Abstract
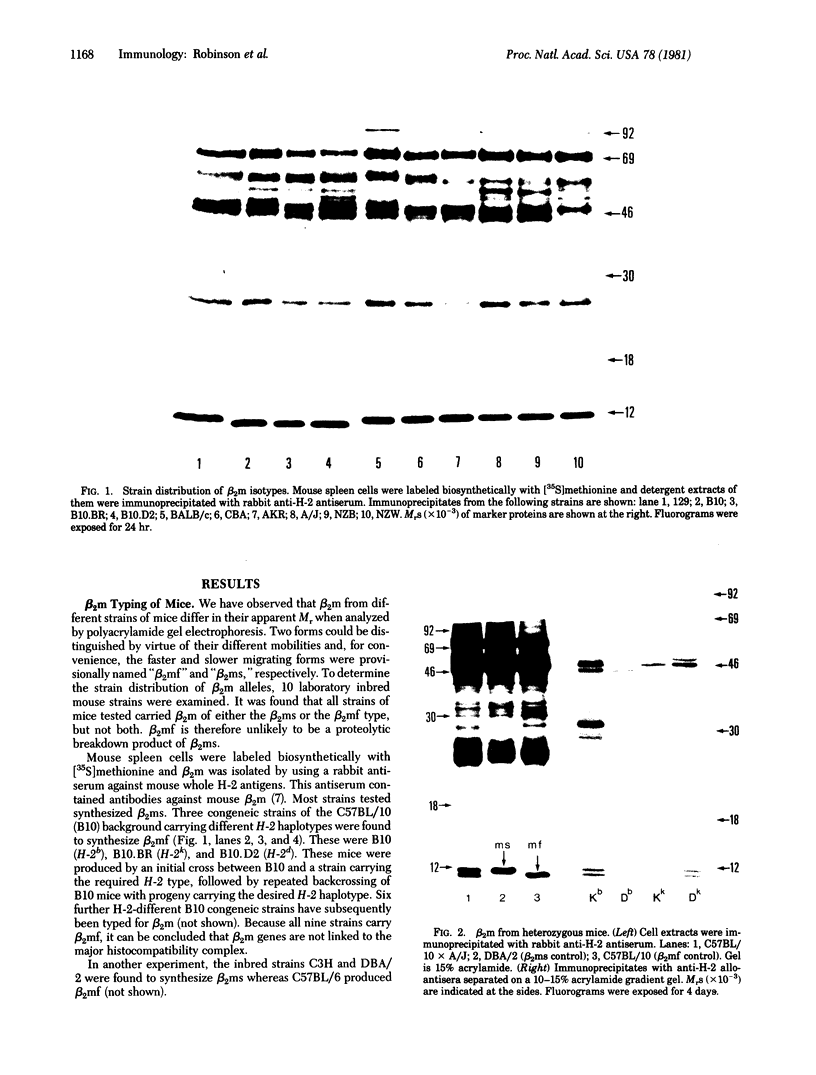
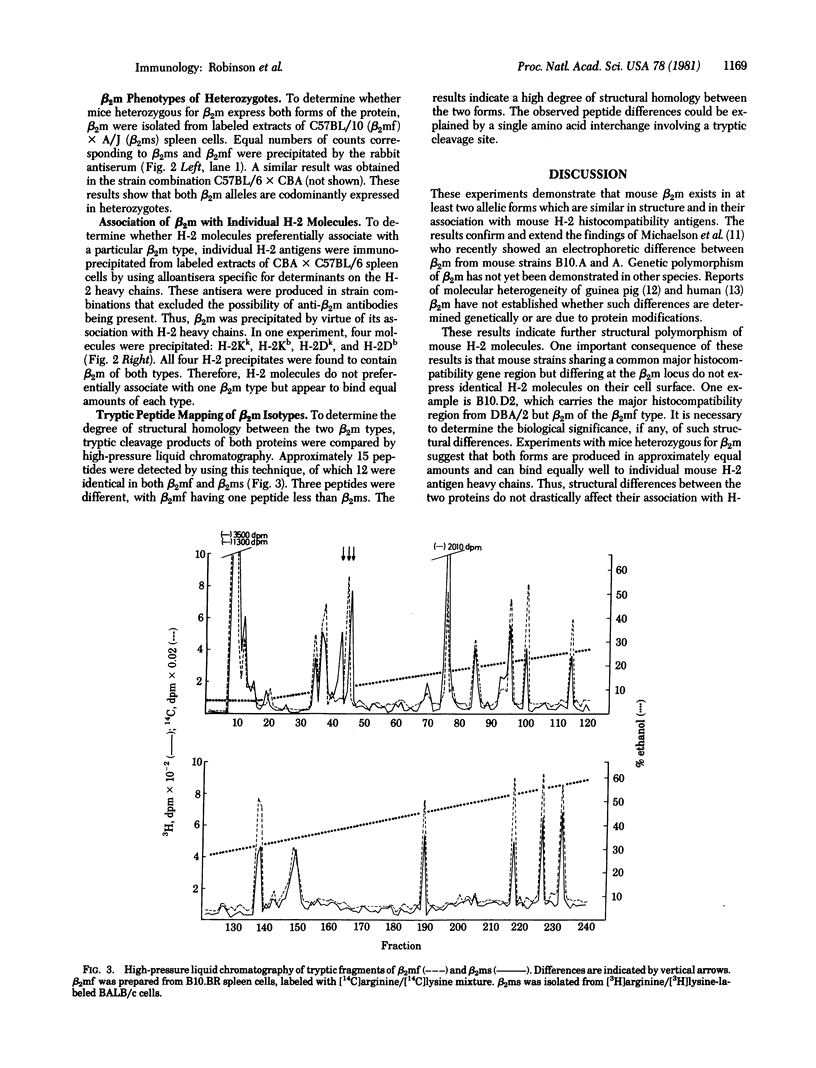
Two allelic forms of mouse beta 2-microglobulin (beta 2m), the small polypeptide chain of H-2 histocompatibility antigens, have been identified. The two forms can be distinguished by NaDodSO4/polyacrylamide gel electrophoresis. All inbred mouse strains express a single beta 2m isotype. Mice heterozygous for beta 2m synthesize both forms, showing codominant expression of beta 2m alleles. In mice heterozygous for both H-2 and beta 3m, individual H-2 histocompatibility antigens associate with both beta 2m forms. Preliminary structural studies indicate differences in peptide composition between the two forms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigén R., Ziffer J. A., Berggård B., Cunningham B. A., Berggård I. Guinea pig beta2-microglobulin. Purification, properties, and partial structure. Biochemistry. 1978 Mar 21;17(6):947–955. doi: 10.1021/bi00599a001. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Hall P. W., Ricanati E. S., Vacca C. V. Characterization of human beta2-microglobulin by isoelectric focusing. Clin Chim Acta. 1977 May 16;77(1):37–42. doi: 10.1016/0009-8981(77)90399-0. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Kvist S., Klareskog L., Peterson P. A. Identification of H-2 and Ia-antigen analogues in several species by immunological crossreactions of xenoantisera. Scand J Immunol. 1978;7(6):447–452. doi: 10.1111/j.1365-3083.1978.