Abstract
Although mesenchymal stem cells (MSCs) are the natural source for bone regeneration, the exact mechanisms governing MSC crosstalk with collagen I have not yet been uncovered. Cell adhesion to collagen I is mostly mediated by three integrin receptors – α1β1, α2β1 and α11β1. Using human MSC (hMSC), we show that α11 subunit exhibited the highest basal expression levels but on osteogenic stimulation, both α2 and α11 integrins were significantly upregulated. To elucidate the possible roles of collagen-binding integrins, we applied short hairpin RNA (shRNA)-mediated knockdown in hMSC and found that α2 or α11 deficiency, but not α1, results in a tremendous reduction of hMSC numbers owing to mitochondrial leakage accompanied by Bcl-2-associated X protein upregulation. In order to clarify the signaling conveyed by the collagen-binding integrins in hMSC, we analyzed the activation of focal adhesion kinase, extracellular signal-regulated protein kinase and serine/threonine protein kinase B (PKB/Akt) kinases and detected significantly reduced Akt phosphorylation only in α2- and α11-shRNA hMSC. Finally, experiments with hMSC from osteoporotic patients revealed a significant downregulation of α2 integrin concomitant with an augmented mitochondrial permeability. In conclusion, our study describes for the first time that disturbance of α2β1- or α11β1-mediated interactions to collagen I results in the cell death of MSCs and urges for further investigations examining the impact of MSCs in bone conditions with abnormal collagen I.
Keywords: mesenchymal stem cells, integrin receptor, apoptosis, osteogenic differentiation
Mesenchymal stem cells (MSCs), derived from bone marrow, have been shown to differentiate into the osteoblast lineage in vitro as well as in vivo.1 As a result of this ability, these cells are considered to be the main cellular players in the processes of bone regeneration and healing. In order to survive, amplify or differentiate into cells with a specialized function, MSC have to establish appropriate contacts with the extracellular matrix (ECM) in the bone marrow, which is mainly composed of collagen type I, IV and fibronectin.2 One of the most important and ubiquitous receptor families mediating cell–ECM interactions is the integrin family. In vertebrates, there exist 18 α- and 8 β-integrin subunits that can assemble into 24 different heterodimers.3, 4 Generally, the amount of α-subunit determines the amount of receptor that will go to the cell surface as no free α- or β-integrin can be present on the plasmalemma.5 On the basis of the ligand-binding properties, the integrins can be grouped into subgroups as the collagen I-binding group consists of three receptors – α1β1, α2β1 and α11β1.5
Integrins do not only establish cell bonds with a range of ligands but also regulate cytoskeletal dynamics and initiate various signals that are essential for the regulation of cellular processes, such as cell adhesion, migration and differentiation.6, 7 Integrin signaling is initiated at the focal adhesion sites, which are membrane-associated platforms consisting of clustered, ECM-bound integrins as well as various enzymes (e.g., focal adhesion kinase, FAK), cytoskeletal and adaptor proteins (e.g., paxillin, p130cas) in the cytoplasm. Integrin adhesion triggers ‘outside-in' signaling, which frequently synergizes with growth factor-dependent cascades and activates downstream proteins such as extracellular signal-regulated protein kinases 1 and 2 (Erk1/2). Furthermore, integrin-mediated anchorage and signaling can also regulate cell survival processes and prevent cells entering anoikis, which is a special form of apoptosis triggered by inappropriate cell–ECM interactions.8, 9 For instance, the integrin-dependent adhesion of mammary and intestinal epithelial cells to the ECM is pivotal to avert anoikis through the activation of protein kinase B (PKB/Akt) survival pathway and the prevention of mitochondrial translocation of the pro-apoptotic protein Bcl-2-associated X protein (Bax).10, 11, 12
Although collagen I is one of the major proteins in the bone marrow, the precise roles of collagen-binding integrin receptors in human MSC (hMSC) behavior have not been investigated. In this study, we addressed the functions of α1β1, α2β1 and α11β1 integrins in hMSC by applying short hairpin RNA (shRNA)-mediated knockdown (KD) of the corresponding α-subunits and performing all functional analysis on native human collagen type I. Our major finding was that the deficiency of α2 or α11 integrin in hMSC leads to initiation of cell apoptosis via Akt pathway, while the deficiency of α1 integrin was entirely permissible for hMSC survival on collagen I. Furthermore, α2- and α11-shRNA hMSC completely failed to osteogenically differentiate because of the profound loss of cells throughout in vitro osteogenic stimulation (OS). Finally, a pilot investigation with hMSC derived from osteoporotic patients showed a significant downregulation of α2 integrin in these cells. Taken together, our study reports for the first time that two of the collagen I-binding integrins, α2β1 and α11β1, transmit essential and non-redundant signaling for hMSCs inhabiting collagen I-rich cell niches and that disturbance of the collagen I–integrin interactions results in stem cell death. Hence, we believe that our findings can open a novel way to interpret, understand and further investigate bone conditions in which the cell access to normal collagen I is affected.
Results
hMSC adhesion and proliferation are enhanced on collagen I and two of the collagen I-binding integrins are significantly upregulated in osteogenically differentiated hMSC
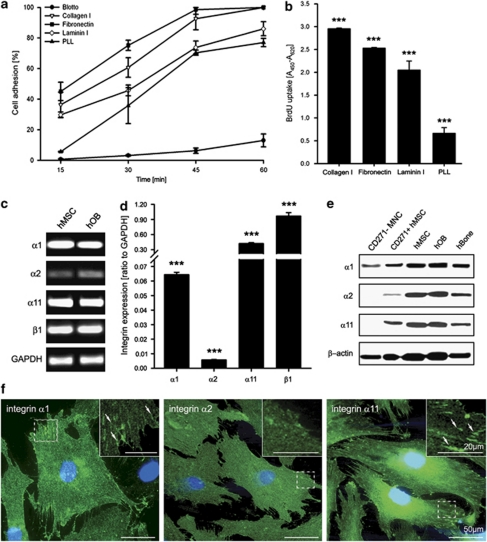
First, we investigated the effect of collagen type I on hMSC adhesion and proliferation. For hMSC adhesion (Figure 1a) and proliferation analysis (Figure 1b), cells were cultivated on collagen I, fibronectin, laminin I, poly--lysine (PLL) or 5% milk/PBS (blotto). After 45 min, hMSC attachment to collagen I and fibronectin entered an adhesion plateau (92.6±7.2% and 98.1±2.9%, respectively) whereas the cell adhesion to laminin I and PLL remained lower at all-time points (Figure 1a). Similar to the adhesion data, hMSC proliferation was significantly higher on collagen I and fibronectin demonstrated by bromodeoxyuridine (BrdU) incorporation assay (Figure 1b).
