Abstract
Introduction
Pure squamous cell carcinoma of the breast [SCCB] is rare.
Presentation of Case
We report a case of primary squamous cell carcinoma of breast with ipsilateral axillary lymph node metastasis in a 58year old woman.
Discussion
It is a breast carcinoma entirely composed of metaplastic squamous cells that may be keratinized, non-keratinized or spindled. The pure squamous cell carcinoma usually present with central cystic cavity, which we found in our case, also supported by immunohistochemical evidence.
Conclusion
Although a rare breast cancer subtype, SCCB is of considerable interest due to its pathological heterogeneity and differences in clinical behavior and less reported occurrence of nodal metastasis.
Keywords: Squamous cell carcinoma, Breast, Metastatic lymph node
1. Introduction
Squamous cell carcinoma is a well known malignancy of the skin and other organs composed of squamous cells. Squamous cell carcinoma of the breast is very rare. It is important to discriminate this entity from malignancies of the skin of the breast or metastasis of a squamous cell carcinoma somewhere else in the body. In the literature only some small series are reported.1–3 Reported incidences of primary squamous cell carcinoma of the breast vary between 0.1% to less than 0.04% of all breast carcinomas.1–3 Clinical and radiographic characteristics are not specific, and tumors are usually hormone receptor negative. In general, these are very aggressive, treatment-refractory tumors, with a poor prognosis. We report a case of this rare breast malignancy with metastasis to ipsilateral axillary lymph node and review the literature.
2. Case report
2.1. Clinical presentation
A 58-year-old, previously healthy female presented with a mass in the right breast of four months duration. Physical examination revealed firm, non tender mobile lesion in her right breast with axillary lymphadenopathy. Mammography revealed a tumor (without microcalcifications). Modified radical mastectomy with axillary clearance was performed. The specimens demonstrated a cystic mass of 3 cm × 4 cm containing blood mixed necrotic material [Fig. 1a]. HP section showed tumoural structure composed of atypical keratinocytes in different sizes with narrow eosinophillic cytoplasm and moderate mitotic activity on a background of fibrolipomatous breast stroma [Fig. 2a]. There was also presence of a large central necrotic area [Fig. 1c]. In some areas abortive pearl formation also was noted [Fig. 2c]. The overlying skin was also unremarkable [Fig. 1a]. Estrogen receptors and progesterone receptors were negative in tumor cells with positive internal control of normal ducts [Fig. 3]. Ipsilateral axillary lymph node showed metastatic deposit of squamous cell carcinoma [Fig. 1d]. Metastatic disease to the breast was ruled out. CT scan thorax and abdomen was within normal limits. Other routine laboratory parameters at the point of diagnosis were unremarkable. The patient had no history of skin cancer, nor did she have any skin, oral pharynx, or anal lesions. According to all these findings the patient was diagnosed with primary large cell keratinizing squamous cell carcinoma [moderately differentiated] of the breast with metastasis to axillary lymph node. After the operation patient suffered from severe dyselectrolitemia for this she was kept in intensive care unit and supportive treatment was given. On the 3rd post operative day patient died due to cardio-respiratory failure.
Fig. 1.
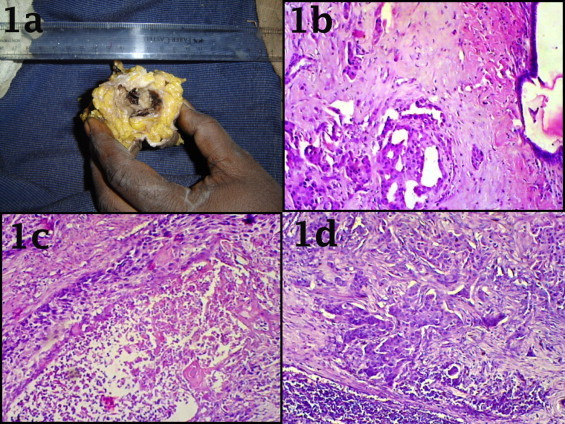
[a] Gross picture of breast specimen having a cystic mass of 3 cm × 4 cm size containing blood mixed necrotic material with attached skin grossly appeared free from tumor. Photomicrograph showing Tumoural area of breast with dilated duct showing squamoid metaplastic changes [b], cystic area containing necrotic material, inflammatory cells, giant cells and lined by malignant squamous cells [c]. Metastatic tumor within the lymph node [d] [H&E stain −40×].
Fig. 2.
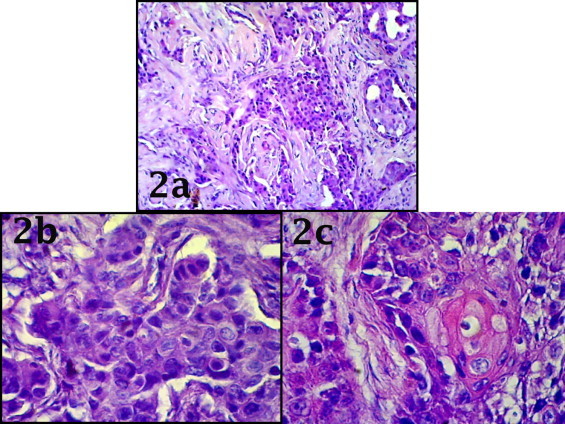
Photomicrograph showing tumor proper composed of atypical keratinocytes with moderate mitotic activity on a background of fibrous breast stroma [a]. Individual cells having abundant eosinophillic cytoplasm and hyperchromatic pleomorphic nuclei [b] with abortive squamous pearl formation. [c] [H&E stain 40×].
