Abstract
Introduction
Rice body formation has been traditionally observed in the joint and tendon sheaths of patients with tuberculosis. Few case reports exist that describe rice body formation in patients with rheumatoid arthritis. We describe a case report of bilateral recurrent wrist flexor tenosynovitis with rice body formation in a patient with sero-negative rheumatoid arthritis.
Presentation of case
This case report describes a 72 year old lady presenting with severe bilateral, flexor tenosynovitis of the wrists. Ultrasonography revealed significant echogenic fluid on the palmer aspect of wrist joint surrounding flexor tendons with intact neurovascular bundles and no bony erosion. Laboratory tests demonstrated elevated erythrocyte sedimentation rate (50 mm/h) and negative rheumatoid factor. A sequential subtotal flexor tenosynovectomy was carried out with decompression of the carpal tunnel. During the operation, multiple rice bodies among the flexor tendons with adherent synovitis were found. Histology revealed disrupted synovial tissue containing several areas of fibrinoid necrosis, bounded by a layer of vaguely pallisaded histiocytes but no epitheloid granulomata or germinal centre. A revision surgery with debulking of the fibro-osseous canal was undertaken following recurrence. The patient presently has complete resolution of symptoms at one year follow-up.
Discussion
The combined clinical, laboratory, ultrasound and histology findings of the patient indicated that the cause of the rice body formation was due to a sero-negative arthritis rather than tuberculosis.
Conclusion
Rice body formation can be caused by sero-negative arthritis. Bilateral wrist flexor tensosynovitis can recur within five months of a previous synovectomy in a patient with sero-negative arthritis.
Keywords: Wrist, Flexor tenosynovitis, Rice bodies, Ultrasonography, Tenosynovectomy
1. Introduction
Rice body formation was first and is traditionally observed in the joint and tendon sheaths among patients with tuberculous arthritis.1,2 These have also been noted in patients with rheumatoid arthritis3 however only a few cases with rice bodies in tendon sheaths have been mentioned in the literature.4,5 We present a case of bilateral recurrent wrist flexor tenosynovitis with rice body formation in a patient with sero-negative rheumatoid arthritis. The clinical, radiological, histo pathological features with operative findings have been discussed with review of literature.
2. Presentation of case
In May 2008, a 72 year old lady presented to the rheumatologist with a 6-month history of progressive painful swelling of both of her wrists. The pain, had started insidiously, and gradually worsened with time. This was associated with pins and needles affecting the digits in the median nerve distribution. There was no associated history of fever, loss of weight/appetite, night sweats, malaise or fatigue. There was no history of trauma, other joint involvement or morning stiffness. The patient had no significant past medical history.
Physical examination revealed bilateral volar wrist swellings, extending across the wrist crease. Each of the swelling was non-tender, firm in consistency, approximately 8 cm × 6 cm in size and non-compressible. It was mobile at right angles to the plane of the wrist joint but not longitudinally. There was wasting of thenar eminence especially the abductor pollicis with positive Tinel's and Phalen's test consistent with median nerve neuropathy. There was terminal restriction of dorsiflexion and palmarflexion of the wrist.
Laboratory tests were normal except for an elevated erythrocyte sedimentation rate (50 mm/h). Rheumatoid factor was negative. Wrist joint radiographs showed no erosive changes. Ultrasound of the wrists showed significant echogenic fluid surrounding flexor tendons suggestive of tenosynovitis. The tendons were intact with normal appearance of the neurovascular bundles. There was no significant Doppler flow. With these features a diagnosis of sero-negative inflammatory arthritis was made and the patient was started on Methotrexate.
The patient was referred six months later to our hand surgery department as there was no respite in her symptoms despite being on Methotrexate and two previous local corticosteroid injections. The patient underwent sequential subtotal synovectomy of her wrists using an extended volar approach. The flexor tendons were encased with fibronous tissue which was debrided. During the operation, multiple rice bodies among the flexor tendons with adherent synovitis were found. The median nerve was decompressed with external neurolysis. Immobilization with a splint was carried out in initial two post-operative weeks for patient comfort and wound healing. Thereafter no splint was used. The patient underwent both surgeries under the cover of Methotrexate. Methotrexate was administered before and continued following surgery under rheumatologist supervision.
