Abstract
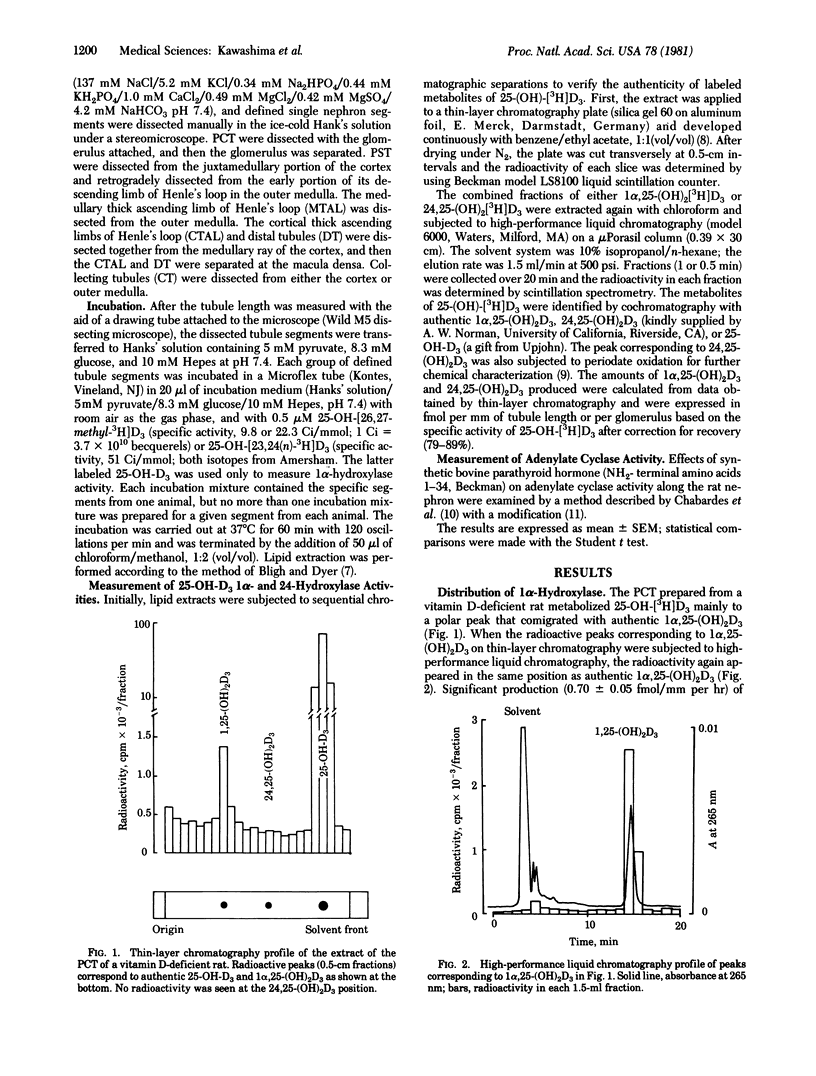
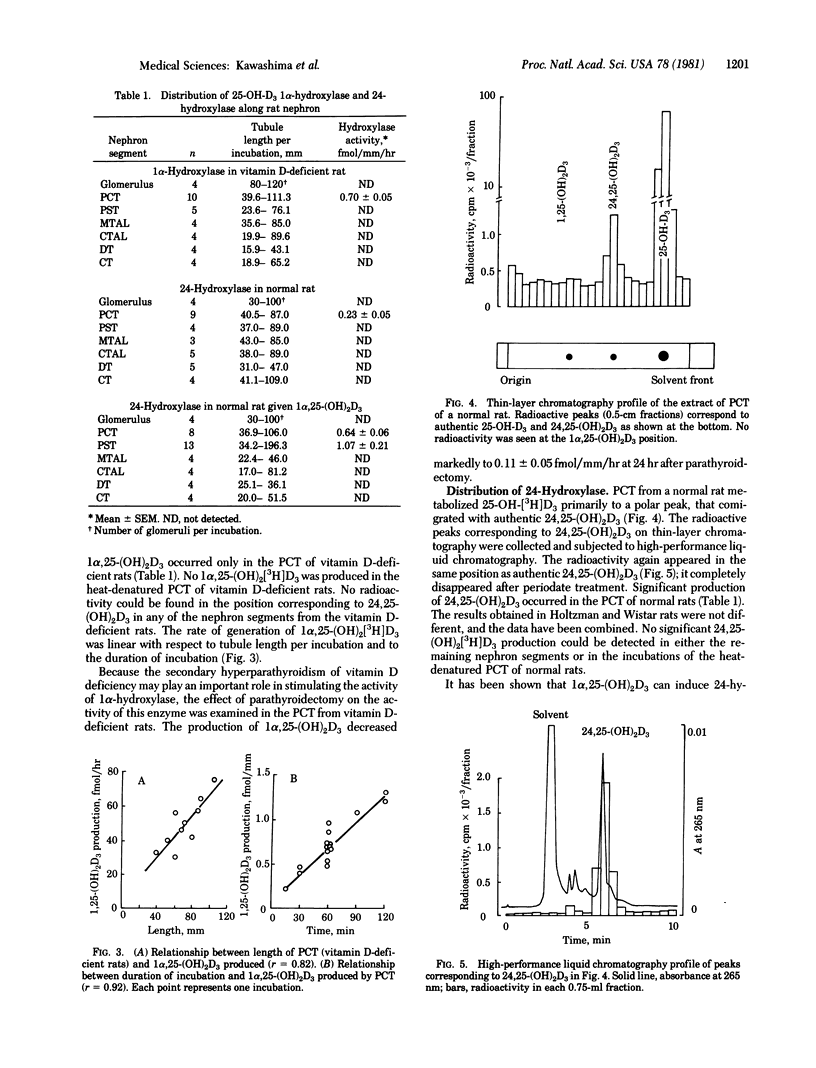
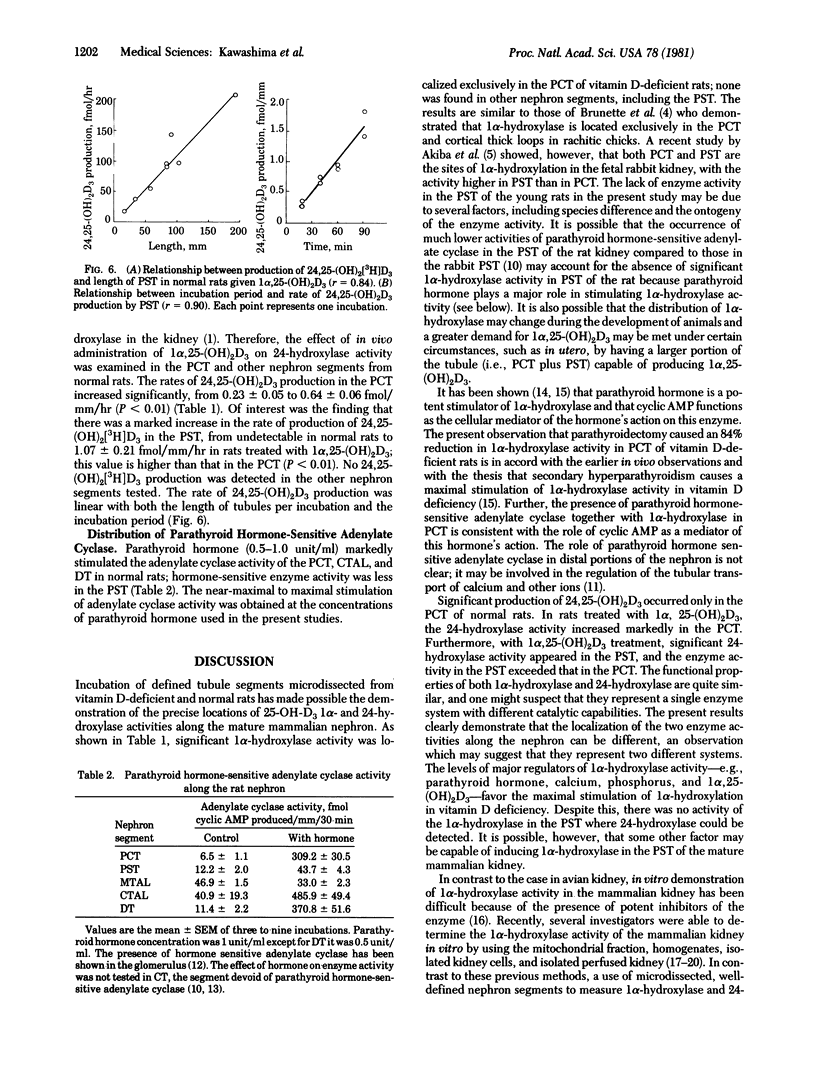
Defined nephron segments were microdissected from the kidney of vitamin D-deficient rats, normal rats, and normal rats treated with 1 alpha, 25-dihydroxyvitamin D3 [1 alpha, 25-(OH)2D3]. Tubule segments were incubated with 3H]labeled 25-hydroxyvitamin D3 and the rates of production of 3H]labeled 1 alpha, 25-(OH)2D3 and 24,25-dihydroxyvitamin D3 [24,25-(OH)2D3] were determined. Nephron segments tested include the glomerulus, proximal convoluted tubules (PCT), proximal straight tubules (PST), medullary and cortical thick ascending limbs of Henle's loop, distal tubules, and collecting tubules. Production of 1 alpha, 25-(OH)2D3 was detected only in PCT of vitamin D-deficient rats (mean +/- SEM, 0.70 +/- 0.05 fmol/mm per hr); the value decreased to 0.11 +/- 0.05 after parathyroidectomy. By contrast, significant 24,25-(OH)2D3 production occurred in PCT of normal rats (0.23 +/- 0.05 fmol/mm per hr). After administration of 1 alpha, 25-(OH)2D3 to normal rats, the rate of production of 24,25-(OH)2D3 in PCT increased to 0.64 +/- 0.06 fmol/mm per hr and also became apparent in PST (1.07 +/- 0.21). The rates of production of 1 alpha, 25-(OH)2D3 and 24,25-(OH)2D3 in these nephron segments were linear with tubule length over a wide range of lengths per incubation and with the incubation time. The results define the localization of 25-hydroxyvitamin D3 1 alpha-hydroxylase and 24-hydroxylase along the rat nephron: PCT is capable of producing both 1 alpha, 25-(OH)2D3 and 24,25-(OH)2D3 and PST can produce 24,25-(OH)2D3. the use of defined nephron segments may be useful for study of the distribution and regulation of 25-hydroxyvitamin D3 hydroxylases in the kidney.