Abstract
Seven out of 31 (23%) human malignant tumor cell lines had no detectable methylthioadenosine phosphorylase activity (less than 0.001 nmol/min per mg of protein), assayed with 5'-chloroadenosine as substrate. The enzyme-deficient cell lines were derived from five leukemias, one melanoma, and one breast cancer. None of 16 cell lines of nonmalignant origin, derived from lymphocytes, fibroblasts, and epithelial cells, lacked the enzyme (range, 0.156-1.447 nmol/min per mg of protein). As detected by autoradiography, intact enzyme-positive cell lines normal immature bone marrow cells, and four specimens of malignant tumor cells incorporated the adenine moiety of 5'-chloroadenosine into nucleic acids; however, no enzyme-deficient cell lines used 5'-chloroadenosine. When both types of cell lines were cultured in a medium containing 0.4 microM methotrexate, 16 microM uridine, and 16 microM thymidine (or 10 microM azaserine alone), no cells grew. If methylthioadenosine was added to the same medium, only enzyme-positive cells increased in number; most enzyme-deficient cells were dead after 3 days. Thus, human malignant tumor cell lines naturally deficient in methylthioadenosine phosphorylase could be selectively killed when de novo purine synthesis was inhibited and methylthioadenosine was the only exogenous source of purines.
Full text
PDF
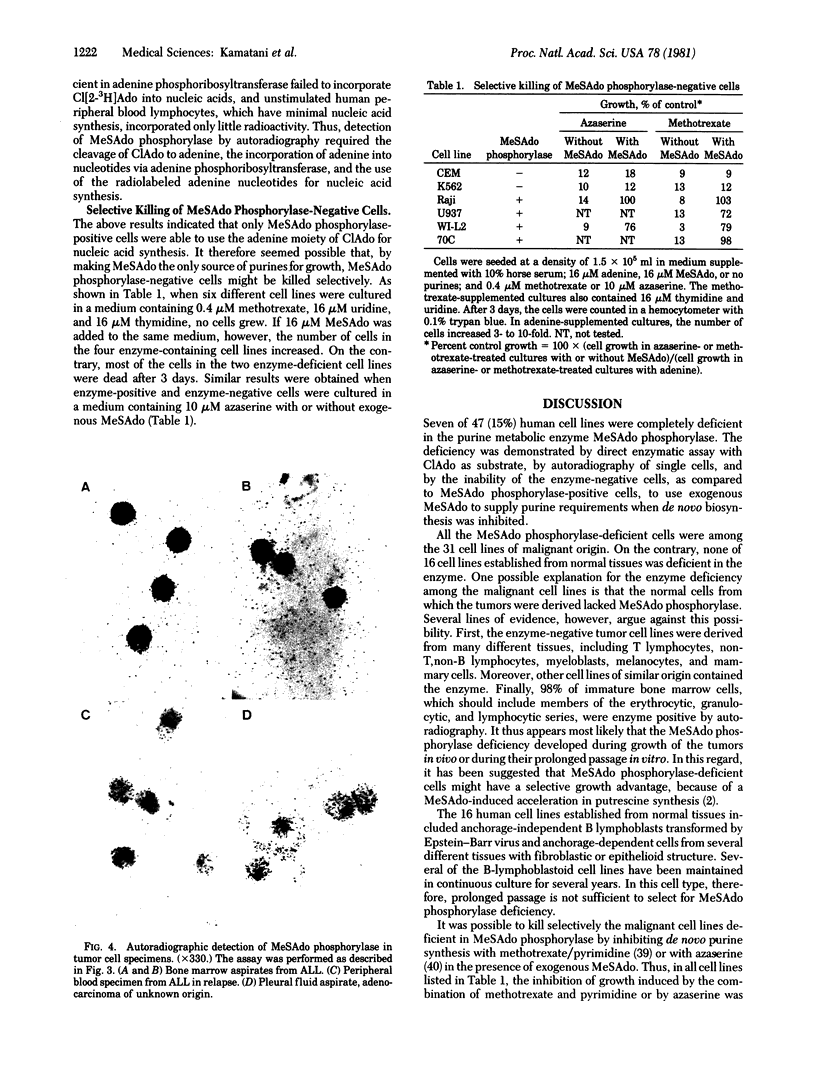

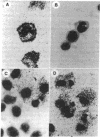
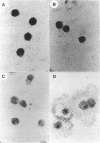
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnstein P., Taylor D. O., Nelson-Rees W. A., Huebner R. J., Lennette E. H. Propagation of human tumors in antithymocyte serum-treated mice. J Natl Cancer Inst. 1974 Jan;52(1):71–84. doi: 10.1093/jnci/52.1.71. [DOI] [PubMed] [Google Scholar]
- BENNETT L. L., Jr, SCHABEL F. M., Jr, SKIPPER H. E. Studies on the mode of action of azaserine. Arch Biochem Biophys. 1956 Oct;64(2):423–436. doi: 10.1016/0003-9861(56)90286-7. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Seegmiller J. E. Effect of adenosine deaminase inhibition upon human lymphocyte blastogenesis. J Clin Invest. 1976 Feb;57(2):274–282. doi: 10.1172/JCI108278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasey A. A., Smith H. S., Hackett A. J., Fukuyama K., Epstein W. L., Madin S. H. Biological properties of human melanoma cells in culture. In Vitro. 1979 May;15(5):342–350. doi: 10.1007/BF02616140. [DOI] [PubMed] [Google Scholar]
- Crooks P. A., Dreyer R. N., Coward J. K. Metabolism of S-adenosylhomocysteine and S-tubercidinylhomocysteine in neuroblastoma cells. Biochemistry. 1979 Jun 12;18(12):2601–2609. doi: 10.1021/bi00579a026. [DOI] [PubMed] [Google Scholar]