Figure 1.
hMSC adhesion and proliferation on ECM proteins, and integrin expression profile. (a) Quantitative adhesion assay on collagen I, fibronectin, laminin I, PLL (poly--lysine) and blotto (5% milk/PBS). The highest hMSC adhesion was toward fibronectin and collagen I. The data represent two independent experiments. (b) Proliferation analysis. Cells were cultured for 24 h in the presence of 10 μM BrdU. The highest BrdU uptake was detected when hMSC were grown on collagen I. Three independent experiments, each consisting of triplicates, were performed (n=9; ***P<0.001; one-way ANOVA). (c) Semi-quantitative PCR analysis of the basal expression of α1, α2, α11 and β1 integrin in hMSC and hOB. Shown is a representative experiment of three independent repeats. (d) Quantitative PCR analysis of collagen I-binding integrin expression revealed that α11 is the most strongly expressed integrin subunit in hMSC cultivated on collagen I. Data consist of three independent quantitative measurements with three donors (n=9; ***P<0.001; one-way ANOVA). (e) Representative western blots for integrin α1, α2 and α11 in freshly isolated CD271-MNC and CD271+hMSC, cultivated hMSC and hOB, and hBone protein extracts confirmed integrin protein expression in vivo and in vitro. (f) Immunoflourescent stainings of α1, α2 and α11 integrins of hMSC cultured on collagen I. Integrin α11 showed the most pronounced focal adhesions (indicated by arrows). The staining was reproduced twice, bar 50 μm and in the inset 20 μm
Next, we investigated the basal expression of α1, α2, α11 and β1 subunits in hMSC and human osteoblasts (hOBs) by semi-quantitative PCR (Figure 1c). Both cell types were found to express all integrins. Quantitative PCR performed in hMSC revealed that, among the α-subunits, α11 had the strongest basal expression on collagen I and the α1 mRNA levels were higher than α2 (Figure 1d). In addition, we performed western blot analyses of hMSC, hOB and native bone tissue (Figure 1e). The signal for α1, α2 and α11 was strong in cultured hMSC and hOB, while the freshly isolated low-affinity nerve growth factor receptor (CD271)-positive, non-cultured hMSC and human bone tissue revealed a weaker signal. However, we demonstrated for the first time the protein expression of the three collagen I-binding integrins in native hMSC, which were directly isolated and lysed from the bone marrow. Finally, we performed immunostaining for integrins α1, α2 and α11 in hMSC cultured on collagen I (Figure 1f). The cells were positive for all subunits; however, the most robust focal adhesions were observed in the case of α11-specific staining.
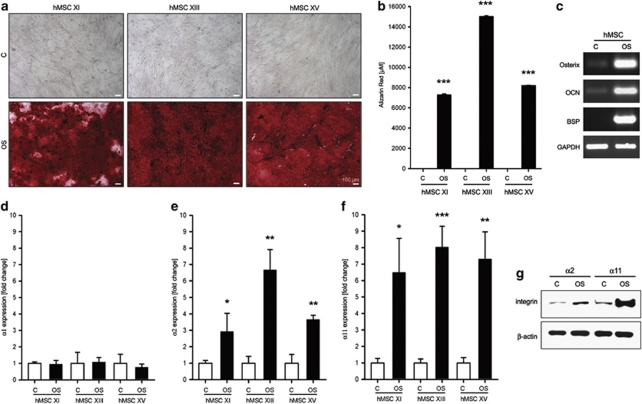
In order to investigate if the collagen-binding integrin levels are altered during hMSC commitment into OB linage, hMSC were osteogenically stimulated. Figures 2a and b demonstrate the osteogenic differentiation, judged by Alizarin Red (AR) cytochemistry, and quantification, of three hMSC donors at day 21. The osteogenic differentiation was further validated by semi-quantitative PCR for osteoblast-specific genes namely, Osterix, osteocalcin and bone sialoprotein (Figure 2c). Quantitative RT-PCR analyses of α1, α2 and α11 integrin expression revealed no changes for α1 (Figure 2d), while α2 and α11 were significantly upregulated on hMSC OS (Figures 2e and f). The increase in α2 and α11 expression on protein level was confirmed by western blotting (Figure 2g).
Figure 2.
Expression changes in collagen I-binding integrins on hMSC commitment into osteoblasts. (a) hMSC were osteogenically stimulated for 21 days and the deposited calcified matrix was visualized by AR staining. (b) AR quantification. Experiments were performed with three donors (n=3; ***P<0.001; unpaired t-test for each donor). (c) RT-PCR analysis of Osterix, osteocalcin (OCN) and bone sialoprotein (BSP) demonstrated the upregulation of bone-specific genes. Shown is a representative experiment of three independent repeats. Quantitative RT-PCR for integrin α1 (d), α2 (e) and α11 (f) revealed that α2 and α11 integrins significantly upregulate on OS. Data shown are for all donors as three independent quantitative RT-PCR measurements were performed per donor, (n=9; *P<0.05; **P<0.01; ***P<0.001; unpaired t-test for each donor). (g) Western blot analysis for α2 and α11 protein expression confirmed the previous findings. A representative experiment of two independent western blots is shown
Taken together, these findings demonstrate that first, collagen I promotes hMSC adhesion and proliferation; second, among the three collagen-binding integrins, α11 has the most pronounced basal expression; and third, when hMSC differentiate toward OB, α2 and α11 integrins are significantly upregulated, thus suggesting an important role for hMSC functions on collagen I.
Stable and efficient gene KD of α1, α2 and α11 integrins in MSC from human bone marrow
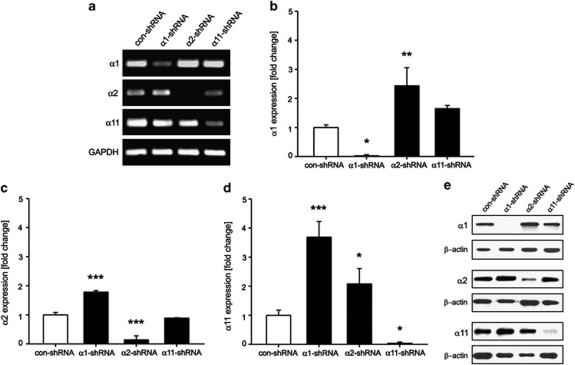
To examine the functional role of collagen-binding integrins, we applied small interference RNA technology. hMSC were stably transduced with control, α1, α2 or α11 shRNAs and cells, which had no pro-virus integration were eliminated by antibiotic selection. RT-PCR and western blot analyses were used to estimate the degree of integrin KD (Figure 3). The overall efficiency of integrin KD was >85% in all independent infections of each hMSC donor. Using the quantitative RT-PCR data, we analyzed if the downregulation of a particular integrin subunit leads to expression changes in the other two subunits (Figures 3b–d). We found that following integrin α1 KD, the expression levels of α2 and α11 increased with 1.7±0.1 and 3.7±0.5-fold, respectively. Similar changes occurred when integrin α2 was diminished (α1 with 2.4±0.6 and α11 with 2.1±0.5-fold increase). In contrast, the KD of integrin α11 led only to 1.7±0.1-fold increase in α1 mRNA whereas the level of α2 remained unchanged. These results were also apparent on protein levels (Figure 3e).
Figure 3.
