Abstract
Botanicals (herbal materials and extracts) are widely used in traditional medicines throughout the world. Many have an extensive history of safe use over several hundreds of years. There is now a growing consumer interest in food and cosmetic products, which contain botanicals. There are many publications describing the safety assessment approaches for botanicals, based on the history of safe use. However, they do not define what constitutes a history of safe use, a decision that is ultimately a subjective one. The multi-criteria decision analysis (MCDA), is a model that has been developed, which assesses the safety of botanical ingredients using a history of use approach. The model evaluates the similarity of the botanical ingredient of interest to its historic counterpart – the comparator, the evidence supporting the history of use, and any evidence of concern. The assessment made is whether a botanical ingredient is as safe as its comparator botanical, which has a history of use. In order to establish compositional similarity between the botanical ingredient and its comparator, an analytical ‘similarity scoring’ approach has been developed. Applicability of the model is discussed with an example, Brahmi ( Bacopa monnieri).
This evolution of the risk assessment of botanicals gives an objective, transparent, and transferable safety assessment approach.
Keywords: Botanicals, Brahmi, history of safe use, multi multi-criteria decision analysis, safety assessment, similarity score
INTRODUCTION
Herbal materials and extracts (botanicals) form a major component in indigenous peoples’ traditional medicine and are a fundamental element in Ayurvedic, naturopathic, traditional oriental, and Native American Indian medicine. In Africa and Asia, 80% of the population use traditional medicine routinely.[1] In India over 70% of the population relies on some form of traditional medicine, mainly Ayurveda, Unani, and Siddha.[2] These materials, which are either a single herb or a synergistic mix, are complex and contain hundreds of different chemical constituents that may be responsible for any therapeutic effects observed.[3]
Over the last decade there has been a significant increase in the number of foods and cosmetic products that contain botanicals, many of which are marketed with a health benefit claim, but which are not intended to be marketed as medicines. Previously reported safety assessment approaches for botanicals in foods and food supplements have used a decision flow diagram approach, to capture the history of safe use with an appropriate comparator.[4–6] This approach has also been described as presumed safety.[7,8] The output of such an approach does not define what constitutes sufficient evidence to demonstrate a history of safe use. Ultimately, a judgment is required by the risk assessor. Additionally the absence of a clearly recorded output for each of the history of use criteria may result in inconsistent or irreproducible assessments.
This article describes a model of expert toxicological judgment that has been developed to enable a history of safe use assessment of botanicals using multi-criteria decision analysis (MCDA). The model utilizes a comparative approach with the botanical material of interest and an appropriate comparator botanical material, for which there is a history of safe use. It evaluates the history of use together with an evidence for toxicological concern of the botanical of interest and the comparator botanical. The compositional similarity of the botanical of interest with the comparator is also evaluated. An analytical ‘similarity scoring’ approach has been developed, which uses quantitative and qualitative chemical analysis of the botanical and its comparator. Applicability of the model and the data required for the safety assessment to be made is discussed with a worked example Bacopa monnieri (Brahmi).
The types of ingredients that this assessment approach has been designed for are:
Botanicals and botanical preparations, for example, traditional Chinese medicines, Ayurvedics, and the like — these are usually complex mixtures.
Extracts of botanicals with enhanced levels of active components.
Single components derived by extraction from botanicals or synthetic versions of these components.
The model compares the botanical to be safety assessed with a comparator that people have historically already been exposed to. However, this approach cannot be used if the proposed substance is intended to be used at a substantially higher level than the level of the comparator to which people have historically been exposed (as there is no history of exposure at that level) or if the proposed substance is a drug (because it is likely to have significant pharmacological activity).
The model can be used to assess the systemic safety of botanicals for both food and cosmetic products if the history of use of the comparator relates to systemic exposure. Considerations of local toxicity (e.g., skin and eye irritation, sensitization, etc.) are not addressed and require a separate risk assessment approach, outside of the model.
For botanicals where there is no history of safe use for a relevant comparator or where the evidence of previous human exposure is inadequate, the model cannot be used, and the safety of the material needs to be assessed using toxicological data alone. The level of toxicological data required depends upon the botanical ingredient and the type and level of exposure to the ingredient.