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiba T., Endou H., Koseki C., Sakai F., Horiuchi N., Suda T. Localization of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the mammalian kidney. Biochem Biophys Res Commun. 1980 May 14;94(1):313–318. doi: 10.1016/s0006-291x(80)80222-1. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Botham K. M., Tanaka Y., DeLuca H. F. 25-Hydroxyvitamin D3-1-hydroxylase. Inhibition in vitro by rat and pig tissues. Biochemistry. 1974 Nov 19;13(24):4961–4966. doi: 10.1021/bi00721a014. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Chan M., Ferriere C., Roberts K. D. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978 Nov 16;276(5685):287–289. doi: 10.1038/276287a0. [DOI] [PubMed] [Google Scholar]
- Chabardès D., Gagnan-Brunette M., Imbert-Teboul M., Gontcharevskaia O., Montégut M., Clique A., Morel F. Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest. 1980 Feb;65(2):439–448. doi: 10.1172/JCI109687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabardès D., Imbert M., Clique A., Montégut M., Morel F. PTH sensitive adenyl cyclase activity in different segments of the rabbit nephron. Pflugers Arch. 1975;354(3):229–239. doi: 10.1007/BF00584646. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Gray R. W., Boyle I. T., Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972 Nov 7;11(23):4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- Horiuchi N., Suda T., Takahashi H., Shimazawa E., Ogata E. In vivo evidence for the intermediary role of 3',5'-cyclic AMP in parathyroid hormone-induced stimulation of 1alpha,25-dihydroxyvitamin D3 synthesis in rats. Endocrinology. 1977 Sep;101(3):969–974. doi: 10.1210/endo-101-3-969. [DOI] [PubMed] [Google Scholar]
- Kumar R., Schnoes H. K., DeLuca H. F. Rat intestinal 25-hydroxyvitamin D3- and 1alpha,25-dihydroxyvitamin D3-24-hydroxylase. J Biol Chem. 1978 Jun 10;253(11):3804–3809. [PubMed] [Google Scholar]
