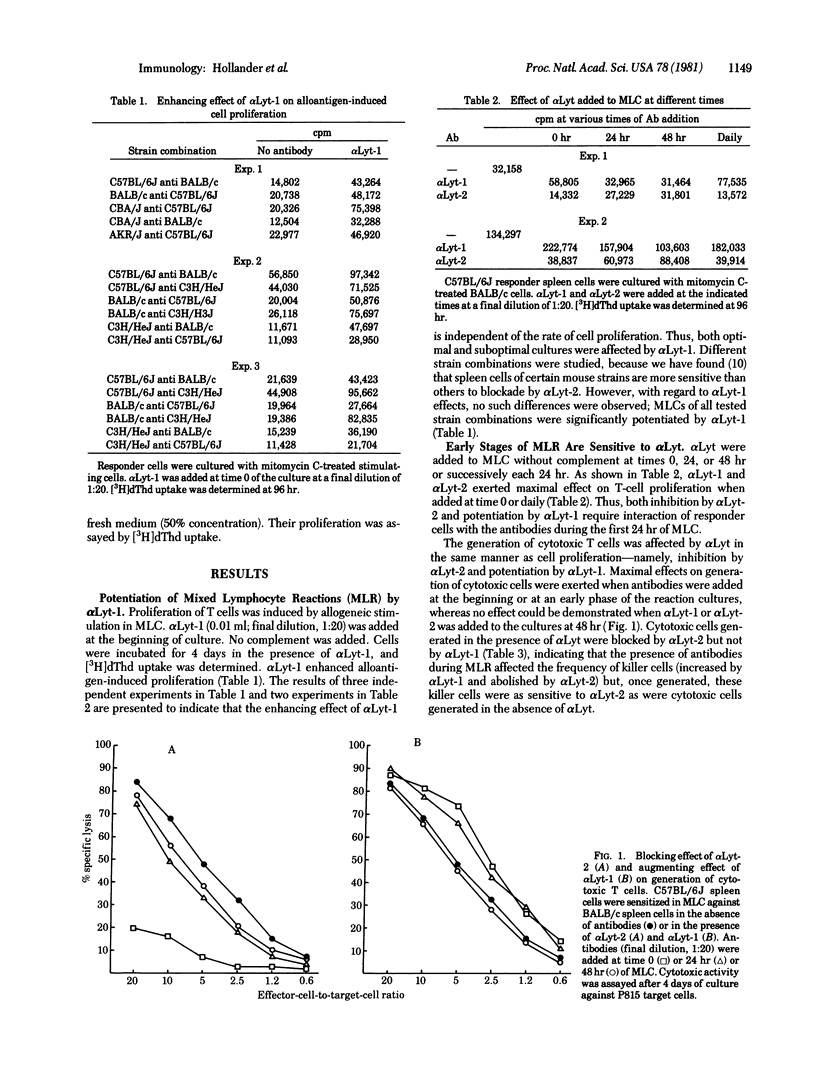
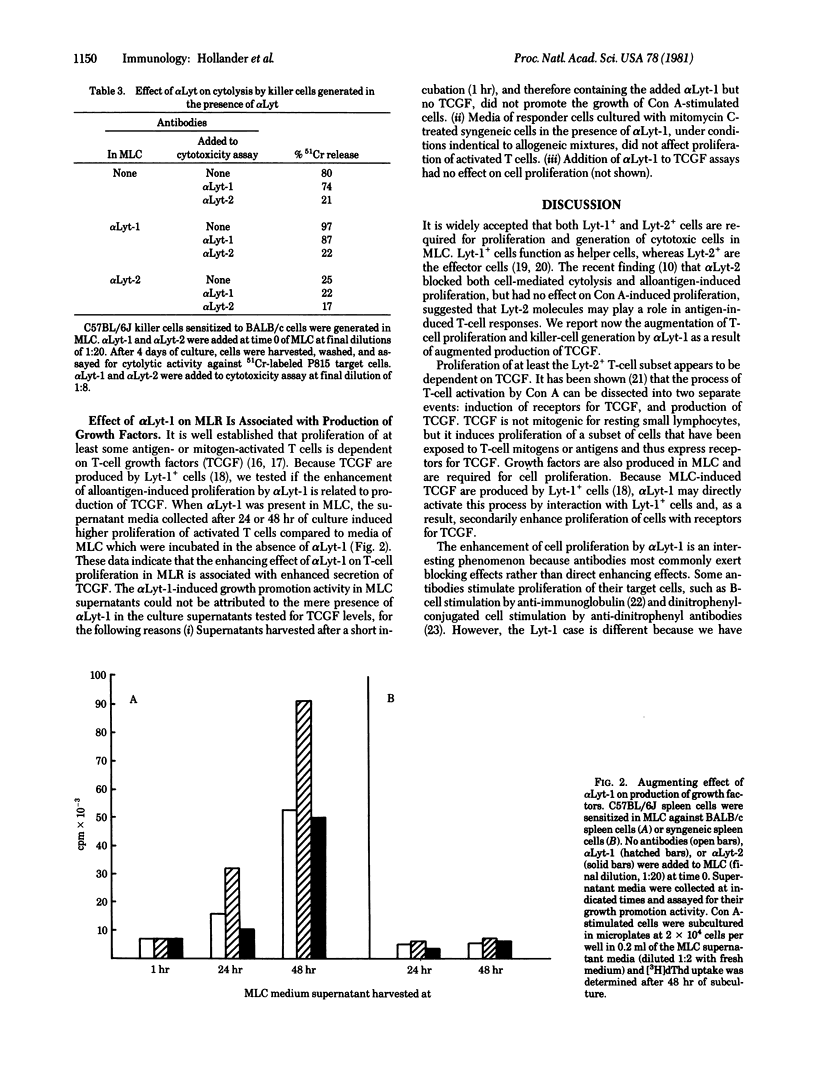
Monoclonal anti-Lyt-1 (alpha Lyt-1) and alpha Lyt-2 manifest inverse effects on allogeneic mixed lymphocyte reactions when added to the reaction mixtures without complement. alpha Lyt-1 augments cell proliferation and generation of cytotoxic cells but has no effect on cell-mediated cytolysis, whereas alpha Lyt-2 blocks cell proliferation, generation of killer cells, and cytolytic activity of killer cells. The augmenting effect of alpha Lyt-1 cannot be attributed to a direct mitogenic effect on T cells. Both inhibition by alpha Lyt-2 and potentiated by alpha Lyt-1 require interaction of responder cells with the antibodies during the first 24 hr of the mixed lymphocyte reaction, indicating that early stages of the reaction are sensitive to Lyt antibodies. The enhancing effect of alpha Lyt-1 on alloantigen-induced T-cell proliferation is associated with augmented production of T-cell growth factors. When alpha Lyt-1 is present in mixed lymphocyte cultures, the supernatant media collected after 24 or 48 hr of culture induce higher proliferation of activated T cells compared to media of mixed lymphocyte cultures incubated in the absence of antibodies or in the presence of alpha Lyt-2 which has no effect on secretion of growth factors. The differences in the effects of alpha Lyt-1 and alpha Lyt-2 could not be attributed to differences in heavy chain constant region functions because both were of the same lambda 2A immunoglobulin class and were used at the same concentration. The data suggest a possible role for Lyt-1 molecules in early activation and mitogenesis processes such as production of growth factors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Grönvik K. O., Larsson E. L., Coutinho A. Studies on T lymphocyte activation. I. Requirements for the mitogen-dependent production of T cell growth factors. Eur J Immunol. 1979 Aug;9(8):581–587. doi: 10.1002/eji.1830090802. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Bach M. L., Sondel P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976 Jan 29;259(5541):273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Itakura K., Stockert E., Iritani C. A., Miura M. Ly-C: a third locus specifying alloantigens expressed only on thymocytes and lymphocytes. Transplantation. 1971 Mar;11(3):351–353. [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Burakoff S. J., Finberg R., Glimcher L., Lemonnier F., Benacerraf B., Cantor H. The biologic significance of alloreactivity. The ontogeny of T-cell sets specific for alloantigens or modified self antigens. J Exp Med. 1978 Nov 1;148(5):1414–1422. doi: 10.1084/jem.148.5.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Larsson E. L., Grönvik K. O., Andersson J. Studies on T lymphocyte activation II. The target cells for concanavalin A-induced growth factors. Eur J Immunol. 1979 Aug;9(8):587–592. doi: 10.1002/eji.1830090803. [DOI] [PubMed] [Google Scholar]