Assessment methods
In the development of this particular history-of-safe-use model, the collective knowledge of a group of toxicological and chemical analysis experts within the Safety and Environmental Assurance Center in Unilever was captured, both through selection of the decision criteria and the weighting system, which together embody the value judgments of the group. These experts have many years of experience of making risk assessment decisions by weighing evidence for historical use of botanicals against any known or potential safety concerns.
The primary driver for developing a formal model was to capture this knowledge and experience within a safety assessment approach for botanicals, to enable a more standardized approach to be utilized and greater consistency in the safety of decision-making on botanical materials. However, the model does not replace the need for expert toxicological knowledge and judgment that is required when reviewing the data that is put in as well as the output of the model.
The advantages of the model are that new substances can be evaluated quickly, using the expert consensus on the key criteria for establishing the history of safe use embodied within the model, and that these evaluations are justifiable and consistent over time. The approach also negates in many cases the need for new data generation, for example from animal studies.
The multi-criteria decision analysis method
The Multi-Criteria Decision Analysis (MCDA) is a scientifically sound framework for making choices where there is no single best solution and where, due to the large number and complexity of factors to consider, it is difficult for decision makers to use all the available information effectively. MCDA enables users to synthesize a wide variety of information, both semi-quantitative and qualitative, to overcome the limitations of unstructured individual or group decision-making. A detailed analysis of the theoretical underpinning of MCDA methods is available in many publications, one of which is presented in French.[9]
MCDA models are generally based on the decision maker's relative preferences for each option (expressed as a relative score for each option), repeated for each criterion identified as being relevant to the decision. Each criterion has a potentially different weight on the decision outcome. Multiplying each score by its criterion weight and aggregating the resulting outcomes gives the net score for each option. When MCDA is used to identify the best choice, generally the option that has the most net ‘pro’ compared to net ‘con’ aggregate values, is selected.[10]
The Multi-Criteria Decision Analysis is well-established and widely used in a variety of decision-making contexts, such as, structuring decisions for managing ecotoxicological risks.[11,12]
Application of MCDA to Botanicals Toxicology assessment
Generally MCDA models describe a decision-making situation at a fixed point in time when all options are known (so that all options can be compared for relative preferences). However, the purpose of the history-of-safe-use model is not to choose the ‘best’ substance at a particular point in time, but to create a model of the ‘world of botanicals,’ which can be used as a reference when new substances are considered. The model was created by encoding ‘preferences’ as fixed, discrete values for the entire range of potential responses to each criterion, and by permanently retaining the weights for each criterion. These parameters were derived through elicitation of expert judgment by considering both theoretical and known benchmark substances.
Elicitation of criteria utilized within the model
Elicitation of criteria was through plenary idea generation with the expert group. The candidate criteria were first arranged using influence diagrams to help clarify the most important elements in the assessment. The resulting criteria are similar to those identified in the previous publications. In the approach described in this article, they were logically arranged into clusters of either ‘history of use’ or ‘evidence of concern’ branches, by constructing a hierarchical ‘value tree’ [Figure 1].
Figure 1.
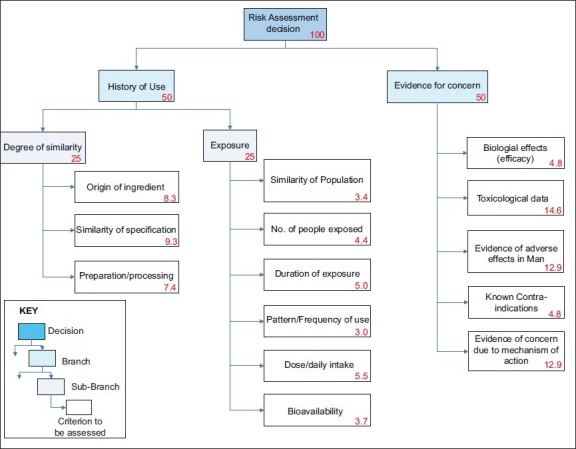
Value tree for the Botanicals Toxicology model, with the relative weight of each criterion, and the cumulative weights for each branch and sub-branch
The first major branch of the value tree compares what is known about the botanical ingredient of interest and its comparator, focusing on the degree of similarity between the two materials and also what is known about the historical exposure to the comparator.