Synovial histology revealed several areas of fibrinoid necrosis, bounded by a layer of pallisaded histiocytes but no epitheloid granulomata or germinal centre (Fig. 1). There were no epithelioid granulomata or giant cells. The rice bodies consisted of fibrino-necrotic material. Special stains for fungi (DPAS-Diastase resistant periodic acid Schiff) and acid fast bacilli (Ziehl–Neelsen) were negative (Fig. 2).
Fig. 1.
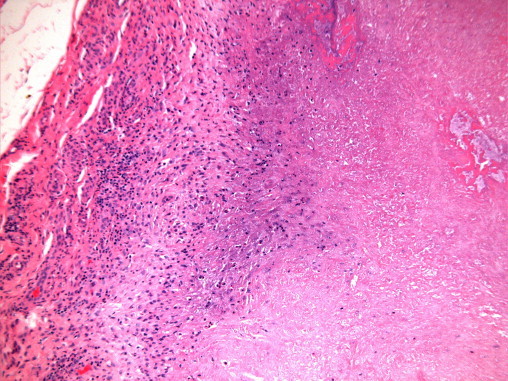
Amorphous, necrotic material (right) separated from inflamed synovium (left) by a rather pale zone of histiocytes.
Fig. 2.
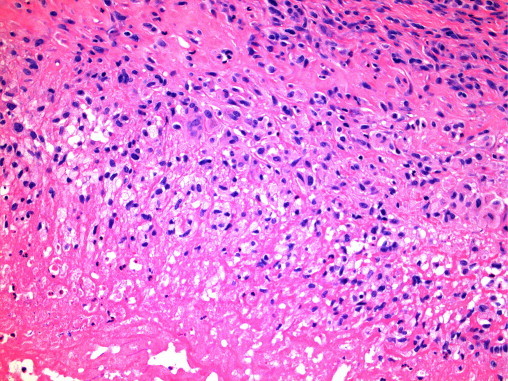
Amorphous, pink, necrotic material (bottom) bounded by a layer of vaguely pallisaded histiocytes. (Stains for mycobacteria and fungi were negative.)
The patient's initial post-operative period was uneventful with primary healing of the wounds. Symptoms of median neuropathy improved. However, five months following the index operation, the symptoms recurred with reappearance of the wrist swellings and paraesthesia. A re-synovectomy of both the wrists was undertaken using the extended volar approach. The median nerve was identified and protected using loupe magnification. Recurrent synovitis in the carpal tunnel and around the flexor tendons was removed with radical debridement and debulking of the fibro-osseous canal. This time, there was yellowish altered synovial fluid with pellet like rice bodies encasing the flexor tendons (Fig. 3).
Fig. 3.
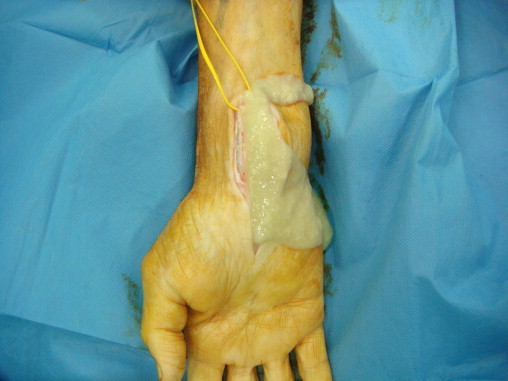
Yellowish discharge of altered synovial fluid with pellet like rice bodies following synovectomy.
Following re-synovectomy the patient regained her pre-operative range of movement with resolution of symptoms at one year follow-up.
3. Discussion
Rice bodies’ formation was first and is traditionally observed in the joint and tendon sheaths among patients with tuberculous arthritis.1,2
Rice bodies in joints are also known to occur in rheumatic diseases such as rheumatoid arthritis, osteoarthritis, systemic lupus erythematous, seronegative arthritidies, as well as infectious diseases such as nonspecific arthritis, tuberculosis and atypical mycobacterial infections.3–7 However, rice bodies in tendon sheaths are rarely seen among non-tuberculosis patients.8,9
The pathogenesis of rice body formation has not yet been completely determined. Several theories exist regarding the formation of rice bodies including micro-infarction of the synovium with release of tissue into the joint,3 chronic bursitis,10 encasement of synovial shedding following inflammation,11 encasement of fibrin12 and alteration in viscosity and content of synovial fluid.13 Some of these factors seem to have influenced the development of rice body formation in our patient.