Gene silencing of α1, α2 and α11 integrins in hMSC. hMSC were transduced with α1-, α2- and α11-specific shRNA sequences as well as with con-shRNA. (a) Semi-quantitative PCR demonstrated a downregulation of the three targeted integrins. Data are representative of three independent experiments. Quantitative RT-PCR analysis for α1 (b), α2 (c) and α11 (d) integrins in shRNA-transduced hMSC. The analysis showed a significant and reproducible integrin silencing in hMSC. Additionally, a compensatory upregulation was evident between the three α-subunits. Data consist of quantitative RT-PCR measurements from three independent infections (n=3; *P<0.05; **P<0.01; ***P<0.001; ANOVA tested versus con-shRNA). (e) Confirmation of the integrin α1, α2 and α11 KD on protein level. Shown is a representative experiment from two independent western blots of all donors
In conclusion, a very efficient and reproducible KD of α1, α2 and α11 integrins was successfully established in hMSC. In addition, a compensatory upregulation among some of the integrin subunits was observed.
hMSC deficient for α2 or α11 integrins exhibit a clear defect in adhesion, spreading and migration
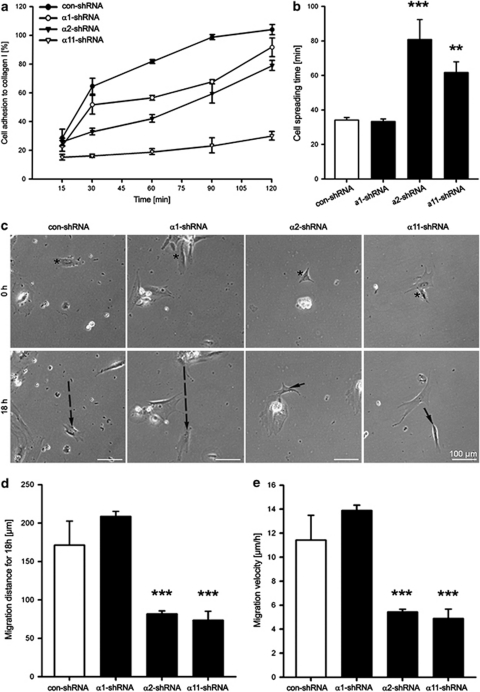
Subsequent to the establishment of integrin KD in hMSC, we investigated for changes in their cell attachment (Figure 4a), spreading (Supplementary Figures 2 and 4b) and migration on collagen I (Figures 4c–e). After 120 min, all control-shRNA (con-shRNA) and 91.6±6.5% of α1-shRNA hMSC were attached versus only 79.1±2.5% of α2-shRNA hMSC. The lowest cell adhesion was detected in α11-shRNA hMSC as these cells reached a maximal adhesion of just 30.1±3%. Con- and α1-shRNA cells required approximately 30 min to fully spread while α2- and α11-shRNA hMSC exhibited a significantly delayed spreading. More precisely, cells with α2 and α11 KD needed 80.8±11.5 min and 61.7±6.3 min to spread, respectively. Similar to the adhesion and spreading data, the α2- and α11-shRNA hMSC had also a migratory deficit. The average path con-shRNA cells took was 171.3±31.3 μm with mean velocity of 11.4±2.1 μm/h (Figure 4c). Similarly, α1-shRNA hMSC migrated to approximately 200 μm distances with 13.9±0.4 μm/h of average speed. In comparison, the migration distance and velocity of α2- and α11-shRNA hMSC were significantly reduced to approximately 50% (Figures 4d and e). In conclusion, hMSC deficient for α2 and α11 integrin receptors exhibited a reduced adhesion, delayed spreading and lower migration velocity.
Figure 4.
Investigation of adhesion, spreading and migration of integrin-deficient hMSC on collagen I. (a) Quantitative adhesion assay demonstrated that α2- and α11-shRNA-transduced hMSC have reduced attachment to collagen I in comparison with α1- and con-shRNA hMSC. Data consist of two independent infections; experiments were performed in triplicate. (b) Time lapse analysis of cells spreading on collagen I showed significant delay of hMSC deficient for α2 or α11 subunits. (c) Shown is a panel of images representative of the migration distance taken by a single control or shRNA-transduced hMSC. Cell tracks are indicated with black arrows. Quantification of migration distance (d) and velocity (e) revealed significantly lower motility of α2- and α11-shRNA hMSC. Time-lapse analysis was performed after two independent infections. Data in b, d and e are representative of three independent movies as a total of 30 cells per type were evaluated (n=3; **P<0.01; ***P<0.001; ANOVA tested versus con-shRNA)
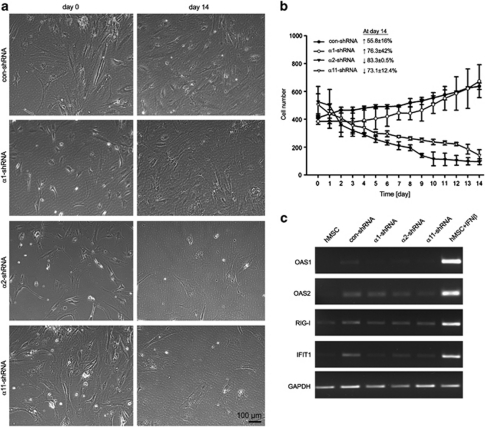
KD of α2 or α11 integrin results in a dramatic loss of hMSC numbers and impaired osteogenic differentiation.
Throughout hMSC cultivation on collagen I, we detected a persistent decrease in cell numbers within the population of α2- and α11-shRNA-transduced cells (Figures 5a and b). In contrast, the con- and α1-shRNA hMSC continuously increased. In order to estimate the rate of cell regression, cell quantification was performed and the percentage of cell gain or loss between days 0 and 14 was determined (Figure 5b). At day 14, con- and α1-shRNA hMSC increased with 55.8±16% and 76.3±42%, whereas α2- and α11-shRNA significantly reduced to 83.3±0.5% and 73.1±12.4%, respectively of the original seeding density.
Figure 5.
Cell regression of hMSC after integrin KD and investigation of IFN pathway-related genes. (a) Subsequent to the antibiotic selection, cells were pictured every 24 h for a period of 14 days. Shown are representative images at day 0 and 14. (b) hMSC deficient for α2 or α11 integrin decrease in number during cultivation in vitro. In contrast, α1-shRNA and con-shRNA hMSC were able to expand. In the text inset, the total cell gain or cell loss between day 0 and 14 is shown in percentage. The experiment was performed in duplicates with two donors as the total cell number in 1.1 cm2 area was automatically counted by ImageJ software. (c) RT-PCR was performed for OAS1, OAS2, retinoic acid-inducible gene I (RIG-I) and IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), genes involved in IFN-related cell death. The PCR results showed that none of the tested genes were upregulated after viral transduction of shRNA. Shown is a representative experiment of three independent infections. Positive control: hMSC treated with 2 × 103U/ml IFNβ for 72 h
A viral infection or a presence of double-stranded RNA within the cells can lead to an activation of interferon (IFN) signaling, which then can result in IFN-mediated cell apoptosis.13 In order to exclude that the observed reduction of α2- and α11-shRNA hMSC numbers is due to a triggered IFN pathway, we performed semi-quantitative PCR analyses of genes related to IFN pathway – OAS1, OAS2, retinoic acid-inducible gene I and IFN-induced protein with tetratricopeptide repeats 1. hMSC stimulated with IFNβ were used as a positive control. As shown in Figure 5c, none of the tested genes showed distinctive upregulation in the shRNA-transduced hMSC.