The second major branch of the value tree focuses on any adverse data that may exist on the historical comparator.
All criteria are assessed semi-qualitatively, based on the available evidence, except for the similarity of specification between the botanical of interest and its comparator. The semi-quantitative method employed to assess this is detailed in section 2.4.
Assessment of degree of similarity of the material and comparator
The need for effective sample characterization is critical to assure the safety of any herbal preparation, which is extremely challenging due to its complexity. An important contributor to the evidence for a history of safe use is demonstration of the chemical similarity that the botanical of interest has with the comparator (botanical that has a history of safe use). The multi-component nature of the majority of plant or natural product extracts, in comparison to a chemically synthetic drug requires a different strategy to the conventional analysis conducted on a single or a few marker compounds. To isolate and identify every component from each herb, which may contain hundreds of natural constituents in the traditional reductive manner, would require tremendous time and resource, with no guarantee of success.
To demonstrate the link between a traditional preparation with an established history of safe use and a potential production scale material within this model, the chemical fingerprint analysis has been employed. Fingerprint analysis offers a more logical approach for providing a comparison between materials proposed for use. Although definitions of an analytical fingerprint vary, for the purposes of this study it is defined as a ‘unique visual pattern representing the presence of known and / or unknown characteristic chemical components’. This approach has been used and to some degree accepted as a suitable means for the quality control of natural materials.[13–15]
In order to produce an effective information-rich fingerprint, the analytical method should include a highly sensitive universal detection system, which can detect or give a response to as many components in the material as possible. The system should be robust and reproducible to ensure that experimental error is minimized. Therefore, the most commonly used technique is high performance liquid chromatography (HPLC), although spectroscopic methods such as Fourier transform infra red (FT-IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy provide additional fingerprint possibilities. Within the example presented here, HPLC and attenuated total reflectance (ATR) FT-IR have been used.
Despite plant and natural materials being extremely complex, with a vast number of differing components, it is usually possible from visual inspection, to identify whether a component signal is added or missing and if relative intensities can be assessed. However, in order to confirm and document whether a material is similar, objective criteria are required. Multivariate analysis can assist with this,[16] although in the majority of cases, when trying to establish a link with a proposed material and a historical sample there are only two variables to consider, which are the proposed material and the comparator
Similarity scoring has been devised and is suggested as a simple method to compare analytical data from two samples and provide a number that can be used in conjunction with the raw data to define similarity. The score is essentially a distance measure that is traceable, objective, and obtained in a transparent, easy-to-understand manner, which is appealing to the non-expert. It accounts for qualitative and quantitative differences when using concentration-related signal intensities. For example, if a chromatographic peak is present in sample 1, but absent in sample 2, then the intensity of the peak in sample 2 is assigned as zero. Similarity scoring has been applied to compare the two samples. One will be the comparator material (sample 1), an extract of a botanical that has been prepared using traditional methods and which has a recorded history of safe use, and the other will be the material to be safety assessed (sample 2).
Similarity score:
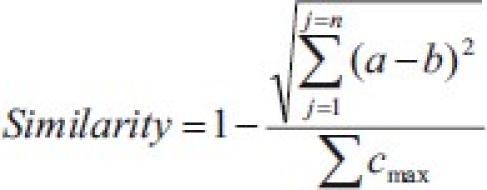
Where:
a is the Y value of variable j relating to Sample 1
b is the Y value of variable j relating to Sample 2
n is the number of variables considered in the comparison of the samples
∑cmax is the sum of all Y values for Sample 1
If two samples are identical, similarity score = 1
If two samples are entirely dissimilar, similarity score = 0
The advantage of this method is that it can use any number of data points from chromatographic or spectroscopic data and even individual chemical tests (i.e., moisture, pH, etc.). The more data that is put in, the more effective the measure is in providing a holistic view of the samples compared. It is recommended that the data is normalized prior to applying the calculation.
To produce an effective similarity score it is critical that the data are matched between samples for chromatographic retention time and concentration, although it is easier to correct the latter. It is noted that the method does not account for how much of the sample is being represented by the analytical measurement, and hence, does not negate the need for expert evaluation of data.