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Michaelson J., Rothenberg E., Boyse E. A. Genetic polymorphism of murine beta 2-microglobulin detected biochemically. Immunogenetics. 1980 Jul;11(1):93–95. doi: 10.1007/BF01567773. [DOI] [PubMed] [Google Scholar]
- Rask L., Ostberg L., Lindblom B., Fernstedt Y., Peterson P. A. The subunit structure of transplantation antigens. Transplant Rev. 1974;21(0):85–105. doi: 10.1111/j.1600-065x.1974.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Snell G. D., Cherry M., McKenzie I. F., Bailey D. W. Ly-4, a new locus determining a lymphocyte cell-surface alloantigen in mice. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1108–1111. doi: 10.1073/pnas.70.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki N., Pressman D. The basic structure and the antigenic characteristics of HL-A antigens. Transplant Rev. 1974;21(0):15–34. doi: 10.1111/j.1600-065x.1974.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Trägärdh L., Rask L., Wiman K., Fohlman J., Peterson P. A. Amino acid sequence of an immunoglobulin-like HLA antigen heavy chain domain. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5839–5842. doi: 10.1073/pnas.76.11.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. Thymus and lymphohemopoietic cells: their role in T cell maturation in selection of T cells' H-2-restriction-specificity and in H-2 linked Ir gene control. Immunol Rev. 1978;42:224–270. doi: 10.1111/j.1600-065x.1978.tb00264.x. [DOI] [PubMed] [Google Scholar]