Finally, we induced osteogenic differentiation of hMSC and observed that con- and α1-shRNA cells were able to differentiate into osteoblasts indicated by positive AR staining, whereas α2- and α11-shRNA hMSC did not deposit mineralized matrix shown by the lack of AR staining. Instead, during the stimulation period, a further significant regression in the cell numbers of both cell types was observed (Supplementary Figure 3).
Hence, we concluded that viral treatment and shRNA transduction in hMSC had no stimulatory effect on IFN pathway and that only the gene KD of α2 and α11 integrins leads to a remarkable reduction of hMSC numbers during cultivation as well as OS.
hMSC deficient for α2 or α11 integrins exhibit a mitochondrial permeability, BAX upregulation and reduced activation of Akt
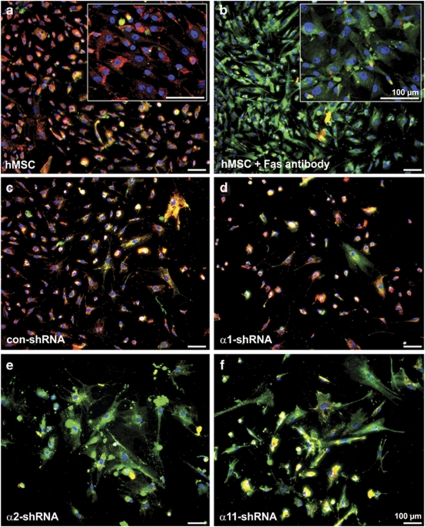
The observed decline in α2- or α11-shRNA KD hMSC numbers suggested a survival defect on collagen I; therefore apoptosis was evaluated first by 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) staining (Figure 6). In viable cells, JC-1 aggregates in the intact mitochondria and produces red fluorescent signal (Figure 6a). In apoptotic cells, which have a mitochondrial leakage, JC-1 resides in the cytoplasm in a monomeric form and stains the cell cytoplasm in green (Figure 6b). Our results showed that con- and α1-shRNA hMSC populations consisted of mostly non-apoptotic cells (Figures 6c and d). In contrast, α2- and α11-shRNA hMSC, similarly to the positive control (hMSC treated with CD95 death receptor (FAS) antibody), were predominantly stained in green indicating mitochondrial leakage (Figures 6e and f).
Figure 6.
Investigation of the mitochondrial integrity of integrin-deficient hMSC. Representative microphotographs of hMSC cultivated on collagen I and stained with JC-1. JC-1 dye aggregates and emits red color in cells with intact mitochondria such as non-treated hMSC (a), con- (c) and α1-shRNA hMSC (d) whereas apoptotic cells are labeled in green as in the positive control – hMSC treated with 1 μg/ml FAS antibody (b). hMSC with α2 (e) or α11 (f) KD are labeled in green indicating that their mitochondria are permeabilized. Cytochemistry was performed twice independently with two donors
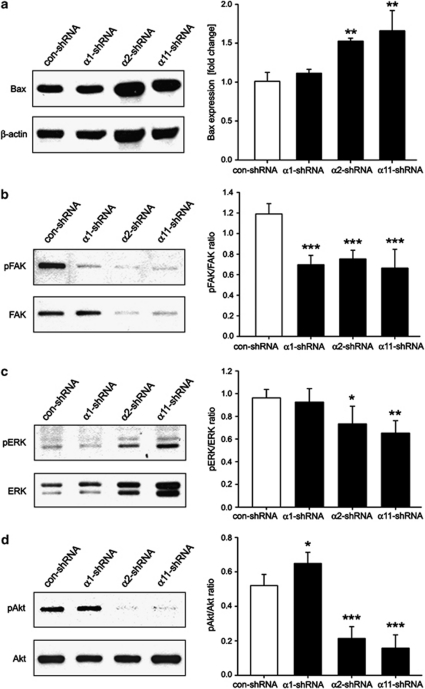
Next, we carried out a western blot analysis for the pro-apoptotic factor Bax, which is responsible for mitochondrial membrane permeabilization.14 A significantly elevated Bax expression was observed only in α2- and α11-shRNA hMSC (Figure 7a).
Figure 7.
Investigation of cell apoptosis. (a) Western blot analysis for Bax, a pro-apoptotic marker, revealed a significant elevation of this protein in α2- or α11-deficient hMSC (n=3; **P<0.01; ANOVA tested versus con-shRNA). Quantification of three independent western blot experiments is shown. (b–d) Representative western blots of phosphorylated and total FAK, ERK and Akt in control and integrin KD hMSC. pFAK was significantly reduced in all integrin-deficient hMSC whereas a slight reduction of pERK and almost a complete loss of pAkt was detected only in α2- or α11-shRNA hMSC. Quantification analyses were performed from two independent infections and western blotting (n=2, *P<0.05; **P<0.01; ***P<0.001; ANOVA tested versus con-shRNA)
Finally, we analyzed the activation of the integrin downstream targets FAK (Figure 7b), ERK (Figure 7c) and Akt (Figure 7d) using phospho-specific antibodies. The pFAK levels were significantly reduced by approximately 50% in all integrin-deficient hMSC, whereas a slight reduction of pERK and almost a complete loss of pAkt were detected only in α2- and α11-KD hMSC. Thus, we concluded that the hMSC shortfall observed after α2 or α11 KD is the consequence of cell apoptosis because of the lack of adhesion-dependent activation of the major survival effector – Akt.
A significant downregulation of α2 integrin revealed in hMSC derived from osteoporotic patients
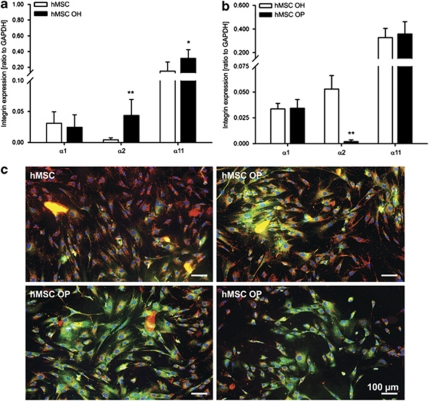
To investigate a hypothetic relationship between osteoporosis and integrin dysregulation, a pilot study with hMSC derived from older healthy (hMSC OH, n = 3) and older osteoporotic donors (hMSC OP, n = 6) (Supplementary Table 1) was performed. We analyzed the expression profile of the collagen-binding integrins in these cells and compared it with that of hMSC from the younger healthy donors. Hence, an intriguing trend was found: first, α2 and α11 integrins were upregulated during aging (Figure 8a) and second, no changes were detected in α1 expression in hMSC OP whereas α11 was slightly upregulated in hMSC OP (Figure 8b). Most strikingly, a significant, 17-fold downregulation of α2 was detected in hMSC OP (Figure 8b) as well as a notable number of cells exhibited a mitochondrial leakage (Figure 8c) in comparison with hMSC OH (Figure 8b).