It is important that this similarity score approach should only be used as a ‘top line’ indicator of similarity and should always be used in conjunction with raw data and a visual inspection of the chromatogram or spectrum. Within this model, a sliding scale of statements has been used, to assign a similarity descriptor, based on the similarity index. When using chromatographic peak area data, similarity scores of 0 to 0.4 would be assigned as ‘different’, 0.4 to 0.75 ‘similar,’ and 0.75 to 1.0 ‘essentially the same’. For FT-IR spectroscopy data it is proposed that similarity scores of ≥ 0.95 are assigned as ‘essentially the same’. These ranges are based on the experience obtained by comparing many materials in this manner and are subject to constant review. The use of such descriptors reduces the emphasis on the number itself, and hence, the risk of misinterpretation by the non-expert is also reduced. However, a suitable level of objectivity for the comparison is retained.
The ATR-FT-IR data provides notably higher similarity scores, because it is acquired as a continuous sequential scan, whereas, HPLC has increased resolution, resulting in marked independent changes in response. ATR-FT-IR, although lower in sensitivity, provides a more holistic view of the material as, unlike HPLC, it does not suffer from variability in component sample dissolution, selective detector response or retention time shift. Identification of individual components is not possible by FT-IR although under the definition of an analytical fingerprint described previously this is not required.
The similarity score, used alongside expert evaluation of the analytical data, is a significant contributor to the overall history-of-use assessment. There also needs to be consideration and confirmation of an absence of batch-to-batch variability in specification, which can also be evaluated by fingerprint approaches. The importance of adequate specification, identification, and confirmation of batch-to-batch reproducibility has been previously identified.[17,18]
MCDA model construction
For each criterion identified as relevant to the history-of-safe-use model, fixed (discrete) values were set for each potential response, with a maximum (100) score. For instance for the criterion ‘Duration of exposure to comparator’, for the purposes of the history-of-safe-use model, ‘thousands of years’ of exposure is not significantly better than ‘centuries’ of exposure, so ‘centuries (or longer)’ is set as the maximum value (100) of the scale for this criterion. This represents the ‘best’ score on that criterion for a material. A minimum (0 point) on the scale describes a situation where the quality or quantity of the information about the substance is inadequate to form a judgment. This represents the ‘worst’ score for a material on this criterion. Intermediate points on the scale were set which, for the expert group, gave a meaningful discrimination.
Having fixed responses ensures that each new substance being evaluated can quickly be assigned a ‘value’ through examination of available evidence, without having to directly assess the preference for this new material compared to all the other materials / options in the model. The approach ensures consistency of judgment across individuals (who can choose only from a limited number of responses) and consistency over time, which is important, because the model is intended to help evaluate successive new substances.
Prototype models were refined because, for instance, they may not have given sufficient distinction between substances that were known to have favorable versus unfavorable safety decision outcomes, or the outcomes for substances assessed in the model did not correspond with the prior ‘real world’ judgment. Fine tuning of the criteria weightings and the preference values of responses within the criterion ensured correct discrimination. During construction additional criteria were identified that had originally been overlooked, and the understanding of each criterion and its responses was honed, to make it even more explicit and acceptable to the entire group.
The group was eventually able to come to a consensus view, which embodied the entire range of expert opinion. A corollary of the model construction was a greatly increased understanding of different experts’ values within the group. This confirmed that, as is common with MCDA, the process of constructing the model could be as important as (and in some cases more important than) the model outcome.
Validation of the model
The MCDA model is intended to structure and harmonize the qualitative decision-making of individual experts. Validation of the model consists of checking the model outcomes against the expert judgment of the group, which in this case consisted of thirteen individuals with long and wide-ranging experience in the safety risk assessment of botanical substances.
Validation was carried out using well-characterized botanicals as benchmarks, upon which safety decisions had previously been made outside of the model. The benchmark substances were carefully chosen to reflect the widest possible examples, both materials that had favorable safety decision outcomes as well as those that had not, and for which the reasons for the outcome (favorable or not) were also varied. A training set of eighteen botanical substances was used.
Validation was complete when the experts were confident that the model embodied their expert knowledge and values.
Output from the model
Once validated, the model was used to evaluate new substances, which had not been previously risk-assessed. Information on new substances was collected by completing a proforma for later input into the model. The evidence and expert reasoning behind choosing a particular response was also recorded.