- Norman A. W., Wong R. G. Biological activity of the vitamin D metabolite 1,25-dihydroxycholecalciferol in chickens and rats. J Nutr. 1972 Dec;102(12):1709–1718. doi: 10.1093/jn/102.12.1709. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. M., Jones G., Kooh S. W., Fraser D. 25-hydroxyvitamin D3 metabolism by isolated perfused rat kidney. Am J Physiol. 1980 Jul;239(1):E12–E20. doi: 10.1152/ajpendo.1980.239.1.E12. [DOI] [PubMed] [Google Scholar]
- Sommerville B. A., Fox J., Care A. D., Swaminathan R. The in vitro metabolism of 25-hydroxycholecalciferol by pig kidney: effect of low dietary levels of calcium and phosphorus. Br J Nutr. 1978 Jul;40(1):159–162. doi: 10.1079/bjn19780107. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Mawer E. B., Wallace J. E., St John J., Cochran M., Russell R. G., Kanis J. A. The absence of 24,25-dihydroxycholecalciferol in anephric patients. Clin Sci Mol Med Suppl. 1978 Dec;55(6):541–547. doi: 10.1042/cs0550541. [DOI] [PubMed] [Google Scholar]
- Torikai S., Imai M. A simple method to determine adenylate cyclase activity in isolated single nephron segments by radioimmunoassay for succinyl adenosine 3',5'-cyclic monophosphate. Tohoku J Exp Med. 1979 Sep;129(1):91–99. doi: 10.1620/tjem.129.91. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Bottemiller B. L., Howard G. A., Baylink D. J. In vitro metabolism of 25-hydroxyvitamin D3 by isolated rat kidney cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1537–1540. doi: 10.1073/pnas.77.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth R., Fraser D. Kinetic behavior of 25-hydroxyvitamin D-1-hydroxylase and -24-hydroxylase in rat kidney mitochondria. J Biol Chem. 1979 Dec 25;254(24):12455–12460. [PubMed] [Google Scholar]