- Ensminger W. D., Frei E., 3rd The prevention of methotrexate toxicity by thymidine infusions in humans. Cancer Res. 1977 Jun;37(6):1857–1863. [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L. Demonstration of 5'-methylthioadenosine phosphorylase activity in various rat tissues. Some properties of the enzyme from rat lung. Biochim Biophys Acta. 1978 Mar 14;523(1):82–93. doi: 10.1016/0005-2744(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Ikeda R. M., Erickson K., Owens R. Enhancement of heterotransplanted human tumor graft survival in nude mice treated with antilymphocyte serum and in congenitally athymic-asplenic (Lasat) mice. J Natl Cancer Inst. 1978 Jul;61(1):245–248. doi: 10.1093/jnci/61.1.245. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T. Prevention of toxicity of amethopterin for sarcoma-180 cells in tissue culture. Science. 1957 Aug 9;126(3267):255–255. doi: 10.1126/science.126.3267.255. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Smith H. S., Springer E. L., Owens R. B., Nelson-Rees W. A., Riggs J. L., Gardner M. B. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J Natl Cancer Inst. 1977 Jun;58(6):1795–1806. doi: 10.1093/jnci/58.6.1795. [DOI] [PubMed] [Google Scholar]
- Hiraki S., Miyoshi I., Kubonishi I., Matsuda Y., Nakayama T., Kishimoto H., Masuji H. Human leukemic "null" cell line (NALL-1). Cancer. 1977 Nov;40(5):2131–2135. doi: 10.1002/1097-0142(197711)40:5<2131::aid-cncr2820400523>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Howell S. B., Ensminger W. D., Krishan A., Frei E., 3rd Thymidine rescue of high-dose methotrexate in humans. Cancer Res. 1978 Feb;38(2):325–330. [PubMed] [Google Scholar]
- Kamatani N., Carson D. A. Abnormal regulation of methylthioadenosine and polyamine metabolism in methylthioadenosine phosphorylase-deficient human leukemic cell lines. Cancer Res. 1980 Nov;40(11):4178–4182. [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- LASFARGUES E. Y., OZZELLO L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958 Dec;21(6):1131–1147. [PubMed] [Google Scholar]
- Levy J. A., Virolainen M., Defendi V. Human lymphoblastoid lines from lymph node and spleen. Cancer. 1968 Sep;22(3):517–524. doi: 10.1002/1097-0142(196809)22:3<517::aid-cncr2820220305>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lotan R. Different susceptibilities of human melanoma and breast carcinoma cell lines to retinoic acid-induced growth inhibition. Cancer Res. 1979 Mar;39(3):1014–1019. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Cytotoxicity of a factor isolated from human spleen. J Natl Cancer Inst. 1973 Feb;50(2):535–538. doi: 10.1093/jnci/50.2.535. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Miyoshi I., Hiraki S., Tsubota T., Kubonishi I., Matsuda Y., Nakayama T., Kishimoto H., Kimura I., Masuji H. Human B cell, T cell and null cell leukaemic cell lines derived from acute lymphoblastic leukaemias. Nature. 1977 Jun 30;267(5614):843–844. doi: 10.1038/267843a0. [DOI] [PubMed] [Google Scholar]
