Abstract
Oncocytomas are rare tumours of the adrenal glands whose malignant potential is difficult to assess with certainty. We report a case of an adrenal oncocytoma and present a review of the literature particularly with regards to the radiological and histopathological features and their impact on the management.
Adrenal oncocytomas are usually identified incidentally on imaging and can achieve large sizes. They should be considered in the differential diagnosis of any large upper abdominal lesion including those apparently arising from the liver as in this case report. MRI scan appears to be the ideal imaging modality to characterise such lesions. There seems to be little benefit in biopsying these masses and surgery remains the most optimal management. It remains difficult to predict metastatic behaviour based on histological findings and so long term surveillance is advisable.
Keywords: Adrenal oncocytoma, Adrenocortical oncocytoma, Adrenal tumour, Adrenal incidentaloma
1. Introduction
Oncocytoma is a rare tumour of the adrenal gland (adrenocortical oncocytoma). Approximately 50 cases have been reported in world literature. The distinguishing histological feature of oncocytoma is the presence of epithelial cells with abundant mitochondria within a granular eosinophilic cytoplasm.
Oncocytomas have been reported in numerous other organs, notably the kidney, thyroid, pituitary, salivary, parathyroid and lacrimal glands as well as the skin, respiratory and gastrointestinal tracts.1 They are usually bulky, benign lesions but have an unpredictable malignant potential.1
We describe a case of a woman who was incidentally found to have a large mass in the abdomen which was subsequently found to be an adrenal oncocytoma. A review of the literature with regards to histological, radiological and clinical features of adrenal oncocytomas is presented.
2. Case report
A 31 year-old woman was referred to the hepato-biliary unit after a large right sided abdominal mass was incidentally detected on a CT scan while being investigated for haematuria. Apart from a mild back ache and some early satiety there were no other specific clinical complaints. She was normotensive, fit and well with the only past medical history of vaginal hysterectomy for endometriosis. She had previously been on the oral contraceptive pill but now took no regular medication. There was no family history of note. In particular, there was no history of weight loss or gain. On the basis of the CT scan appearance, the mass was initially thought to be arising from the liver. There was no history suggestive of liver disease and she was a non smoker with a minimal alcohol intake. All the blood tests including liver function tests were within the normal range. Tumours markers were not raised.
The CT scan (Fig. 1) revealed a 10 cm × 9 cm × 9 cm mass which seemed to be arising from the posterior aspect of the right hepatic lobe. The mass contained prominent blood vessels and areas of hypoattenuation consistent with necrosis. The remaining liver parenchyma was normal. A gadolinium-enhanced MRI (Fig. 2) scan was organised to further elucidate the nature of the lesion. This showed features indicative of primary liver tumours such as enhancement in the arterial phase followed by contrast washout in the portal venous phase. However the right adrenal gland could not be identified separately from the lesion. On both imaging modalities a differential diagnosis of primary hepatic tumour or giant adrenal neoplasm was made. Subsequently, plasma and 24-h urinary metanephrines and full adrenal functional profile was measured which was normal.
Fig. 1.
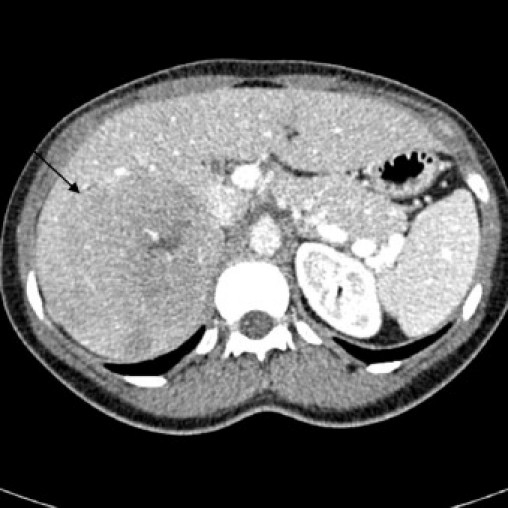
CT demonstrating large right sided lesion suggestive of a liver lesion (arrow).