The score for each new substance was obtained by multiplying the criterion response value by the weight of that criterion in the model, and aggregating the values. This was calculated for each major branch of the model, thus there was a summary score for the ‘History of use’ (pro) criteria and another summary score for all the ‘Evidence for Concern’ (con) criteria. The summary values for each substance were then mapped onto an x-y graph. An example of the type of output from the tool is shown in Figure 5.
Figure 5.
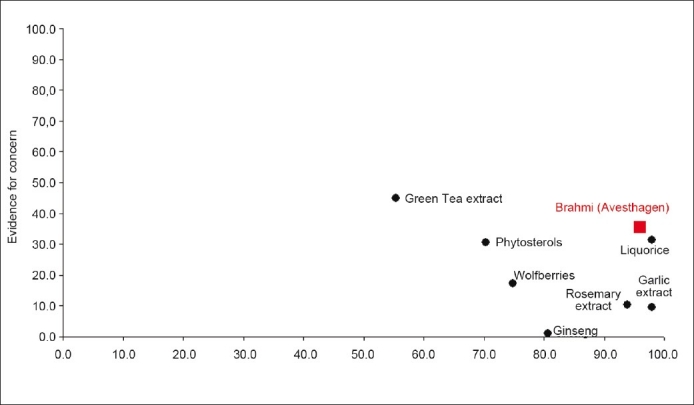
History of safe use map for benchmark botanicals and Brahmi
Those substances which have all the necessary evidence, and have both high scores on the ‘history of use’ criteria and low scores on the ‘Evidence for Concern’ criteria are more likely to be judged as safe for use in consumer products, without the need for further data generation.
When or if new information comes to light for a material, reassessments and refinements can be made on how the material is scored in the model. The model has been built to reflect a conservative judgment, so lack of information defaults to an unfavorable value. Once an initial evaluation has been made it is possible to identify where fuller or higher quality evidence against the criteria would enable a more refined evaluation to be made.
Application of the approach — Safety assessment of Bacopa monnieri (Brahmi)
An example botanical, Bacopa monnieri (L.) of the Scrophulariaceae family, or Brahmi as it is commonly referred to, has been assessed using the model, and the results are presented. Brahmi is an Indian Ayurvedic botanical, with a number of health benefits reported within Ayurveda. The key components present in Bacopa monnieri are saponin glycosides[19] referred to as Bacosides,[20] which have been linked to enhanced cognitive performance in clinical trials.[21,22] Its use as an ingredient in foods and / or cosmetic products can therefore be assessed by utilizing the body of historical evidence that exists around the consumption of the ayurvedic material. The history-of-use proforma results for Brahmi are presented in Table 1.