Wrist flexor tenosynovitis is often seen in patients with rheumatoid and seronegative arthritidies. The sheaths of the tendons of the wrist have been reported as a site for rice body formation.5,8,9,14 However, rice bodies in tendon sheaths of patient with seronegative rheumatoid arthritis are rare. The interesting feature in our patient was the bilateral affliction with signs of median nerve neuropathy.
Rheumatoid factor is helpful in the diagnosis of Rheumatoid Arthritis however only 60–70% of Rheumatoid Arthritis patients are Rheumatoid Factor positive.15 Recently anticyclic citrullinated peptide (anti-CCP) antibodies have been found to show significantly high sensitivity and specificity for early rheumatoid arthritis and seronegative rheumatoid arthritis patients.16 Unfortunately this test was not available in our NHS trust when the patient presented to us.
Though Magnetic Resonance (MR) imaging has been shown to be a better modality at delineating flexor tenosynovitis in the wrist,17 real time Ultrasound imaging has proven to be highly effective in detecting rheumatoid soft tissue changes as well.18 Ultrasonography is also proposed as an effective first-line approach and as a periodical follow-up survey in these patients. The finding of an inflamed synovial sheath with relatively little synovial fluid is characteristic of tuberculous pathology, while significant echogenic fluid surrounding flexor tendons suggestive of non tubercular tenosynovitis as found in our patient.19–21 Ultrasound can also demonstrate the tendon discontinuity in cases of tendon rupture which fortunately this patient did not have.
With similar clinical features and a negative rheumatoid factor, one has to keep a differential diagnosis of tubercular aetiology in such patients. Herein, histo-pathological diagnosis plays a key role in guiding the clinical management. A classical tubercular lesion is tubercular granulomata. Tubercular granulomata consist of a central area of caseation necrosis, surrounded by epitheloid cells and characteristic Langerhan's multinucleate giant cells.
These characteristic tubercular histological features may not be present in some patients.22 Synovial histology in our patient revealed features of fibrinoid necrosis, some pallisaded histiocytes and mild inflammatory cells with no epitheloid granulomata. These appearances were consistent with those of rheumatoid nodules within the synovium, found on two separate histological specimens examined following both procedures. Overall, a combination of clinical, radiological and histological features was strongly suggestive of seronegative rheumatoid disease in our case.
4. Conclusion
Synovitis can destroy tendon, cartilage and bone when in a closed space either by attrition or by direct invasion by hypertrophic tenosynovium. A rupture due to invasive tenosynovitis within the joint predicts an unfavourable prognosis such that prevention is one of the main objectives in management strategies in these patients.23 Treatment of such patients involves a combined surgical and medical approach. We feel in the presence of compressive neuropathy, surgical decompression is essential to relieve the pressure on the nerve and prevent further deterioration. Radical debridement and debulking forms an integral part of the surgery to reduce en mass load of the affected synovium. Prevention of tendon ruptures by early tenosynovectomy and removal of bone spurs if any, is one of the cornerstones of treatment.24 However, it is still imperative that medical treatment is administered before, during and after surgery.25
Flexor tenosynovectomy with tenolysis is a useful procedure with a low rate of recurrence in patients with chronic tenosynovitis of the wrist.26 Surgery does not avoid local recurrence but decreases the progression of the destructive osteocartilaginous process and deterioration of nerve function as was evident in our patient.27 One study has demonstrated a clinical recurrence rate of 31% after flexor teno-synovectomy with a re-operation rate of 15% but the unfortunately did not state a time period. Furthermore the report also has stated that debulking the fibro-osseous canal by excising a slip of flexor digitorum superficialis was associated with a reduction in the recurrence and re-operation rate.28 However, to the best of our knowledge, the authors of this report are unaware of a case of bilateral wrist flexor tensosynovitis that has recurred within five months of a previous synovectomy. The severity of the patient's inflammatory process probably predicts that the tenosynovitis of the wrists may recur. However, following the principle of radical debridement and debulking of the fibro-osseous canal while conducting our revision surgery will hopefully prevent any further recurrence.