Figure 8.
Pilot investigation of the mRNA levels of collagen I-binding integrins in hMSC derived from younger healthy (n=3), older healthy (hMSC OH, n=3) and older osteoporotic patients (hMSC OP, n=6). For donor information refer to Supplementary Table 1. (a) Quantitative RT-PCR showed an upregulation of α2 and α11 integrins during aging (young hMSC versus hMSC OH; n=9; *P<0.05; **P<0.01; unpaired t-test for each α-integrin subunit). (b) Comparison between hMSC OH and hMSC OP revealed that integrin α2 was significantly downregulated in hMSC OP (n=15; **P<0.01; unpaired t-test for each α-integrin subunit). (c) JC-1 staining demonstrated in hMSC OP a prominent number of cells with mitochondrial permeabilization (green-labeled cells) in contrast to control hMSC, which had mostly intact mitochondria (red-labeled cells). Photomicrographs represent three different hMSC OP donors
Discussion
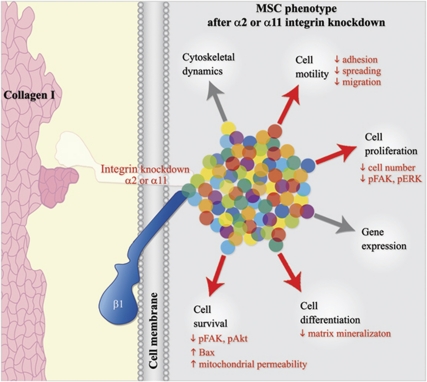
In this study, we investigated the role of the collagen I-binding integrins, α1β, α2β and α11β, in human, bone marrow-derived MSC. We applied a stable, shRNA-based integrin silencing and observed significant changes only in the behavior of hMSC deficient for α2 or α11 integrin subunits. The major cellular changes comprises (Figure 9): (i) reduced adhesion, spreading and motility on collagen I; (ii) increased apoptosis associated with mitochondrial leakage and Bax upregulation; and (iii) affected integrin-mediated signaling to Akt, FAK and ERK.
Figure 9.
Schematic model summarizing the cell phenotype of human MSC deficient for integrin α2 or α11. Integrins are heterodimeric receptors (white and blue forms) having central roles in establishment of cell to extracellular matrix interactions (e.g., collagen type I, pink shape) and in regulation of crucial cell processes (arrows) by engaging various integrin-, membrane-, actin- and signaling-associated proteins (multi-color dots). As the amount of α-subunit determines the amount of receptor present on the plasmalemma, we applied here a lentiviral delivery of α-subunit-specific shRNA in hMSC. Thereby, highly efficient and stable KD of integrin α1, α2 and α11 was achieved (represented by the translucent α-subunit). Our functional analyses showed that only the deficiency of α2 or α11 integrin leads to significant changes in hMSC behavior on collagen I (red arrows). Most importantly, hMSC maintenance was affected as these cells displayed features typical for cell apoptosis. Taken together, this study reports for the first time that disturbance of collagen I–integrin interactions results in the cell death of MSCs. The image is adapted from Docheva et al.7
Owing to the high rates of hMSC adhesion and proliferation on collagen I, we first examined the expression levels of the collagen I-binding integrins in hMSC cultivate on collagen I and found that among the three different collagen I-binding α-subunits, α11 integrin had the highest basal expression. Our study also reported for the first time the in vivo expression of the three α1, α2 and α11 integrins by analyzing freshly isolated, non-cultured hMSC. Furthermore, we clearly demonstrated that α2 and α11 integrins, but not α1, were significantly upregulated on OS of hMSC. Forster et al.15 have previously demonstrated by quantitative proteomic analysis that the amount of collagen I, as well as α2 and α11 integrins, increases during hMSC osteogenic differentiation. Hence, we suggest that the elevation of α2 and α11 might be a consequence of the changes in collagen I production in the differentiating cells.
To investigate the role of these three receptors in hMSC, we next performed a loss-of-function analysis. After the accomplishment of almost complete silencing of each integrin subunit, we observed that only hMSC deficient for α2 or α11 integrins exhibited adhesion, spreading and migration deficits on collagen I while α1-deficient cells behaved similarly to control hMSC. Changes in adhesion and migration efficacy after the loss of collagen I-binding integrins have been described only in mouse cells derived from integrin knockout models.16, 17, 18, 19 However, the phenotype of bone marrow-derived MSC has not been investigated so far. Using α2-knockout keratinocytes and dermal fibroblasts, it was shown that α2 integrin regulates the cell attachment and migration on collagen I, whereas adhesion to collagen IV and laminin I is permissive.16, 17 Popova et al.18, 19 reported a positive effect of integrin α11 on embryonic fibroblast adhesion and spreading on collagen I. In contrast, the investigators observed that this cell type was actually able to migrate faster on various collagens when α11 was missing.18 The reported discrepancy in the migratory behavior of integrin-deficient cells could be explained with the notion that collagen-binding integrins might exert different effects on cell adhesion and migration depending on the cell type or on the availability of other compensatory integrin subunits. In our study with human MSC, we observed that in the absence of α2 integrin, α1 and α11 subunits were significantly upregulated, while only a slight increase in α1 was detected subsequent to α11 KD. Hence, we suggest that the adhesion deficit of α2-deficient cells was not as severe as that of α11 because one or both remaining integrins could have a compensatory role.
Integrins have been implicated also in the control of cell differentiation and survival. Salasznyk et al.20, 21 demonstrated that FAK and ERK signaling regulate the osteogenic differentiation of hMSC on collagen I, however, in these studies the upstream pathway or an integrin involvement was not examined. Integrins are also known to trigger pro-survival signals when cells interact with ECM proteins, but when such contacts are disturbed, integrins can as well initiate cell death.22 It has been reported in human lung fibroblasts that the disruption of ECM–integrin β1 interactions leads to reduced cell survival because of negatively affected FAK and Akt signaling.23 Several studies have also coupled integrin signaling with Bcl-2/Bax-dependent apoptotic machinery in keratinocytes24 and calvaria-derived fibroblasts.25 It has been shown in rat MSC26 that transfection of integrin-linked kinase, a downstream effector of β1 integrin, results in reduction of the pro-apoptotic Bax levels and thus, assists the MSC survival in hypoxic conditions. However, our study is the first one to demonstrate that the loss of collagen I–integrin interactions triggers stem cell death; using hMSC, we showed that the downregulation of α2 or α11 integrin, but not α1, leads to induction of apoptosis. As no activation of the IFN pathway was detected, we concluded that the observed cell apoptosis is indeed specific to the KD of α2 or α11 integrin. These cell populations were unable to expand in vitro. Moreover, as a predominant fraction of adherent cells exhibited mitochondrial leakage and a significant elevation of Bax protein levels, we suggest that the cell apoptosis was initiated before cell detachment. We also detected a reduction in the phosphorylation of FAK and ERK in the three integrin KDs. However, the most pronounced reduction of pERK was found in the α2- or α11-deficient hMSC. Furthermore, both cell populations showed a dramatic reduction of pAkt levels. Finally, as α2-deficient cells underwent cell death, despite the upregulation of α1 and α11, we propose that each integrin triggers a specific, non-dismissive downstream signaling cascade. Further studies, however, are required to dissect the exact signaling pathways mastered by α2 and α11 integrins in hMSC and other cell types.