Table 1.
Information required for assessment and scoring of history of safe use for Brahmi
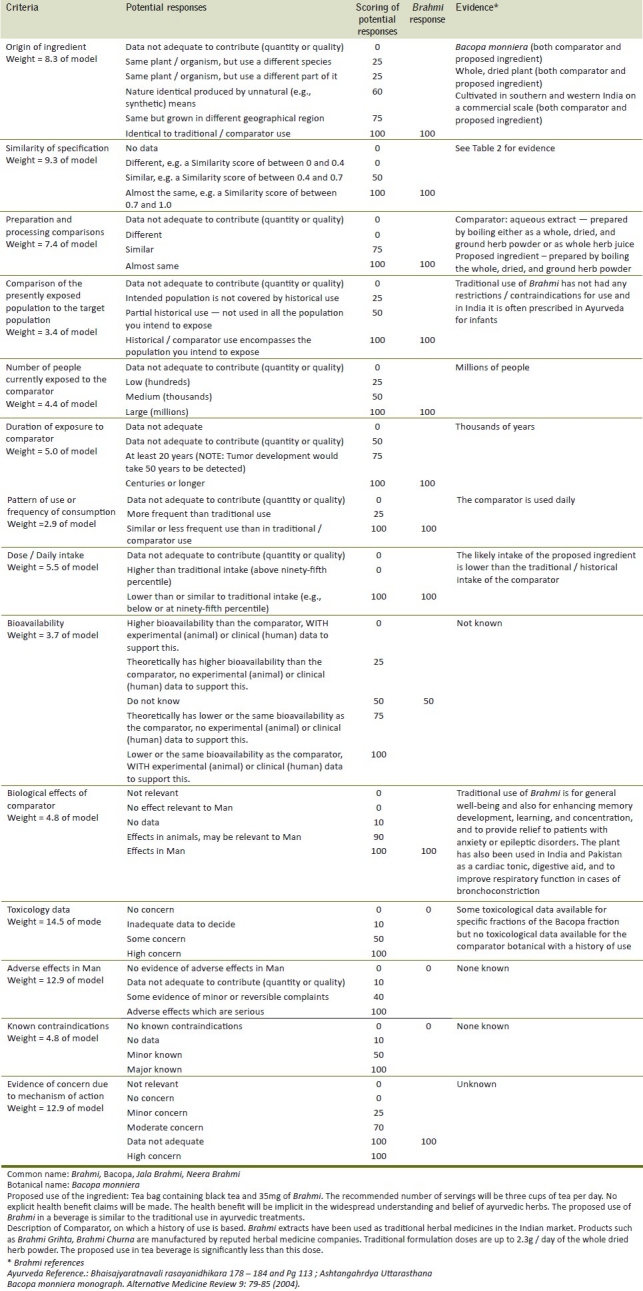
The similarity scores for the proposed Brahmi material in comparison to that prepared using traditional methods are shown in Table 2 with the classification for both HPLC and FT-IR being’ essentially the same’. Examination of the ATR-FT-IR spectra [Figure 2] which was reconstructed in Microsoft Excel using the exported wave number versus response dataset shows no significant differences between the materials.
Table 2.
Similarity scores for HPLC and FT-IR fingerprint data for the proposed Brahmi material in comparison to the comparator sample

Figure 2.
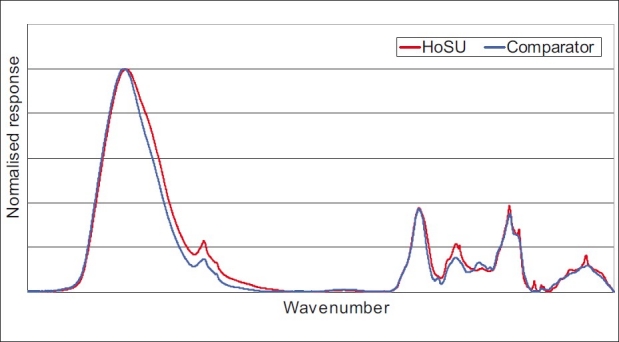
Reconstructed FT-IR spectra of Brahmi sample with a history of safe use (comparator) and the proposed Brahmi material. Spectra reconstructed in Microsoft Excel
The history-of-use scores, incorporating the similarity scores, are then used to populate the history-of-use assessment [Figure 3] and evidence for concern assessment [Figure 4]. These two assessments can then be used to plot an overall position within a history-of-safe-use map [Figure 5]. A number of ‘benchmark’ materials have also been assessed using the model (garlic extract, ginseng, green tea extract, liquorice, phytosterols, rosemary extract, and wolfberries). These materials have been previously risk-assessed outside of the model. The assessments for these materials both within and outside of the model can therefore be used to identify a history-of-safe-use outcome within the model. The position of Brahmi can then be compared with the previously assessed benchmark materials and a final risk assessment made.
Figure 3.
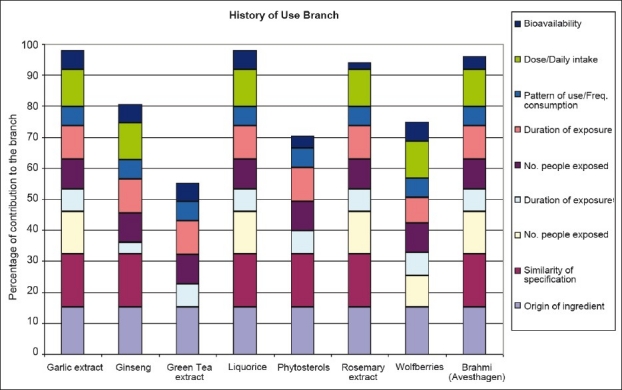
Assessments for criteria driving history of use
Figure 4.