Fig. 2.
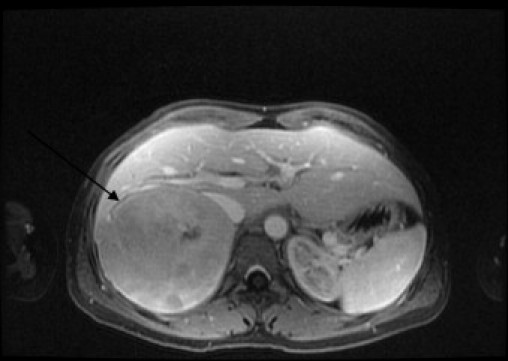
MRI demonstrating the lesion (arrow) to arise from the adrenal gland.
At laparotomy, the mass was found to be abutting but separate from the liver. Adrenal gland could not be separately identified. The lesion was completely excised. In the post-operative period there were no complications or any sign of adrenal insufficiency. The histology revealed adrenal oncocytoma. She remains well three months later. There is a plan for follow up with interval CT imaging given the uncertainty over the long term malignant potential of these neoplasms.1
3. Histology
Macroscopic examination showed a 10.5 cm × 9.5 cm × 9 cm rounded, smooth mass weighing 547 grams. The cut surface was brown and homogenous with a thick fibrous capsule. A compressed rim of adrenal gland was identified at one edge of the tumour (Fig. 3).
Fig. 3.
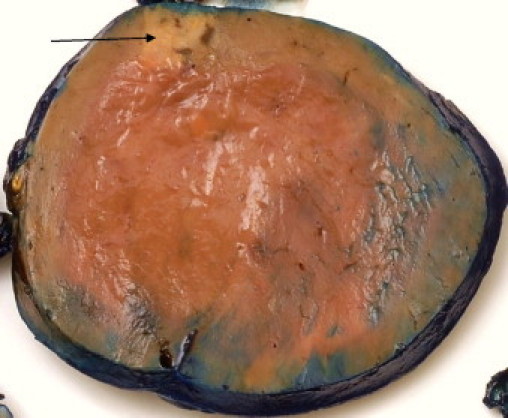
Macroscopic appearance of the oncocytoma with compressed residual adrenal (arrow).
Microscopic findings showed a well-circumscribed partly encapsulated tumour composed of nests and trabeculae of large polygonal cells with abundant eosinophilic granular cytoplasm (Fig. 4). There was random nuclear atypia with pleomorphic nuclei containing prominent nucleoli. No mitotic figures were identified. There was no evidence of capsular or vascular invasion. Residual adrenal gland was identified adjacent to the tumour and appeared unremarkable.
Fig. 4.
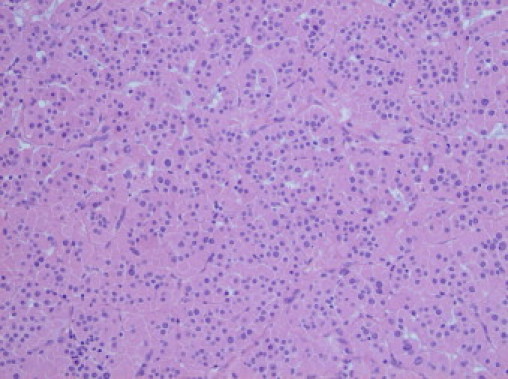
High power view (40×) showing polygonal adrenal cortical cells with abundant eosinophilic cytoplasm. (Haematoxylin and eosin, H&E stain).
The tumour cells were diffusely and strongly positive for Calretinin and Melan A (adrenal cortical markers). There was strong expression of synaptophysin and patchy but strong chromogranin A positivity. Renal and hepatic markers were negative. The tumour was negative for TTF1 and inhibin. The MIB index was approximately 5%. Electron microscopy confirmed the presence of abundant mitochondria in the cytoplasm of tumour cells. Although patchy chromogranin positivity was unusual neurosecretory granules were not identified. The morphological features in conjunction with immunohistochemistry and electron microscopy were typical for an adrenocortical oncocytoma.
