Abstract
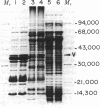
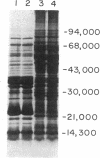
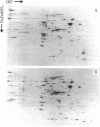
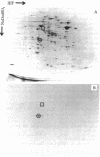
A procedure is described for fractionating Yersinia grown in small cultures into inner and outer membranes and soluble cytoplasmic proteins. The procedure was applied to the three recognized species of the genus grown under conditions simulating mammalian intracellular fluid with respect to Ca2+ and Mg2+. These conditions are known to elicit the production of the plague virulence antigen V. Isolates capable of making this antigen were compared with virulence-antigen-negative derivatives by two-dimensional electrophoresis. The V antigen was localized to the soluble protein fraction as a peptide that comigrates with the major component of a specific immunoprecipitate. This peptide had an apparent molecular weight of 38,000 and was not found in either apparent molecular weight of 38,000 and was not found in either membrane fraction. The comparison of virulence antigen-producers and nonproducers of Y. pseudotuberculosis and Y. enterocolitica revealed large qualitative and quantitative differences in outer membrane protein patterns, whereas the same comparison for Y. pestis showed only minor differences. The complexity of changes in the various protein fractions corroborate data in the literature indicating that extensive physiological changes occur in virulent organisms cultivated under simulated intracellular conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. The effects of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br J Exp Pathol. 1958 Jun;39(3):278–291. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960 Feb;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W. Virulence of Pasteurella pestis. Nature. 1957 Jun 15;179(4572):1246–1247. doi: 10.1038/1791246a0. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Varga C. F., Keet E. E. New strain of Yersinia enterocolitica pathogenic for rodents. Appl Microbiol. 1973 Dec;26(6):1016–1018. doi: 10.1128/am.26.6.1016-1018.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWTON W. D., ERDMAN R. L., SURGALLA M. J. BIOSYNTHESIS AND PURIFICATION OF V AND W ANTIGEN IN PASTEURELLA PESTIS. J Immunol. 1963 Aug;91:179–184. doi: 10.21236/ad0299868. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Sato T., Ito K., Yura T. Membrane proteins of Escherichia coli K-12: two-dimensional polyacrylamide gel electrophoresis of inner and outer membranes. Eur J Biochem. 1977 Sep;78(2):557–567. doi: 10.1111/j.1432-1033.1977.tb11769.x. [DOI] [PubMed] [Google Scholar]
- Zahorchak R. J., Charnetzky W. T., Little R. V., Brubaker R. R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979 Sep;139(3):792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]