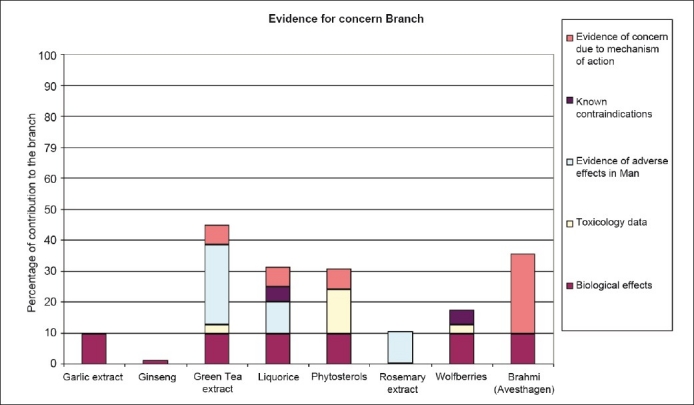
Assessments for criteria driving evidence for concern
As described previously and as shown in Figure 5, the area considered to be acceptable is defined by the benchmark materials that have been previously assessed and judged to be safe, using the history-of-use assessments, outside of the model. The overall history of safe use output for Brahmi falls within this area and it is therefore judged to be safe within the exposure considerations detailed in Table 1.
DISCUSSION
There is general recognition for the need to incorporate previous human experience and exposure to botanical materials in safety decision-making, thus helping to avoid any unnecessary animal testing on what can be complex botanical mixtures. As described, a safety assessment approach using the MCDA model of history-of-safe-use has been developed for botanicals and botanical components. The model provides a framework for consistent and transparent decision-making, based on the data supporting a history of safe use. The advantage of approaching risk assessment of botanicals through this MCDA model is that it captures a consensus of opinion among an expert group about those criteria that are most relevant, and their relative weight on the decision. This reasoning is easily transferred to new individuals, ensuring consistency of judgment between individual experts and over time. The reasoning and information associated with any particular decision is explicit and documented, and can be shared with non-toxicologists. Decision-making for each new substance is considerably faster using this structured approach.
In order to support the history-of-safe use approach a similarity scoring has been devised and applied as a simple and transparent method of comparing two analytical fingerprints in an objective manner. The level of similarity index to be adopted as an acceptable threshold to demonstrate substantial equivalence is proposed as a sliding scale. It is used so that chromatographic data 0 to 0.4 is assigned as ‘different’, 0.4 to 0.75 ‘similar,’ and 0.75 to 1.0 ‘essentially the same’ (≥ 0.95 for FT-IR data). This approach retains the objectivity of the comparison without placing excessive emphasis on the number itself, which can lead to misinterpretation by the non-expert. Similarity scoring can also be used to establish the reproducibility of batch specifications, particularly with regard to potential seasonal variation.
It is possible that this approach may be used to risk assess the initial limited exposure trials, which themselves will generate some ‘history of safe use’ data. The subsequent assessment of further studies may therefore change, depending on the data generated within the earlier trial. Therefore, in such instances, it is important to fully understand the testing and marketing strategy for any botanical that is going to be assessed using this approach. This will ensure that the most appropriate safety assessment strategy has been used from the beginning of any development activity.
CONCLUSION
This evolution of the MCDA model for history-of-safe-use risk assessment of botanicals gives an objective, transparent, and transferable system. Additionally its use within regulatory decision-making requires consideration. The European Novel Food Regulation is currently being revised. Part of that revision is the adoption of an accelerated authorization procedure for traditional foods from countries outside of Europe. Using this model to establish history-of-safe-use for a botanical would be desirable for regulators as it would provide a standardized and more rapid approach to safety assessment than currently exists. Equally, potential benefits for applicants would be, a faster time to market for botanical ingredients that have an acceptable history of safe use.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
Bobbie Bradford, Matt Dent, Julia Fedyk, Sabrina Gill, Jeanne-Marie Membré, Mary Spurgeon, Tonia Walters, Sarah Ward, Andy Scott, and Dave Edwards — the Unilever expert group. Prof. L Phillip, London School of Economics, for discussion of the MCDA theory and application.
Footnotes
Source of Support: Nil.