Conflicts of interest statement
None
Funding
None
Ethical approval
Consent was obtained from the patient using the appropriate form. The patient's details have been kept anonymous in the manuscript.
Author contributions
Mr. Karthikeyan Iyengar – 1st Author of case report, performed the operation, involved in writing 1st draft of case report, literature search.
Dr. Tharjan Manickavasagar – 2nd Author of case report, assisted in the operation, involved in writing 1st draft of case report, literature search.
Mr. Jayant Nadkarni – 3rd Author of case report – involved in 2nd draft of case report and reviewing draft.
Mr. William Loh – Supervising Orthopaedic Consultant, performed the operation, reviewed 2nd draft of case report.
Dr. Paul Mansour – Interpretation of histology slides.
References
- 1.Reise H. Die Reiskorpschen in tuberculs erranken synovalsacken. Dtsch Z hir. 1895;42:1. [Google Scholar]
- 2.Pimm L.H., Waugh W. Tuberculous tenosynovitis. J Bone Joint Surg. 1957;39B:91–101. doi: 10.1302/0301-620X.39B1.91. [DOI] [PubMed] [Google Scholar]
- 3.Cheung H.S., Ryan L.M., Kozin F., McCarty D.J. Synovial origins of rice bodies in joint fluid. Arthritis Rheum. 1980;23:72–76. doi: 10.1002/art.1780230112. [DOI] [PubMed] [Google Scholar]
- 4.Amrami K.K., Ruggleri A.P., Sundaram M. Radiologic case study. Rheumatoid arthritis with rice bodies. Orthopaedics. 2004;27(350):426–427. doi: 10.3928/0147-7447-20040401-01. [DOI] [PubMed] [Google Scholar]
- 5.Chau C.L., Griffith J.F., Chan P.T., Lui T.H., Yu K.S., Ngai W.K. Rice body formation in atypical mycobacterial tenosynovitis and bursitis: findings on sonography and MR imaging. Am J Roentgenol. 2003;180:1455–1459. doi: 10.2214/ajr.180.5.1801455. [DOI] [PubMed] [Google Scholar]
- 6.Sanger J.R., Stampfi D.A., Franson T.R. Recurrent granulomatous synovitis due to Mycobacterium kansasii in a renal transplant recipient. J Hand Surg Am. 1987;12:436–441. doi: 10.1016/s0363-5023(87)80019-9. [DOI] [PubMed] [Google Scholar]
- 7.Bucki B., Lansaman J., Janson X., Billion-Galland M.A., Marty C., Ruel M. Osteoarthritis with rice bodies rich in calcium microcrystals. 4 cases with ultrastructural study. Rev Rhum Ed Fr. 1994;61:415–420. [PubMed] [Google Scholar]
- 8.Nagasawa H., Okada K., Senma S., Chida S., Shimada Y. Tenosynovitis with rice body formation in a non-tuberculous patient: a case report. Ups J Med Sci. 2009;114:184–188. doi: 10.1080/03009730902931408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ergun T., Lakadamyali H., Aydin O. Multiple rice body formation accompanying the chronic non-specific tenosynovitis of the flexor tendons of the wrist. Radiat Med. 2008;26:545–548. doi: 10.1007/s11604-008-0270-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen A., Wong L.Y., Sheu C.Y., Chen B.F. Distinguishing multiple rice body formation in chronic subacromial bursitis from synovial chondromatosis. Skeletal Radiol. 2002;31:119–121. doi: 10.1007/s002560100412. [DOI] [PubMed] [Google Scholar]
- 11.Geiler G., Mehlhorn U. Vasculitis with anaemia infarcts of the villi of the synovial membrane in rheumatoid arthritis. Z Rheumatol. 1989;48:63–67. [PubMed] [Google Scholar]
- 12.Popert A.J., Scott D.L., Wainwright A.C., Walton K.W., Williamson N., Chapman J.H. Frequency of occurrence, mode of development and significance of rice bodies in rheumatoid joints. Ann Rheum Dis. 1982;41:109–117. doi: 10.1136/ard.41.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altmann S., Regul M., Zeider H., Hartmann F. Time-dependent flow behaviour and fibrinogen content of synovial fluid. Z Rheumatol. 1985;44:64–71. [PubMed] [Google Scholar]