A recent study reported that α11 integrin deficiency in mice results in disorganized periodontal ligament, halted incisor eruption and tooth-dependent dwarfism.19 The investigators identified a number of apoptotic cells in the periodontal ligaments. This tissue is highly enriched with collagen I and α11β1 integrin is the only collagen receptor. Interestingly, when fed with soft food, the α11 knockouts only partially overcame their growth retardation. We propose that this tooth-independent growth defect might be linked to insufficient skeletal development because of MSC deficit. In contrast to α11, integrin α1 and α2 knockout mice show no gross developmental abnormalities.27, 28 Taken together, the relatively mild phenotypes of α1, α2 and α11 mutant mice suggest that, under normal physiological conditions, there is a functional redundancy between the individual collagen receptors. However, in certain tissues or cell types, or under disease-like conditions, these receptors might execute indispensable functions. Therefore, our study urges further investigation of the bone marrow niche of the single or double knockout mice as well as on their involvement in experimental models challenging bone development or healing.
Another possible field for further investigation includes bone conditions associated with collagen I abnormalities such as osteogenesis imperfecta and osteoporosis. A recent study has reported mutations causing osteogenesis imperfecta in type I collagen, which are located in the binding sites for integrins and proteoglycans.29 Another study found in patients with specific collagen I polymorphism that the collagen I α1 and α2 chains were made in an aberrant ratio and suggested that this is relevant for the onset of osteoporosis.30 Furthermore, it has been shown that the total amount of collagen I protein is decreased in hMSC derived from osteoporotic patients31 and that osteoblasts isolated from patients with osteoporotic or osteoarthritic condition have reduced attachment and spreading as well as decreased FAK activation.32 However, several other studies have found no dramatic changes in the hMSC proliferative and differentiation capacity from osteoporotic patients.33, 34 One possible explanation for this discrepancy is the variability in hMSC preparation and validation. On the basis of our results and the literature, we now speculate that in such conditions, one additional factor might be that the MSC population is being gradually exhausted in vivo because of loss of ECM interactions and subsequent cell death. Therefore, we performed a pilot analysis with hMSC derived from osteoporosis-suffering patients and found that, in comparison with hMSC from healthy old patients, the osteoporotic cells have significantly reduced mRNA levels of α2 integrin. Importantly, further experiments involving higher number of patients will be necessary to clarify what role integrin expression and signaling undertake in bone diseases. Such studies are currently ongoing.
In conclusion, our study contains the first cellular and molecular analysis demonstrating the role of collagen I-binding integrins in the survival of MSCs in collagen I-rich environment and calls for future investigations examining the impact of MSCs in bone conditions with abnormal collagen I.
Materials and Methods
Human cells and cell culture
The summary of hMSC donors used in this study is shown in Supplementary Table1. hMSC were purchased from Lonza (Basel, Switzerland) or were isolated from bone marrow by ficoll gradient centrifugation (the procedure was approved by the LMU ethical commission, grant 311–04). The hMSC characteristics were verified by the producer or by us according to Dominici et al.35 Briefly, hMSC were proven by FACS to be positive (>98%) for the MSC-related markers, 5′-nucleotidase, GPI-linked glycoprotein and endoglin, and negative for the hematopoietic-related markers, monocytes/macrophages receptor, hematopoietic progenitor marker and protein tyrosine phosphatase receptor type C and by two-lineage differentiation to be multipotent (data not shown). hMSC were cultured in MEM Alpha GlutaMAX medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (Sigma, Taufkirchen, Germany) and were grown on plastic or 10 μg/ml native human collagen I-coated surfaces (BD Biosciences, San Jose, CA, USA). hMSC at passages 5–9 were used in the experiments. hOBs were purchased from PromoCell (Heidelberg, Germany) and maintained in OB-specific growth medium (PromoCell). Cells were maintained at 60–80% confluence in T-75 culture flasks, at 37 °C/5% CO2. CD271-negative mononuclear cells (MNCs) and CD271-positive hMSC were isolated with a CD271 MicroBead kit (Miltenyi Biotec, Bergish Gladbach, Germany) from three human bone marrow MNC fractions (Cat. No. 2M-125C) purchased from Lonza and immediately after the cell separation the freshly isolated cells were lysed for protein analysis.
shRNA cloning, lentiviral production and infection of hMSC
shRNA oligonucleotides were designed with Invitrogen's BLOCK-iT RNAi Designer (Invitrogen) against the human integrin α1, α2 and α11 and β-galactosidase genes (Supplementary Table 2). Double-stranded oligonucleotides were annealed and ligated into the pENTR/U6 vector according to the manufacturer's instructions (Invitrogen). The shRNA constructs were transferred to the final pLenti4/BlockIT-DEST expression vector by recombination using LR Clonase (Invitrogen) and were validated by sequencing. The final plasmids were prepared with EndoFree Plasmid Maxi Kit (Qiagen, Hilden, Germany). For viral production and cell infection, Invitrogen's ViraPower lentiviral system was used. The pLenti4/BlockIT-U6-shRNA plasmid was transfected into 293FT cells and viral supernatant was collected after 48 h. hMSC were incubated with the virus-containing supernatant for 24 h in the presence of 8 μg/ml polybrene (Sigma) and were selected with 50 μg/ml Zeocin (Invitrogen) for 10 days. As the three different donor-derived cells represented equivalent hMSC populations (Supplementary Figure 1), we designated hMSC XI as the main donor. hMSC XI was infected independently three times and used in all experiments. The additional two donors, hMSC XIII and hMSC XV, were infected once and were used only in the major experiments to confirm that the observed phenotype is not donor-specific.