4. Discussion
Oncocytomas can occur in various organs, especially the kidneys where oncocytomas represent around 3–7% of all renal neoplasms.2 They are much rarer in the endocrine, respiratory and gastrointestinal tissues. They are predominantly benign tumours which may reach considerable size if unresected. However, there is little data on the natural history of oncocytomas arising from the respective organs with scattered reports of metastatic oncocytomas in the literature.
Oncocytomas of the adrenal gland are particularly rare, although exact overall incidence is unknown. They have been reported in a wide age range between 15 and 77 years. They have been observed to occur more frequently in females (2.5:1) and to congregate on the left side (3.5:1).2 There are no identified environmental or genetic risk factors. They are usually identified incidentally and the majority are non-functioning. Fewer than 10 cases have been reported of hormone secreting adrenal oncocytomas resulting in virilisation and Cushing's syndrome.3
The imaging characteristics of adrenal oncocytomas have been previously discussed by Shah et al.4 Notably, on CT imaging they demonstrate heterogeneous contrast enhancement with areas of hypodensity and fibrous encapsulation. These features are consistent for both benign and malignant histologies. Furthermore, the CT features do not distinguish adrenal oncocytomas from more common adrenocortical carcinoma. These findings are thus non-specific and offer no prognostic information. The diagnostic option of fine needle aspiration has been investigated by Quayle et al.5 They found that histological assessment of incidental adrenal lesions by fine needle aspiration was often unsuccessful in providing a sample of diagnostic value, had a high complication rate (notwithstanding the risks of needle track tumour seeding) and ultimately did not alter the clinical management. Surgical excision followed by a full histopathological assessment is thus best practise for all suspicious lesions. In our case, the MRI scan was particularly useful in delineating the lesion as arising from the adrenal gland.
Several efforts have been made to characterise oncocytomas based on their histological characteristics. Histological scoring systems have been proposed by Weiss for adrenocortical tumours and more recently by Bisceglia et al. specifically with regards to oncocytomas to establish the histological grade.6,7 Bisceglia proposed major and minor criteria as part of a malignancy scoring system. The three major criteria are; high mitotic rate (>5 mitoses per 50 high power fields), atypical mitoses or venous invasion. The four minor criteria are; tumour size >10 cm or >200 g, tumour necrosis, capsular or sinusoidal invasion. Benign lesions would lack all of these features while the presence of any one or more major criteria categorises the lesion as a malignant oncocytoma. The presence of at least one minor criterion yields a diagnosis of “uncertain malignant potential/borderline malignant”. On the basis of this Bisceglia scoring system, 53% of 53 reported cases of oncocytoma can be classed as benign, 19% as malignant and the remaining 28% as borderline/uncertain.2 The tumour in our patient was classified with “borderline” malignant potential and there were no other adverse features such as vascular or capsular invasion.
However, there remains uncertainty over the validity of such scoring systems and their ultimate clinical and prognostic significance. This appears to be due to small numbers and the limited long term follow up data for excised oncocytic lesions and their natural history. This makes the post operative surveillance regime difficult to rationalise. Of the 22 reported benign oncocytomas with long term follow up there was no documented recurrence after resection (follow up ranging 6–96 months; mean 34 months).7,8 In 6 cases of oncocytomas with borderline/uncertain malignant potential the disease-free follow up extends 10–61 months (mean 25.5 months). With limited data, there does not appear to be any instances of recurrence of benign or borderline oncocytomas following excision. In 9 malignant cases with reported follow up 5 were reported to be disease-free with a median reported follow up of 6 months (range 5–32 months) while 4 had developed recurrence.7,9,10 Interestingly in a detailed study of four malignant oncocytomas Hoang et al. concluded that no cytological or mitotic criteria can accurately predict biological behaviour of the tumour.9 They conclude that only tumour size, capsule/vessel invasion, necrosis and metastasis are reliable indicators of malignant disease.