- Morikawa S., Tatsumi E., Baba M., Harada T., Yasuhira K. Two E-rosette-forming lymphoid cell lines. Int J Cancer. 1978 Feb 15;21(2):166–170. doi: 10.1002/ijc.2910210207. [DOI] [PubMed] [Google Scholar]
- Ohsugi Y., Gershwin M. E., Owens R. B., Nelson-Rees W. A. Tumorigenicity of human malignant lymphoblasts: comparative study with unmanipulated nude mice, antilymphocyte serum-treated nude mice, and X-irradiated nude mice. J Natl Cancer Inst. 1980 Oct;65(4):715–718. doi: 10.1093/jnci/65.4.715. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Nelson-Rees W. A., Springer E. L. Epithelial cell cultures from normal and cancerous human tissues. J Natl Cancer Inst. 1976 Apr;56(4):843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Phosphate-stimulated breakdown of 5'-methylthioadenosine by rat ventral prostate. Biochem J. 1969 Nov;115(2):241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue S. W., Kimball R. F., Hsie A. W. A method for combined 3H and 14C autoradiography with a single emulsion tested in cultured mammalian cells. Exp Cell Res. 1977 Jun;107(1):47–54. doi: 10.1016/0014-4827(77)90384-6. [DOI] [PubMed] [Google Scholar]
- RHODES J. B., WILLIAMS-ASHMAN H. G. OBSERVATIONS ON POLYAMINES IN MALE ACCESSORY GLANDS OF REPRODUCTION. Med Exp Int J Exp Med. 1964;10:281–285. doi: 10.1159/000135428. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C., Goutner A., Choquet C., Venuat A. M., Kayibanda B., Pico J. L., Greaves M. F. Phenotypic characterisation of a unique non-T, non-B acute lymphoblastic leukaemia cell line. Nature. 1977 Jun 30;267(5614):841–843. doi: 10.1038/267841a0. [DOI] [PubMed] [Google Scholar]
- Savarese T. M., Crabtree G. W., Parks R. E., Jr Reaction of 5'-deoxyadenosine and related analogs with the 5'-methylthioadenosine cleaving enzyme of sarcoma 180 cells, a possible chemotherapeutic target enzyme. Biochem Pharmacol. 1979 Jul 15;28(14):2227–2230. doi: 10.1016/0006-2952(79)90211-9. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. Autoradiography. Methods Enzymol. 1979;58:279–292. doi: 10.1016/s0076-6879(79)58143-9. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Methylthio group cleavage from methylthioadenosine. Description of an enzyme and its relationship to the methylthio requirement of certain cells in culture. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1273–1280. doi: 10.1016/0006-291x(77)91430-9. [DOI] [PubMed] [Google Scholar]
- Young R. K., Cailleau R. M., Mackay B., Reeves W. J. Establishment of epithelial cell line MDA-MB-157 from metastatic pleural effusion of human breast carcinoma. In Vitro. 1974 Jan-Feb;9(4):239–245. doi: 10.1007/BF02616069. [DOI] [PubMed] [Google Scholar]