Semi-quantitative and real-time PCR
Total RNA was extracted with RNeasy Mini Kit (Qiagen). For cDNA synthesis, 1 μg total RNA and AMV First-Strand cDNA Synthesis Kit (Invitrogen) were used. RT-PCR was performed with Taq DNA Polymerase (Invitrogen) in MGResearch instrument (Bio-Rad, Munich, Germany). Primer pairs and PCR conditions are listed in Supplementary Table 3. For quantitative RT-PCR, LightCycler Fast Start DNA Master SYBR Green kit (Roche, Munich, Germany) and primer kits for α1, α2, α11 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Search-LC, Heidelberg, Germany) were used. The quantitative RT-PCR was performed in LightCycler1.5 instrument (Roche) equipped with LightCycler3.5.3 software. Crossing points for each sample were determined by the second derivative maximum method and relative quantification was performed using the comparative ΔΔCt method according to the manufacturer's protocol. The relative gene expression was calculated as a ratio to GAPDH. All PCR results have been reproduced minimum three times. For PCR analysis of IFN-related gene expression, a positive control was prepared by incubating hMSC with 2 × 103U/ml IFNβ (Cat. No. CYT-26766, Dianova, Germany) for 72 h at 37 °C as demonstrated in Croitoru-Lamoury et al.36
Western blot analysis
Adherent cells were directly lysed in 1x Laemmli buffer (200 m Tris-HCl pH 6.8, 40% glycerol, 10% sodium dodecyl sulfate (SDS), 30% 2-mercaptoethanol, 0.02% bromphenolblue and 0.2 M dithiothreitol). The lysates were homogenized, denatured at 99 °C for 5 min and centrifuged at 4 °C for 10 min. Equal volumes of the protein lysates were loaded on 8% or 15% SDS-PAGE gels and transferred onto polyvinylidene fluoride membranes. For blocking, 5% skim milk (Merck, Darmstadt, Germany) in Tris-buffered saline buffer (0.05% Tween20) was used. The following primary anti-human antibodies were applied: integrins α1 and α11 (both R&D Systems, Minneapolis, MN, USA), integrin α2 (BD Biosciences), Bax (Biolegend, San Diego, CA, USA), phospho-FAK (Invitrogen), total FAK, total and phospho-ERK1/2, and total and phospho-Akt (all Cell Signaling, Danvers, MA, USA). In the western blot analysis, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a loading control allowing for a simultaneous detection with the integrins on the same protein membrane. The antibodies were diluted in the blocking solution and incubated overnight at 4 °C. Secondary horseradish peroxidase-conjugated antibodies (Rockland, Gilbertsville, PA, USA or Santa Cruz) were applied for 1 h at room temperature. Electrochemiluminescence solution (GE Healthcare, Waukesha, WI, USA) and Lumi-detection films (Roche) were used for protein visualization. Band intensities were quantified with ImageProPlus4 software program (Media Cybernetics, Bethesda, MA, USA).
Immunocytochemistry and cytochemistry
hMSC were cultured on 30 μg/ml collagen I-coated glass slides (BD Biosciences) for 48 h. The cells were fixed with methanol or acetone/methanol, treated with Image-iT FX signal enhancer (Invitrogen) and blocked with 10% BSA. Primary antibodies against integrins α1 (R&D Systems), α2 (AbD Serotec, Oxford, UK) and α1137 were applied overnight at 4 °C. Next, secondary Alexa Flour 488-conjugated antibodies and 4′,6-diamidino-2-phenylindole were used (all Invitrogen). For apoptosis analysis, the fluorescent JC-1 dye (Invitrogen) was implicated. shRNA-transduced hMSC (2 × 103 cells/cm2) were cultured on collagen I-coated glass slides for 24 h. On the basis of Kennea et al.,38 to create a positive control for apoptosis, hMSC were treated with 1 μg/ml FAS antibody (BD Biosciences) for 2 h at 37 °C, followed by overnight incubation with 1 μg/ml donkey anti-mouse IgG (Santa Cruz). Next, the cells were incubated with 3 μg/ml JC-1 and 1 μg/ml 2,5′-Bi-1H-benzimidazole (both Invitrogen) solved in complete growth medium for 30 min at 37 °C/5% CO2. Photomicrographs were taken with Axiocam MRm camera (Carl Zeiss, Jena, Germany) on Axioskope 2 or Axiovert100 microscope (Carl Zeiss). Staining procedures were reproduced at least twice with hMSC XI and hMSC XIII.
Proliferation analyses
Long-term cell growth was examined by calculating hMSC cumulative population doubling (PD) and PD time according to Docheva et al.39 S-phase analysis was performed with the Cell Proliferation ELISA, BrdU (colorimetric) kit (Roche) as described in Böker et al.40 Briefly, 96-well dishes were coated with 10 μg/ml collagen I (BD Biosciences), fibronectin, laminin I and PLL (all R&D Systems) and seeded with 3 × 103 hMSC per well. After 12 h, media supplemented with 10 μ BrdU was added. BrdU incorporation was measured after 24 h using microplate reader (Microtek, Overat, Germany) at 450 nm with 690 nm reference wavelength. Three independent experiments were performed in triplicate. For assessment of cell numbers after shRNA transduction, hMSC XI and XV were plated in six-well dishes and microscopically photographed every 24 h for a period of 14 days. Then, two different areas per well (each 1.1 cm2) were used for automated cell counting by ImageJ software program (http://rsb.info.nih.gov/ij).
Adhesion assay
Cell adhesion assay was performed as described in Docheva et al.39 In brief, 96-well plates were coated with 10 μg/ml collagen I, fibronectin, laminin I and blocked with 5% milk/PBS (blotto). hMSC were plated in triplicate (3 × 103 cells per well) and incubated for various time periods (15–120 min) at 37 °C, then non-adherent cells were removed by PBS washing. Cell adhesion was colorimetrically estimated using p-nitrophenyl N-acetyl-beta--glucosaminide (Sigma-Aldrich, Munich, Germany) as a substrate. Absorbance was measured at 405 nm on microtitre-plate reader (Microtek). The percentage of adherent cells was calculated to a maximal reference (suspension of 3 × 103 cells). Two independent experiments were performed.
Live cell imaging
Time lapse experiments were performed with an automated Axiovert100 inverted microscope system (Carl Zeiss) equipped with controlled biochamber (Pecon, Erbach, Germany). hMSC (2 × 104 cells per well) were plated on collagen I-coated six-well dishes. For spreading analysis, cells were imaged immediately after plating with 20 frames/h for 3 h using 20 objective and Axiocam MRm camera (Carl Zeiss). For migration analysis, cells were imaged 3 h after plating for 18 h with 4 frames/h. Three independent movies were produced and approximately 30 cells per type were evaluated. hMSC XI, from two independent viral infections, were used for the time lapse-based experiments. The obtained data were processed with AxioVision LE (Carl Zeiss) and ImageJ software programs.
Osteogenic differentiation
OS was performed as described previously in Böker et al.40 Briefly, hMSC were plated in six-well dishes in a density of 3.5 × 103 cells/cm2. Osteogenic media was applied for 21 days and was changed twice weekly. The osteogenic differentiation was evaluated by AR staining, which visualizes calcium-rich deposits produced by the cells. AR staining and quantification were performed with Osteogenic Quantification kit (Millipore, Billerica, MA, USA) according to the manufacturer's instructions. First, pictures were taken on Axiovert100 microscope with AxioCam ICc3 color camera (Carl Zeiss) and next, AR was extracted with 10% acetic acid and neutralized with 10% NH4OH. Optical density measurements were taken at 405 nm on microtitre-plate reader (Microtek). The AR amount was calculated against an AR standard curve. The experiment was repeated three times.
Statistics
Statistical evaluation was performed using the GraphPrism software (GraphPad, La Jolla, CA, USA). All quantitative data were acquired from two or three independent experiments, each performed with duplicates or triplicates. Graphs and bar charts show mean values and S.D. Unpaired t-test was used for two group analysis and Dunett's one-way ANOVA was applied for multi group statistical testing. A P-value <0.05 was considered statistically significant.