- 14.Sugano H., Nagao T., Tajima Y., Ishida Y., Nagao K., Ohno T. Variation among giant rice bodies: report of four cases and their clinicopathological features. Skeletal Radiol. 2000;29:525–529. doi: 10.1007/s002560000258. [DOI] [PubMed] [Google Scholar]
- 15.Gossec L., Combescure C., Rincheval N., Saraux A., Combe B., Dougados M. Relative clinical influence of clinical, laboratory, and radiological investigations in early arthritis on the diagnosis of rheumatoid arthritis. Data from the French Early Arthritis Cohort ESPOIR. J Rheumatol. 2010;37(December (12)):2486–2492. doi: 10.3899/jrheum.100267. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J., Liu X., Wang Z., Liu R., Li Z. Is it necessary to combine detection of anticitrullinated protein antibodies in the diagnosis of rheumatoid arthritis? J Rheumatol. 2010;37(December (12)):2462–2465. doi: 10.3899/jrheum.100399. [DOI] [PubMed] [Google Scholar]
- 17.Tehranzadeh J., Ashikayan O., Anavim A., Tramma S. Enhanced MR imaging of tensoynovitis of hand and wrist in inflammatory arthritis. Skeletal Radiol. 2006;35:814–822. doi: 10.1007/s00256-006-0129-x. [DOI] [PubMed] [Google Scholar]
- 18.Fornage B.D. Soft tissue changes in the hand in rheumatoid arthritis: evaluation with US. Radiology. 1989;173:735–737. doi: 10.1148/radiology.173.3.2682774. [DOI] [PubMed] [Google Scholar]
- 19.De Flaviis L., Scaglione P., Nessi R., Ventura R., Calori G. Ultrasonography of the hand in rheumatoid arthritis. Acta Radiol. 1988;29(July–August (4)):457–460. [PubMed] [Google Scholar]
- 20.Lall H., Nag S.K., Jain V.K., Khare R., Mittal D. Tuberculous extensor tenosynovitis of the wrist with extensor pollicis longus rupture: a case report. J Med Case Rep. 2009;3:142–147. doi: 10.1186/1752-1947-3-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teo S.C., George J., Kamarul T. Tubercular synovitis mimicking rheumatoid nodules. Med J Malayasia. 2008;63:159–161. [PubMed] [Google Scholar]
- 22.Garrido G., Gomez-Reino J.J., Fernández-Dapica P., Palenque E., Prieto S. A review of peripheral tuberculous arthritis. Semin Arthritis Rheum. 1988;18(November (2)):142–149. doi: 10.1016/0049-0172(88)90007-8. [DOI] [PubMed] [Google Scholar]
- 23.Ertel A.N. Flexor tendon ruptures in rheumatoid arthritis. Hand Clin. 1989;5:177–190. [PubMed] [Google Scholar]
- 24.Ertel A.N., Millender L.H., Nalebuff E., Mckay D., Leslie B. Flexor tendon ruptures in patients with rheumatoid arthritis. J Hand Surg Am. 1998;13:860–866. doi: 10.1016/0363-5023(88)90260-2. [DOI] [PubMed] [Google Scholar]
- 25.Ferlic D.C., Clayton M.L. Synovectomy of the hand and wrist. Ann Chir Gynaecol Suppl. 1985;198:26–30. [PubMed] [Google Scholar]
- 26.Tolat A.R., Stanley J.K., Evans RA Flexor tenosynovectomy and tenolysis in longstanding rheumatoid arthritis. J Hand Surg Br. 1996;21(August (4)):538–543. doi: 10.1016/s0266-7681(96)80061-1. [DOI] [PubMed] [Google Scholar]
- 27.Cozzolino F., Giglotti S., Giuzio E., Angrisani C. Surgical Synovectomy in the treatment of rheumatoid arthritis: the results obtained in a case–control study. Chirugia Degli Organi di Movimento. 1991;76:341–346. [PubMed] [Google Scholar]
- 28.Wheen D.J., Tankin M.A., Green J., Bronkhost M. Long-term results following digital flexor tenosynovitis in rheumatoid arthritis. J Hand Surg. 1995;20:790–794. doi: 10.1016/S0363-5023(05)80433-2. [DOI] [PubMed] [Google Scholar]