Fig. 3.
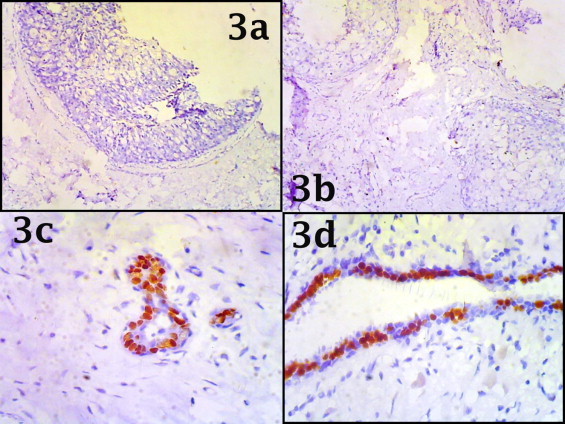
Photomicrograph showing tumoural area with estrogen and progesterone receptor negativity [a and b] [10×] with positive internal control [c and d] [40×].
3. Discussion
The histogenesis of this rare malignancy is unclear.4 Theories include malignant growth of intrinsic epidermal elements (epidermal or dermoid cysts) and metaplasia from breast parenchyma (benign disease, e.g., cystosarcoma phylloides, fibroadenomas, or breast malignancies, e.g., intraductal carcinoma,) or from chronic abscess.5 Perhaps not surprisingly, these tumors have varying degrees of homogeneity. A tumor is considered “pure SCC [Squamous cell carcinoma]” if it meets Macia et al. criteria,6 including: (1) no other neoplastic components are present, such as ductal or mesenchymal elements in tumor, (2) the tumor is independent of adjacent cutaneous structures, (3) no other primary epidermoid tumors are present in the patient (oral cavity bronchus, esophagus, renal pelvis, bladder, ovary, and cervix).6,7
SCCB [Squamous cell carcinoma Breast] has been diagnosed in adult women of ages ranging from 29 years to 90 years,8 with a median of 52 years of age.9 In contrast to most breast cancers these tumors are unusually rapidly growing. Patients typically report a breast mass that enlarged over a period of 2–3 weeks, or in some cases for as long as 18 months.4,8 Primary tumors tend to be relatively large (range 2–5 cm, median 4 cm).4,10 Our case was 58-year-old with duration of the disease four months, which is supported by literature. Approximately two thirds of these tumors are cystic or have a cystic component with central necrosis7,9 which we found in our case. Axillary lymph node metastasis occurs rarely and when it does is usually associated with metaplastic SCCB arising in an invasive ductal carcinoma and we have no such evidence in our case though it showed ipsilateral axillary lymph node metastasis.
Estrogen and progesterone receptors are negative in more than 90% of the cases of pure SCCB8,11,12 which was also proven in our case. The only case of HER-2/neu over expression in SCCB was reported by Karamouzis et al.11 There is only one reported case of a BRCA 1 gene mutation in a patient with SCCB.12
SCCB does not have characteristic mammographic features. Some tumors have been reported to have irregular, indistinct borders, whereas others have been reported to have well circumscribed borders.13 The most consistent feature of SCCB on mammogram is the lack of microcalcifications.4 Only one reported case of SCCB has shown microcalcifications on mammogram.14 Our case followed the norm by not showing microcalcifications on mammogram.
Prognosis appears to be dependent upon several factors, most importantly tumor size and tumor stage.13 The SEER database from 1988 to 2001 included 137 cases of SCCB with a 5-year survival rate of 64%.1
The initial management of SCCB is modified radical mastectomy with adjuvant radiotherapy and or chemotherapy/hormonal therapy. Breast conservation therapy is not usually possible because most patients present with locally advanced disease.15,16 Because squamous cell cancers are often radiosensitive Hennessy et al. proposed early adjuvant radiotherapy despite being unable to demonstrate a difference (presumably because of small numbers) in the loco-regional relapse-free rate of 45% among those receiving vs 33% among those not receiving radiotherapy.15 Adjuvant and neoadjuvant chemotherapy regimens used at M.D. Anderson Cancer Center include 5-flourouracil alone, 5-flourouracil/cisplatin, 5-flourouracil/taxane, 5-flourouracil/cisplatin followed by pacitaxel, and cyclophosphamide plus methotrexate plus luorouracil.15,16 Hennessy et al. reported no benefit to neoadjuvant therapy.15
4. Conclusion
Squamous cell carcinoma of the breast is a rare, generally aggressive disease associated with locoregional and distant relapses. Current surgical management is similar to that for the more common adenocarcinoma. However because effective adjuvant or neoadjuvant therapy is not available, future research should focus on the molecular biology, (e.g., epidermal growth factor receptor), to develop tumor-specific therapy.