Acknowledgments
DD, CP and MS acknowledge the financial support of the German Research Foundation (DFG Grant: DO 1414/1-1). Parts of this work contributed to the PhD thesis of CP at the LMU Munich. DD and MS acknowledge Professor W Mutschler (Head of Surgery Clinic, LMU) for his constant support of the research laboratory and Dr. Katie Bungartz for carefully reading the paper.
Glossary
- hMSC
human mesenchymal stem cell
- shRNA
short hairpin RNA
- Bax
Bcl-2-associated X protein
- FAK
focal adhesion kinase
- ERK
extracellular signal-regulated protein kinase
- PKB/Akt
serine/threonine protein kinase B
- con-shRNA
control shRNA
- ECM
extracellular matrix
- PLL
poly--lysine
- BrdU
bromodeoxyuridine
- hOB
human osteoblast
- CD271
low-affinity nerve growth factor receptor
- KD
knockdown
- IFNβ
interferon β
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- FAS
CD95 death receptor
- hMSC OH
hMSC from older healthy donor
- hMSC OP
hMSC from older osteoporotic donor
- MNC
mononuclear cell
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- SDS
sodium dodecyl sulfate
- PD
population doubling
- PAR
Alizarin Red
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by P Salomoni
Supplementary Material
References
- Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209–214. doi: 10.1111/j.1742-7843.2004.pto950502.x. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46:371–377. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- Stuiver I, O′Toole TE. Regulation of integrin function and cellular adhesion. Stem Cells. 1995;13:250–262. doi: 10.1002/stem.5530130306. [DOI] [PubMed] [Google Scholar]
- Wiesner S, Legate KR, Fassler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–1099. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP. Anoikis. Cell Death Differ. 2005;12:1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- Stupack DG. Integrins as a distinct subtype of dependence receptors. Cell Death Differ. 2005;12:1021–1030. doi: 10.1038/sj.cdd.4401658. [DOI] [PubMed] [Google Scholar]
- Farrelly N, Lee YJ, Oliver J, Dive C, Streuli CH. Extracellular matrix regulates apoptosis in mammary epithelium through a control on insulin signaling. J Cell Biol. 1999;144:1337–1348. doi: 10.1083/jcb.144.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard V, Harnois C, Demers MJ, Thibodeau S, Laquerre V, Gauthier R, et al. B1 integrin/Fak/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis. 2008;13:531–542. doi: 10.1007/s10495-008-0192-y. [DOI] [PubMed] [Google Scholar]
- Benoit YD, Larrivee JF, Groulx JF, Stankova J, Vachon PH, Beaulieu JF. Integrin alpha8beta1 confers anoikis susceptibility to human intestinal epithelial crypt cells. Biochem Biophys Res Commun. 2010;399:434–439. doi: 10.1016/j.bbrc.2010.07.107. [DOI] [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36:355–363. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Foster LJ, Zeemann PA, Li C, Mann M, Jensen ON, Kassem M. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23:1367–1377. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Bothe I, Hirche F, Zweers M, Gullberg D, Pfitzer G, et al. Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: contribution of alpha2beta1 integrin. J Cell Sci. 2006;119:1886–1895. doi: 10.1242/jcs.02921. [DOI] [PubMed] [Google Scholar]
- Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, Zutter MM. Wound healing in the alpha2beta1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol. 2007;127:455–466. doi: 10.1038/sj.jid.5700611. [DOI] [PubMed] [Google Scholar]
- Popova SN, Rodriguez-Sanchez B, Liden A, Betsholtz C, Van Den BT, Gullberg D. The mesenchymal alpha11beta1 integrin attenuates PDGF-BB-stimulated chemotaxis of embryonic fibroblasts on collagens. Dev Biol. 2004;270:427–442. doi: 10.1016/j.ydbio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Popova SN, Barczyk M, Tiger CF, Beertsen W, Zigrino P, Aszodi A, et al. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol Cell Biol. 2007;27:4306–4316. doi: 10.1128/MCB.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004;11:137–153. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Nho RS, Xia H, Kahm J, Kleidon J, Diebold D, Henke CA. Role of integrin-linked kinase in regulating phosphorylation of Akt and fibroblast survival in type I collagen matrices through a beta1 integrin viability signaling pathway. J Biol Chem. 2005;280:26630–26639. doi: 10.1074/jbc.M411798200. [DOI] [PubMed] [Google Scholar]
- Tiberio R, Marconi A, Fila C, Fumelli C, Pignatti M, Krajewski S, et al. Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl-2 dependent manner. FEBS Lett. 2002;524:139–144. doi: 10.1016/s0014-5793(02)03040-5. [DOI] [PubMed] [Google Scholar]
- Ruhl M, Sahin E, Johannsen M, Somasundaram R, Manski D, Riecken EO, et al. Soluble collagen VI drives serum-starved fibroblasts through S phase and prevents apoptosis via down-regulation of Bax. J Biol Chem. 1999;274:34361–34368. doi: 10.1074/jbc.274.48.34361. [DOI] [PubMed] [Google Scholar]
- Song SW, Chang W, Song BW, Song H, Lim S, Kim HJ, et al. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009;27:1358–1365. doi: 10.1002/stem.47. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP, et al. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107:899–907. doi: 10.1172/JCI10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JP, Montecinos L, Rios S, Reyes P, Martinez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79:557–565. doi: 10.1002/1097-4644(20001215)79:4<557::aid-jcb40>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Perinpanayagam H, Zaharias R, Stanford C, Brand R, Keller J, Schneider G. Early cell adhesion events differ between osteoporotic and non-osteoporotic osteoblasts. J Orthop Res. 2001;19:993–1000. doi: 10.1016/S0736-0266(01)00045-6. [DOI] [PubMed] [Google Scholar]
- Stenderup K, Justesen J, Eriksen EF, Rattan SI, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16:1120–1129. doi: 10.1359/jbmr.2001.16.6.1120. [DOI] [PubMed] [Google Scholar]
- Bellantuono I, Aldahmash A, Kassem M. Aging of marrow stromal (skeletal) stem cells and their contribution to age-related bone loss. Biochim Biophys Acta. 2009;1792:364–370. doi: 10.1016/j.bbadis.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J Interferon Cytokine Res. 2007;27:53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D. cDNA cloning and chromosomal localization of human alpha(11) integrin. A collagen-binding, I domain-containing, beta(1)-associated integrin alpha-chain present in muscle tissues. J Biol Chem. 1999;274:25735–25742. doi: 10.1074/jbc.274.36.25735. [DOI] [PubMed] [Google Scholar]
- Kennea NL, Stratou C, Naparus A, Fisk NM, Mehmet H. Functional intrinsic and extrinsic apoptotic pathways in human fetal mesenchymal stem cells. Cell Death Differ. 2005;12:1439–1441. doi: 10.1038/sj.cdd.4401641. [DOI] [PubMed] [Google Scholar]
- Docheva D, Padula D, Popov C, Mutschler W, Clausen-Schaumann H, Schieker M. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J Cell Mol Med. 2008;12:537–552. doi: 10.1111/j.1582-4934.2007.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böker W, Yin Z, Drosse I, Haasters F, Rossmann O, Wierer M, et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J Cell Mol Med. 2008;12:1347–1359. doi: 10.1111/j.1582-4934.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.