Conflict of Interest: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Traditional Medicine (Pamphlet) Geneva: WHO; 2003. WHO. [Google Scholar]
- 2.Mukherjee P K, Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J Ethnopharmacol. 2006;103:25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Xue T, Roy R. Studying Traditional Chinese Medicine. Science. 2003;300:740–1. doi: 10.1126/science.300.5620.740. [DOI] [PubMed] [Google Scholar]
- 4.Schilter B, Andersson C, Anton A, Constable A, Kleiner J, O’Brien J, et al. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem Toxicol. 2003;300:740–1. doi: 10.1016/s0278-6915(03)00221-7. [DOI] [PubMed] [Google Scholar]
- 5.Bast A, Chandler RF, Choy PC, Delmulle LM, Gruenwald J, Halkes SB, et al. Botanical health products, positioning and requirements for effective and safe use. Environ Toxicol Pharmacol. 2002;12:195–211. doi: 10.1016/s1382-6689(02)00035-2. [DOI] [PubMed] [Google Scholar]
- 6.Kroes R, Walker R. Safety issues of botanicals and botanical preparations in functional foods. Toxicology. 2004;198:213–20. doi: 10.1016/j.tox.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 7.EFSA Scientific Cooperation (ESCO) Working Group on Botanicals and Botanical Preparations; Advice on the EFSA guidance document for the safety assessment of botanicals and botanical preparations intended for use as food supplements, based on real case studies on request of EFSA. EFSA J. 2009;7:280. [Google Scholar]
- 8.Conto A. A new approach for the safety assessment of botanicals intended for use as ingredients in food supplements. Agro Food Industry Hi Tech. 2009;20:3. [Google Scholar]
- 9.French S, Maule J, Papamichail N. Cambridge, England: Cambridge University Press; 2009. Decision Behaviour, Analysis and Support. Chapter 7. [Google Scholar]
- 10.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci U S A. 2000;97:1433–7. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linkov I, Satterstrom FK, Kiker G, Seager TP, Bridges T, Gardner KH, et al. Multicriteria decision analysis: A comprehensive decision approach for management of contaminated sediments. Risk Analysis. 2006;26:61–78. doi: 10.1111/j.1539-6924.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 12.Semenzin E, Critto A, Rutgers M, Marcomini A. Integration of bioavailability, ecology and ecotoxicology by three lines of evidence into ecological risk indexes for contaminated soil assessment. Sci Total Environ. 2007;389:71–86. doi: 10.1016/j.scitotenv.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Kelly L. International Symposium on Quality of Traditional Chinese medicine with Chromatographic Fingerprint. Guangzhou. 2001:i4–1. [Google Scholar]
- 14.Chinese Traditional Patent Medicine. Vol. 22. China: State Drug Administration of China; 2002. Technical request about fingerprint technology of injection of traditional medicine; p. 671. [Google Scholar]
- 15.Geneva: WHO; 1991. WHO. World Health Organisation, Guidelines for the assessment of Herbal Medicines (Conference proceedings) Munich. [Google Scholar]
- 16.Liang YZ, Xie P, Chan K. Quality control of herbal medicines. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:53–70. doi: 10.1016/j.jchromb.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 17.Khan IA. Issues related to botanicals. Life Sci. 2006;78:2033–8. doi: 10.1016/j.lfs.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Walker R. Criteria for risk assessment of botanical food supplements. Toxicol Lett. 2004;149:187–95. doi: 10.1016/j.toxlet.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari P, Kumar N, Singh B, Kaul VK. Cucurbitacins from Bacopa monnieri. Phytochemistry. 2007;68:1248–54. doi: 10.1016/j.phytochem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Sivaramakrishna C, Rao CV, Trimurtulu G, Vanisree M, Subbaraju GV. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry. 2005;66:2719–28. doi: 10.1016/j.phytochem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese C, Gregory WL, Leo M, Kraemer D, Bone K, Oken B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;4:707–13. doi: 10.1089/acm.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stough C, Downey LA, Lloyd J, Silber B, Redman S, Hutchison C, et al. Examining the nootropic effects of a special extract of Bacopa monniera on human cognitive functioning: 90 Day double-blind placebo-controlled randomized trial. Phytotherapy Res. 2008;22:1629–34. doi: 10.1002/ptr.2537. [DOI] [PubMed] [Google Scholar]