Surgical excision is curative in the vast majority of adrenal oncocytomas. This may be carried out as an open procedure as in our case or via laparoscopic route for smaller lesions.11 Although there is no established role for chemotherapy or radiotherapy Juliano et al. report the use of empirical chemotherapy and radiotherapy in a 15 year period of follow up of a single patient with metastatic adrenal oncocytoma.10 The initial reported histology in that patient after excision was that of a low malignant potential adrenocortical oncocytoma. Three years later metastases in bone and liver were treated with resection followed by six cycles of chemotherapy. After a further six years a local recurrence at the adrenal bed was treated with radiotherapy.
In conclusion, adrenal oncocytoma is an extremely rare tumour with uncertain malignant potential. We advocate the use of MRI scan for delineation of the tumour characteristics, particularly in case of large right sided lesions. Surgical resection remains the mainstay of the treatment. Although scoring systems exist in order to prognosticate the tumour behaviour, several uncertainties preclude accurate prediction of malignant behaviour. Hence, a long term surveillance policy after surgical resection is advocated.
Conflict of interest statement
No potential conflicts of interests are known for this case report.
Funding
There were no sources of funding sponsoring this study.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- 1.Peppa M., Karamitopoulou E., Nikolopoulos P., Peros G., Economopoulos T., Sotirios A. Large adrenal oncocytoma with uncertain malignant potential. A case report and review of the literature. Endocr Pract. 2010:1–14. doi: 10.4158/EP09309.CR. [DOI] [PubMed] [Google Scholar]
- 2.Dechet C.B., Bostwick D.G., Blute M.L., Bryant S.C., Zincke H. Renal oncocytoma: multifocality, bilateralism, metachronous tumor development and coexistent renal cell carcinoma. J Urol. 1999;162(1):40–42. doi: 10.1097/00005392-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Geramizadeh B., Norouzzadeh B., Bolandparvaz S., Sefidbakht S. Functioning adrenocortical oncocytoma: a case report and review of literature. Indian Pathol Microbiol. 2008;51:237–239. doi: 10.4103/0377-4929.41667. [DOI] [PubMed] [Google Scholar]
- 4.Shah R.K., Oto A., Ozkan O.S., Ernst R.D., Hernandez J.A., Chaudhary H.B. Adrenal Oncocytoma: US and CT findings. JBR-BTR. 2004;87:180–182. [PubMed] [Google Scholar]
- 5.Quayle F.J., Spitler J.A., Pierce R.A., Lairmore T.C., Moley J.F., Brunt L.M. Needle biopsy of incidentally discovered adrenal masses is rarely informative and potentially hazardous. Surgery. 2007;142:497–502. doi: 10.1016/j.surg.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Weiss L.M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumours. Am J Surg Pathol. 1984;8:163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Bisceglia N., Ludovico O., Di Mattia A., Ben-Dor D., Sandbank J., Pasquinelli G. Adrenocortical oncocytic tumours: report of 10 cases and review of the literature. Int J Surg Pathol. 2004;12:231–1231. doi: 10.1177/106689690401200304. [DOI] [PubMed] [Google Scholar]
- 8.Lin B., Bonsib S., Mierau G., Weiss L., Medeiros L.J. Oncocytic adrenocortical neoplasms. Am J Surg Pathol. 1998;22:603–614. doi: 10.1097/00000478-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Hoang M.P., Ayala A.G., Albores-Saavedra J. Oncocytic adrenocortical carcinoma: a morphologic. Immunohistochemical and ultrastructural study of four cases. Mod Pathol. 2002;15(9):973–978. doi: 10.1038/modpathol.3880638. [DOI] [PubMed] [Google Scholar]
- 10.Juliano J.J., Cody R.L., Suh J.H. Metastatic adrenocortical oncocytoma: a case report. Urol Oncol. 2008;26(2):198–201. doi: 10.1016/j.urolonc.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Eldahshan S., Zeccolini G., Guerini A., Breda G. Laparoscopic transperitoneal adrenalectomy for adrenocortical oncocytoma. Arch Ital Urol Androl. 2008;80(2):82–84. [PubMed] [Google Scholar]