- Durda P. J., Gottlieb P. D. The Ly-3 antigens on mouse thymocytes: immune precipitation and molecular weight characterization. J Exp Med. 1976 Aug 1;144(2):476–493. doi: 10.1084/jem.144.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durda P. J., Shapiro C., Gottlieb P. D. Partial molecular characterization of the Ly-1 alloantigen on mouse thymocytes. J Immunol. 1978 Jan;120(1):53–57. [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. M., Taylor B. A., Cherry M. Evidence for close linkage of a mouse light chain marker with the Ly-2,3 locus. J Immunol. 1978 Oct;121(4):1585–1590. [PubMed] [Google Scholar]
- Gottlieb P. D. Genetic correlation of a mouse light chain variable region marker with a thymocyte surface antigen. J Exp Med. 1974 Nov 1;140(5):1432–1437. doi: 10.1084/jem.140.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander N., Pillemer E., Weissman I. L. Blocking effect of lyt-2 antibodies on T cell functions. J Exp Med. 1980 Sep 1;152(3):674–687. doi: 10.1084/jem.152.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Hutton J. J., Boyse E. A., Old L. J. Genetic linkage relationships of loci specifying differentiation alloantigens in the mouse. Transplantation. 1972 Mar;13(3):239–243. doi: 10.1097/00007890-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. Mechanism of T cell activation. I. A screening of "step one" ligands. Eur J Immunol. 1980 Feb;10(2):93–99. doi: 10.1002/eji.1830100205. [DOI] [PubMed] [Google Scholar]
- Ravid A., Novogrodsky A., Wilchek M. Grafting of triggering sites onto lymphocytes: requirement of multivalency in the stimulation of dinitrophenyl-modified thymocytes by anti-dinitrophenyl antibody. Eur J Immunol. 1978 May;8(5):289–294. doi: 10.1002/eji.1830080502. [DOI] [PubMed] [Google Scholar]
- Sell S. Development of restrictions in the expression of immunoglobulin specificities by lymphoid cells. Transplant Rev. 1970;5:19–44. doi: 10.1111/j.1600-065x.1970.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Stutman O., Shen F. W., Boyse E. A. Ly phenotype of T cells cytotoxic for syngeneic mouse mammary tumors: evidence for T cell interactions. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5667–5671. doi: 10.1073/pnas.74.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]