Contributors
Case was attended in the CDC outdoor by Dr. Bhaskar Mitra and Dr. Ashok Maiti, subsequently Dr. Mallika Pal and Dr. Tarak N Saha including the previous two authors who helped in diagnosing the case and lastly Dr. Sharmistha Debnath helped to perform the immunohistochemistry part. Subsequently all the authors collected, compiled the data and formulated the manuscript. The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Conflict of interest
None.
Funding
The source of funding was Institutional funds of Midnapore Medical College & Hospital, Paschim Medinipur.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. Copy of that consent can be produced whenever required.
References
- 1.Gupta G., Malani A.K., Weigand R.T., Rangenini G. Pure primary squamous cell carcinoma of the breast: a rare presentation and clinicopathologic comparison with usual ductal carcinoma of the breast. Pathol Res Pract. 2006;6:465–469. doi: 10.1016/j.prp.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Behranwala K.A., Nasiri N., Abdullah N., Trott P.A., Gui G.P.H. Squamous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol. 2003;29:386–389. doi: 10.1053/ejso.2002.1422. [DOI] [PubMed] [Google Scholar]
- 3.Wrightson W.R., Edwards M.J., McMasters K.M. Primary squamous cell carcinoma of the breast presenting as a breast abscess. Am Surg. 1999;65(12):1153–1155. [PubMed] [Google Scholar]
- 4.Toikkanen S. Primary squamous cell carcinoma of the breast. Cancer. 1981;48:1629–1632. doi: 10.1002/1097-0142(19811001)48:7<1629::aid-cncr2820480726>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Cappellani A., Di Vita M., Zanghì A., De Luca A., Tomarchio G., La Porta D. A pure squamous cell breast carcinoma presenting as a breast abscess: a case report and review of the literature. Ann Ital Chir. 2004;LXXV(2):259–263. [PubMed] [Google Scholar]
- 6.Macia M., Ces J.A., Becerra E., Novo A., Macia M., Ces J.A. Pure squamous carcinoma of the breast; report of a case diagnosed by aspiration cytology. Acta Cytol. 1989;33:201–204. [PubMed] [Google Scholar]
- 7.Shigekawa T., Tsuda H., Sato K., Ueda S., Asakawa H., Shigenaga R. Squamous cell carcinoma of the breast in the form of an intracystic tumor. Breast cancer. 2007;14:109–112. doi: 10.2325/jbcs.14.109. [DOI] [PubMed] [Google Scholar]
- 8.Siegelmann-Danieli N., Murphy T.J., Meschter S.C., Stein M.E., Prichard J. Primary squamous cell carcinoma of the breast. Clinical of breast cancer. 2005;6(3):270–272. doi: 10.3816/CBC.2005.n.030. [DOI] [PubMed] [Google Scholar]
- 9.Weigel R.J., Ikeda D.M., Nowels KW Primary squamous cell carcinoma of the breast. South Med J. 1996;89:511–515. doi: 10.1097/00007611-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson J.T., Graham D.J., Khiyami A., Edward G. Squamous cell carcinoma of the breast: a clinical approach. Annals Surg Oncol. 1996;3(4):367–374. doi: 10.1007/BF02305666. [DOI] [PubMed] [Google Scholar]
- 11.Karamouzis M.V., Fida A., Apostolikas N., Rigatos G. A case of Her-2 positive squamous cell breast carcinoma: an unusual presentation of an unusual clinical entity. EJSO. 2006;32:1250–1251. doi: 10.1016/j.ejso.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Breuer A., Kandel M., Fisseler-Eckhoff A., Sutter C., Schwaab E., Lück H. BRCA-1 germline mutation in a women with metaplastic squamous cell breast cancer. Onkologie. 2007;30:316–318. doi: 10.1159/000101515. [DOI] [PubMed] [Google Scholar]
- 13.Wargotz E.S., Norris H.J. Metaplastic carcinomas of the breast: V. Metaplastic carcinoma with osteoclastic giant cells. Hum Pathol. 1990;21(11):1142–1150. doi: 10.1016/0046-8177(90)90151-t. [DOI] [PubMed] [Google Scholar]
- 14.Bogomoletz W.V. Pure squamous cell carcinoma of breast. Arch Pathol Lab Med. 1982;106:57–59. [PubMed] [Google Scholar]
- 15.Hennessy B.T., Krishnamurthy S., Giordano S., Buchholz T.A., Kau S.W., Zhigang D. Squamous cell carcinoma of the breast. J Clin Oncol. 2005;23:7827–7835. doi: 10.1200/JCO.2004.00.9589. [DOI] [PubMed] [Google Scholar]
- 16.Rosen PR . Lippincott-Raven; Philadelphia, New York: 1997. Rosen's breast pathology. [Chapter 21, p. 397–404] [Google Scholar]


